Abstract
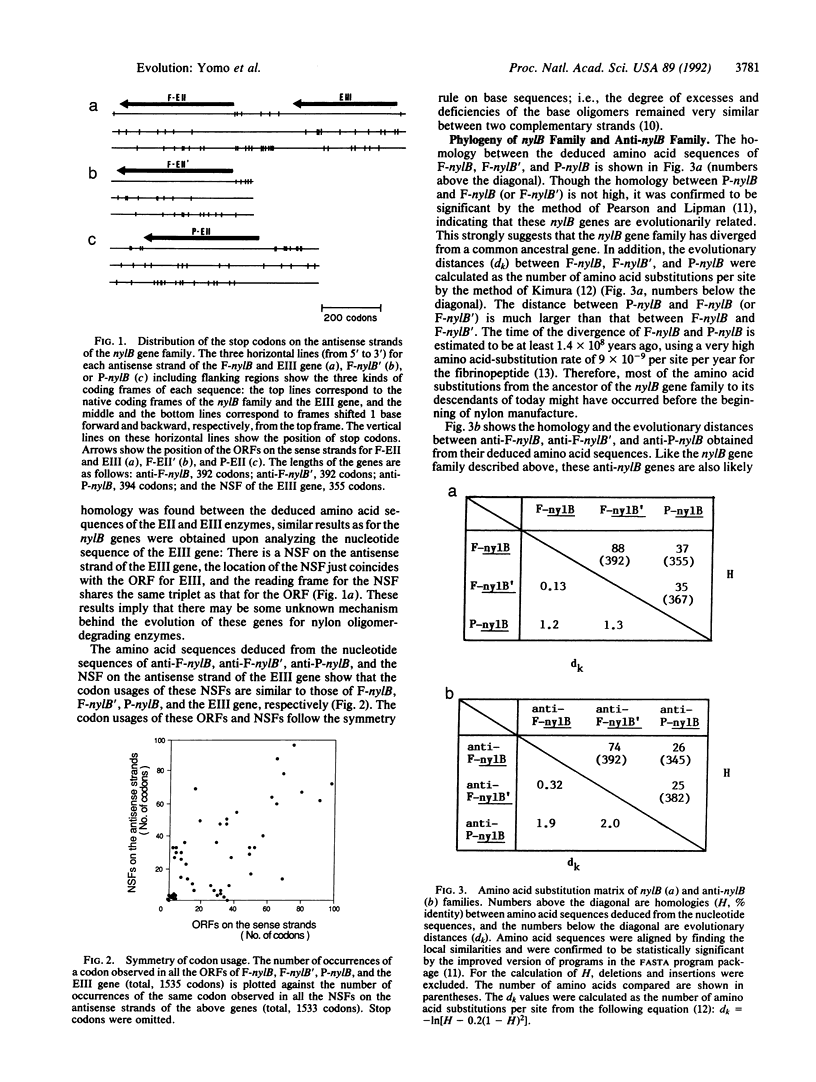
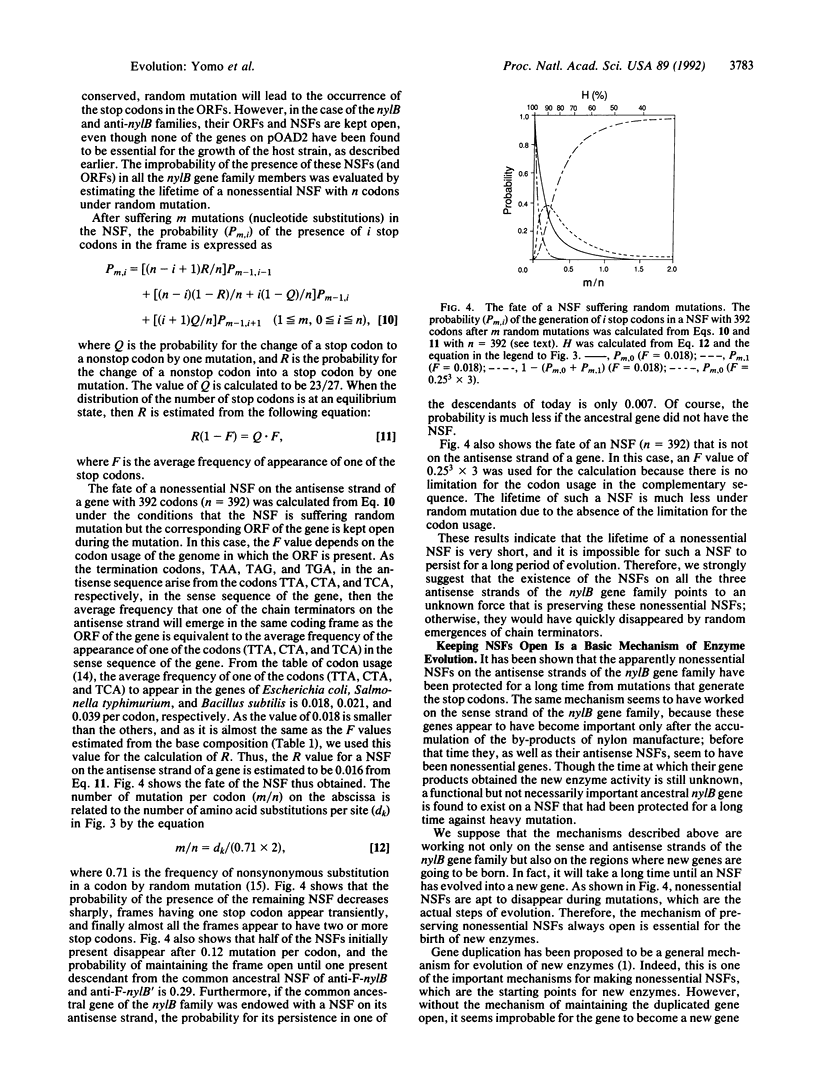
Genes for nylon oligomer-degrading enzymes are unique in the sense that the enzymes encoded by them are found not to have any appropriate substrates during most of the period of their evolution. Furthermore, these nylB genes form a family not related to any other known gene families. The base sequences of these genes were examined and a common characteristic was found: a long stretch of sequence without chain-terminating base triplets, defined as a nonstop frame (NSF), is being maintained on the antisense strand. Moreover, a certain coding frame is open for both the sense and the antisense sequences, while the other frames have many stop codons. The probability of the presence of these NSFs on the antisense strand of a gene is very small (0.0001-0.0018). In addition, another gene for nylon oligomer degradation was found to have a NSF on its antisense strand, and this gene is phylogenically independent of the nylB genes. Therefore, the presence of these NSFs is very rare and improbable. Even if the common ancestral gene of the nylB family was originally endowed with a NSF on its antisense strand, the probability of this original NSF persisting in one of its descendants of today is only 0.007. Unless an unknown force was maintaining the NSF, it would have quickly disappeared by random emergences of chain terminators. Therefore, the presence of such rare NSFs on all three antisense strands of the nylB gene family suggests that there is some special mechanism for protecting these NSFs from mutations that generate the stop codons. Such a mechanism may enable NSFs to evolve into new functional genes and hence seems to be a basic mechanism for the birth of new enzymes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman J. P., Bond C. T., Douglass J., Herbert E. Two mammalian genes transcribed from opposite strands of the same DNA locus. Science. 1987 Mar 20;235(4795):1514–1517. doi: 10.1126/science.3547652. [DOI] [PubMed] [Google Scholar]
