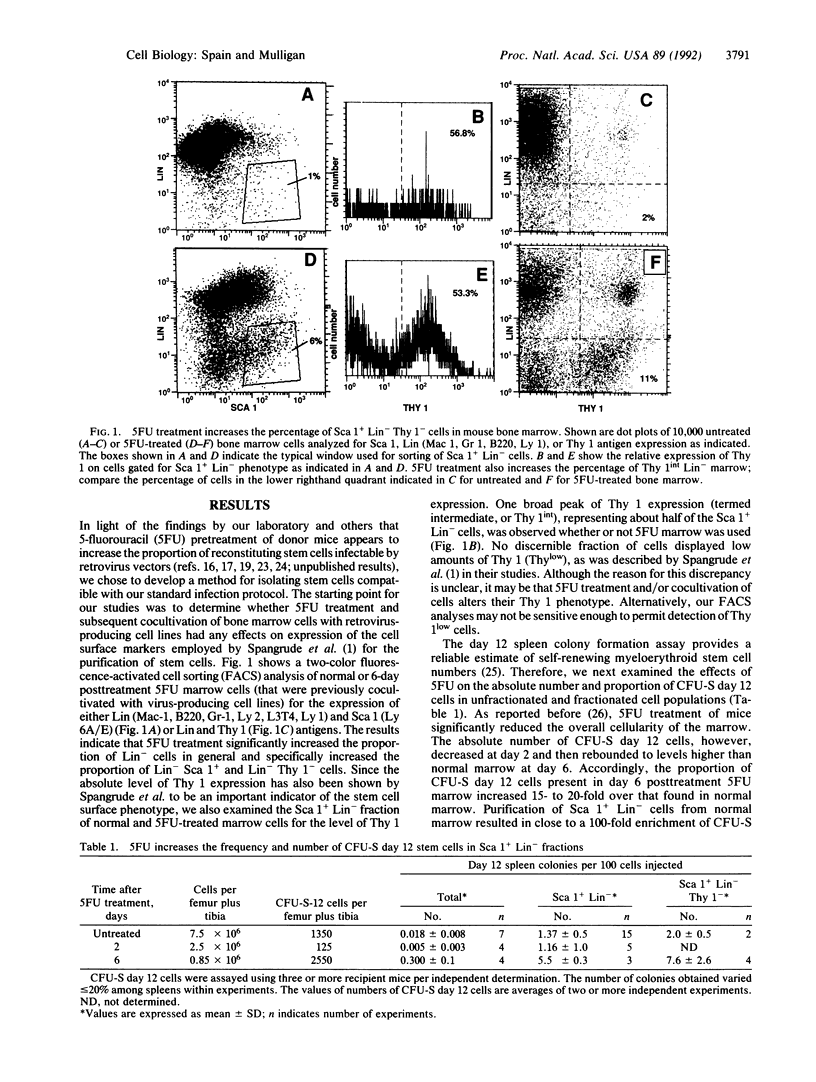
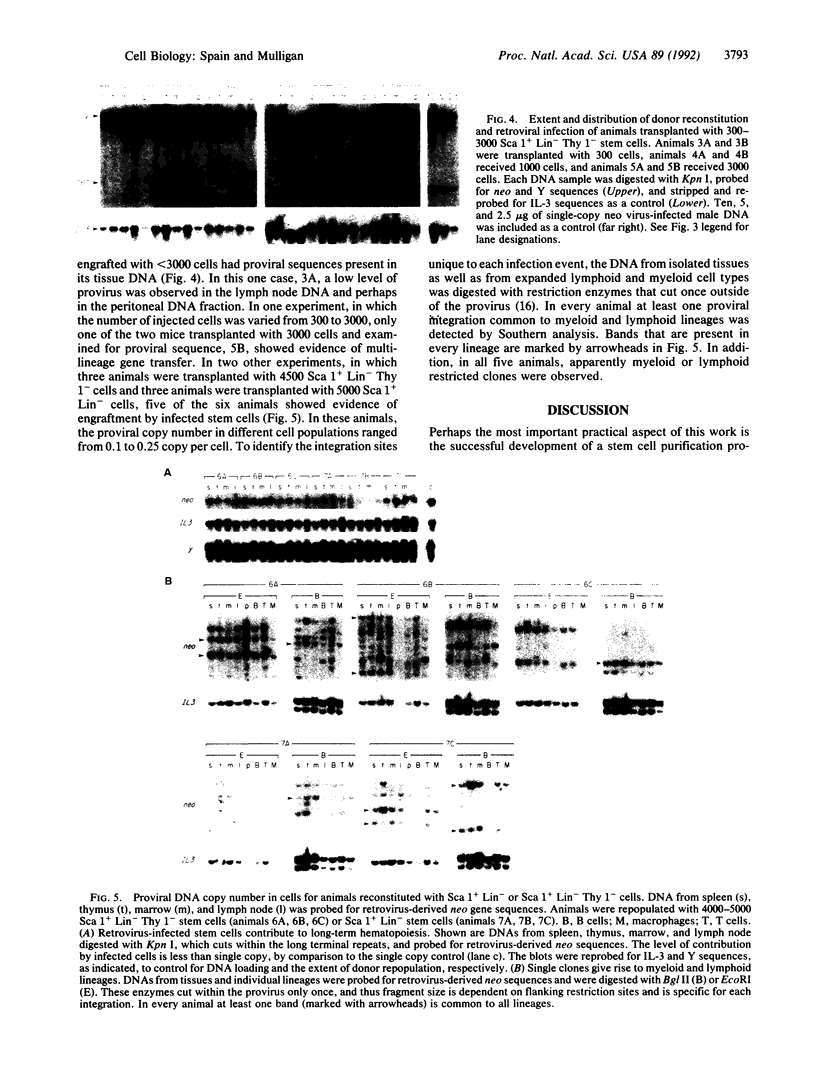
Abstract
We have developed a method for the purification of retrovirally transduced hematopoietic stem cells, based on a modification of the stem cell purification protocols developed by Spangrude et al. [Spangrude, G., Heimfeld, S. & Weissman, I. (1988) Science 241, 58-64] and Spangrude and Scollay [Spangrude, G. & Scollay, R. (1990) Exp. Hematol. 18, 920-926], that depends upon the use of bone marrow cells isolated from 5-fluorouracil-treated mice that have been subsequently cocultivated with recombinant retrovirus-producing cell lines. We found that purified cell populations bearing a Sca 1+ Lin- Thy 1- surface phenotype represent a 50-100% pure population of spleen colony-forming cells (CFU-S day 12). Animals injected with 300 or more purified cells were consistently radioprotected and reconstituted in multiple lineages with donor cells. Sca 1+ Lin- Thy 1- CFU-S day 12 stem cells were shown to be efficiently (100%) transduced by the recombinant retroviruses used in the study. Gene transfer into long-term reconstituting stem cells, as evidenced by Southern blot analysis of mature hematopoietic cell types 3 months after transplantation, was observed only in recipients injected with large numbers (approximately 4000-5000) of the purified cells. The development of methods for purifying retrovirally transduced stem cells should prove extremely useful for various studies in which it is of interest to characterize the activity of a specific gene product (e.g., growth factor, receptor, oncogene) specifically in primitive hematopoietic cell types.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodine D. M., Karlsson S., Nienhuis A. W. Combination of interleukins 3 and 6 preserves stem cell function in culture and enhances retrovirus-mediated gene transfer into hematopoietic stem cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8897–8901. doi: 10.1073/pnas.86.22.8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowtell D. D., Johnson G. R., Kelso A., Cory S. Expression of genes transferred to haemopoietic stem cells by recombinant retroviruses. Mol Biol Med. 1987 Aug;4(4):229–250. [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Dzierzak E. A., Papayannopoulou T., Mulligan R. C. Lineage-specific expression of a human beta-globin gene in murine bone marrow transplant recipients reconstituted with retrovirus-transduced stem cells. Nature. 1988 Jan 7;331(6151):35–41. doi: 10.1038/331035a0. [DOI] [PubMed] [Google Scholar]