- Aota S., Gojobori T., Ishibashi F., Maruyama T., Ikemura T. Codon usage tabulated from the GenBank Genetic Sequence Data. Nucleic Acids Res. 1988;16 (Suppl):r315–r402. doi: 10.1093/nar/16.suppl.r315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock J. E. Complementarity of peptides specified by 'sense' and 'antisense' strands of DNA. Trends Biotechnol. 1990 Jun;8(6):140–144. doi: 10.1016/0167-7799(90)90159-u. [DOI] [PubMed] [Google Scholar]
- Kanagawa K., Negoro S., Takada N., Okada H. Plasmid dependence of Pseudomonas sp. strain NK87 enzymes that degrade 6-aminohexanoate-cyclic dimer. J Bacteriol. 1989 Jun;171(6):3181–3186. doi: 10.1128/jb.171.6.3181-3186.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita S., Negoro S., Muramatsu M., Bisaria V. S., Sawada S., Okada H. 6-Aminohexanoic acid cyclic dimer hydrolase. A new cyclic amide hydrolase produced by Achromobacter guttatus KI74. Eur J Biochem. 1977 Nov 1;80(2):489–495. doi: 10.1111/j.1432-1033.1977.tb11904.x. [DOI] [PubMed] [Google Scholar]
- Kinoshita S., Terada T., Taniguchi T., Takene Y., Masuda S., Matsunaga N., Okada H. Purification and characterization of 6-aminohexanoic-acid-oligomer hydrolase of Flavobacterium sp. Ki72. Eur J Biochem. 1981 Jun 1;116(3):547–551. doi: 10.1111/j.1432-1033.1981.tb05371.x. [DOI] [PubMed] [Google Scholar]
- Miyajima N., Horiuchi R., Shibuya Y., Fukushige S., Matsubara K., Toyoshima K., Yamamoto T. Two erbA homologs encoding proteins with different T3 binding capacities are transcribed from opposite DNA strands of the same genetic locus. Cell. 1989 Apr 7;57(1):31–39. doi: 10.1016/0092-8674(89)90169-4. [DOI] [PubMed] [Google Scholar]
- Negoro S., Nakamura S., Kimura H., Fujiyama K., Zhang Y. Z., Kanzaki N., Okada H. Construction of hybrid genes of 6-aminohexanoic acid-oligomer hydrolase and its analogous enzyme. Estimation of the intramolecular regions important for the enzyme evolution. J Biol Chem. 1984 Nov 25;259(22):13648–13651. [PubMed] [Google Scholar]