- Guild B. C., Finer M. H., Housman D. E., Mulligan R. C. Development of retrovirus vectors useful for expressing genes in cultured murine embryonal cells and hematopoietic cells in vivo. J Virol. 1988 Oct;62(10):3795–3801. doi: 10.1128/jvi.62.10.3795-3801.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. J., Celano P., Sharkis S. J., Sensenbrenner L. L. Two phases of engraftment established by serial bone marrow transplantation in mice. Blood. 1989 Feb;73(2):397–401. [PubMed] [Google Scholar]
- Jones R. J., Wagner J. E., Celano P., Zicha M. S., Sharkis S. J. Separation of pluripotent haematopoietic stem cells from spleen colony-forming cells. Nature. 1990 Sep 13;347(6289):188–189. doi: 10.1038/347188a0. [DOI] [PubMed] [Google Scholar]
- Jordan C. T., Lemischka I. R. Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev. 1990 Feb;4(2):220–232. doi: 10.1101/gad.4.2.220. [DOI] [PubMed] [Google Scholar]
- Jordan C. T., McKearn J. P., Lemischka I. R. Cellular and developmental properties of fetal hematopoietic stem cells. Cell. 1990 Jun 15;61(6):953–963. doi: 10.1016/0092-8674(90)90061-i. [DOI] [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Lord B. I., Spooncer E. Isolation of haemopoietic spleen colony forming cells. Lymphokine Res. 1986 Winter;5(1):59–72. [PubMed] [Google Scholar]
- Mardon G., Mosher R., Disteche C. M., Nishioka Y., McLaren A., Page D. C. Duplication, deletion, and polymorphism in the sex-determining region of the mouse Y chromosome. Science. 1989 Jan 6;243(4887):78–80. doi: 10.1126/science.2563173. [DOI] [PubMed] [Google Scholar]
- McAlister I., Wolf N. S., Pietrzyk M. E., Rabinovitch P. S., Priestley G., Jaeger B. Transplantation of hematopoietic stem cells obtained by a combined dye method fractionation of murine bone marrow. Blood. 1990 Mar 15;75(6):1240–1246. [PubMed] [Google Scholar]
- Ploemacher R. E., Brons N. H. Cells with marrow and spleen repopulating ability and forming spleen colonies on day 16, 12, and 8 are sequentially ordered on the basis of increasing rhodamine 123 retention. J Cell Physiol. 1988 Sep;136(3):531–536. doi: 10.1002/jcp.1041360320. [DOI] [PubMed] [Google Scholar]
- Ploemacher R. E., Brons N. H. Isolation of hemopoietic stem cell subsets from murine bone marrow: I. Radioprotective ability of purified cell suspensions differing in the proportion of day-7 and day-12 CFU-S. Exp Hematol. 1988 Jan;16(1):21–26. [PubMed] [Google Scholar]
- Ploemacher R. E., Brons N. H. Isolation of hemopoietic stem cell subsets from murine bone marrow: II. Evidence for an early precursor of day-12 CFU-S and cells associated with radioprotective ability. Exp Hematol. 1988 Jan;16(1):27–32. [PubMed] [Google Scholar]