- Negoro S., Shinagawa H., Nakata A., Kinoshita S., Hatozaki T., Okada H. Plasmid control of 6-aminohexanoic acid cyclic dimer degradation enzymes of Flavobacterium sp. KI72. J Bacteriol. 1980 Jul;143(1):238–245. doi: 10.1128/jb.143.1.238-245.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negoro S., Taniguchi T., Kanaoka M., Kimura H., Okada H. Plasmid-determined enzymatic degradation of nylon oligomers. J Bacteriol. 1983 Jul;155(1):22–31. doi: 10.1128/jb.155.1.22-31.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepveu A., Marcu K. B. Intragenic pausing and anti-sense transcription within the murine c-myc locus. EMBO J. 1986 Nov;5(11):2859–2865. doi: 10.1002/j.1460-2075.1986.tb04580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S., Matsunaga T. The 48-base-long primordial building block of immunoglobulin light-chain variable regions is complementary to the primordial building block of heavy-chain variable regions. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2338–2341. doi: 10.1073/pnas.79.7.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H., Negoro S., Kimura H., Nakamura S. Evolutionary adaptation of plasmid-encoded enzymes for degrading nylon oligomers. Nature. 1983 Nov 10;306(5939):203–206. doi: 10.1038/306203a0. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk R., Köster M., Pöting A., Hartmann L., Knöchel W. An antisense transcript from the Xenopus laevis bFGF gene coding for an evolutionarily conserved 24 kd protein. EMBO J. 1989 Oct;8(10):2983–2988. doi: 10.1002/j.1460-2075.1989.tb08448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yomo T., Ohno S. Concordant evolution of coding and noncoding regions of DNA made possible by the universal rule of TA/CG deficiency-TG/CT excess. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8452–8456. doi: 10.1073/pnas.86.21.8452. [DOI] [PMC free article] [PubMed] [Google Scholar]