- Roffler-Tarlov S., Graybiel A. M. Weaver mutation has differential effects on the dopamine-containing innervation of the limbic and nonlimbic striatum. Nature. 1984 Jan 5;307(5946):62–66. doi: 10.1038/307062a0. [DOI] [PubMed] [Google Scholar]
- SIMINOVITCH L., MCCULLOCH E. A., TILL J. E. THE DISTRIBUTION OF COLONY-FORMING CELLS AMONG SPLEEN COLONIES. J Cell Physiol. 1963 Dec;62:327–336. doi: 10.1002/jcp.1030620313. [DOI] [PubMed] [Google Scholar]
- Smith L. G., Weissman I. L., Heimfeld S. Clonal analysis of hematopoietic stem-cell differentiation in vivo. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2788–2792. doi: 10.1073/pnas.88.7.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass R., Keller G. Clonal fluctuation within the haematopoietic system of mice reconstituted with retrovirus-infected stem cells. EMBO J. 1987 Dec 20;6(13):3955–3960. doi: 10.1002/j.1460-2075.1987.tb02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Spangrude G. J., Johnson G. R. Resting and activated subsets of mouse multipotent hematopoietic stem cells. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7433–7437. doi: 10.1073/pnas.87.19.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude G. J., Scollay R. A simplified method for enrichment of mouse hematopoietic stem cells. Exp Hematol. 1990 Sep;18(8):920–926. [PubMed] [Google Scholar]
- Szilvassy S. J., Lansdorp P. M., Humphries R. K., Eaves A. C., Eaves C. J. Isolation in a single step of a highly enriched murine hematopoietic stem cell population with competitive long-term repopulating ability. Blood. 1989 Aug 15;74(3):930–939. [PubMed] [Google Scholar]
- Van Zant G. Studies of hematopoietic stem cells spared by 5-fluorouracil. J Exp Med. 1984 Mar 1;159(3):679–690. doi: 10.1084/jem.159.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser J. W., Bauman J. G., Mulder A. H., Eliason J. F., de Leeuw A. M. Isolation of murine pluripotent hemopoietic stem cells. J Exp Med. 1984 Jun 1;159(6):1576–1590. doi: 10.1084/jem.159.6.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser J. W., de Vries P. Isolation of spleen-colony forming cells (CFU-s) using wheat germ agglutinin and rhodamine 123 labeling. Blood Cells. 1988;14(2-3):369–384. [PubMed] [Google Scholar]
- Williams D. A., Lemischka I. R., Nathan D. G., Mulligan R. C. Introduction of new genetic material into pluripotent haematopoietic stem cells of the mouse. Nature. 1984 Aug 9;310(5977):476–480. doi: 10.1038/310476a0. [DOI] [PubMed] [Google Scholar]