Abstract
Objective:
To verify the utility of isolated fermentative microbes from Woodfordia fruticosa flowers for preparation of Arjunāriṣṭa formulation and its comparative evaluation with the same formulation prepared by traditional method.
Methodology:
In the present technique, isolated colonies of microorganisms from Woodfordia fruticosa (Dhātakī) flowers on Saubroad dextrose media were separated and suspended in sterile water. This suspension was aseptically added in previously sterilized mixtures containing all intended ingredients for Arjunāriṣṭa which was thereafter incubated for 20 days at 37°C to achieve optimal fermentation. The formulation thus obtained was further subjected to various evaluation tests.
Result:
Arjunāriṣṭa was prepared using a new approach, and in that, isolated microorganisms from the flowers of Woodfordia fruticosa (Dhātakī) were used. It was found that the new approach was successful in generating approximately same quantities of alcohol content in comparison with traditional methods which have shown varying concentration of alcoholic content. Moreover, the new process prevents the growth of unwanted microbes thus, optimizing standards for purity and safety of the formulation.
Conclusion:
The formulation prepared by a new procedure showed marked uniformity for different parameters such as alcohol production, total phenol content, total solid content as compared to that prepared by the traditional method. Similarly, the results of thin layer chromatography, high performance thin layer chromatography showed marked uniformity related to quality, safety, efficacy, and reproducibility of the new method as compared to the traditional one. Thus, the modern technique was found to show reproducibility and facilitate easier quality assessment.
Keywords: Arjunariṣṭa formulation, Ayurveda, HPTLC, modern technique, TLC
Introduction
Āsavas and ariṣṭas are fermented preparations of medicinal plants. The fermentation procedure adopted to prepare these preparations is termed as ‘sandhāna kalpanā‘ and the ferment used to stimulate fermentation is termed as ‘sandhāna dravya‘. Āsavas are usually prepared by fermenting expressed juice (svarasa), whereas ariṣṭas are prepared from fermentation of decoctions.[1,2,3,4] Sugar or jaggery and powders (cūrṇas) of medicinal plants as required along with a natural ferment are added to these two liquids and they are left in a closed container till the fermentation is completed. Āsava and ariṣṭas can be prepared from svarasa or kvātha (as the case may be) of single plant or from a mixture of ‘svarasa‘ or ‘kvātha‘ from multiple plants. This facilitates the extraction of the active principles contained in the drugs. These are unique liquid dosage forms that contains self generated alcohol, which serves as a self preservative.[5] Both function as weak wines and are rich in active principles.[6]
Fermented biomedicine exhibit high palatability and stability.[7] Ariṣṭas are classical Ayurvedic preparations typically used as digestives and cardiotonics.[8] They are weak spirituous preparations prepared in airtight sealed vessels by anaerobic fermentation of decoction of plant material, sugar and dried flowers of Woodfordia fruticosa (L.) Kurz (Lythraceae) occasionally supplemented with some other powdered dried plant materials.[9,10] Fermentation probably results in the transformation of several phytochemical compounds present in medicinal plants, thereby rendering them less toxic and more potent; besides helping in their absorption.[10] Due to their medicinal value, sweet taste and easy availability, people tend to consume higher doses of these drugs for longer periods.[11]
Arjunāriṣṭa is commonly used oral liquid cardiotonic prepared using Terminalia arjuna as an active constituent. It nourishes and strengthens heart muscles and promotes cardiac functioning by regulating blood pressure and cholesterol.[12] Ayurvedic products available in the market vary in quality and therapeutic efficacy due to the differences in composition of the products. Knowledge about active principles of herbal preparations are not well defined, information on toxicity and adverse effects of these formulations are lacking. Quality is the paramount issue because it can affect the efficacy and/or safety of the products being used. In the traditional fermented formulation of arjunāriṣṭa, the period required for fermentation is around 25 days. Dhātakī and Mahua flowers contain microorganisms which are responsible for fermentation along with other microorganisms which may adversely affect the quality of the formulations. In the present study attempt has been made for formulation of arjunāriṣṭa by a modern method and its quality is assessed.
Methodology
Collection of plant material
Plant materials Terminalia arjuna (Combretaceae) bark, flowers of Madhuca indica (Sapotaceae) and Woodfordia fruticose (Lythraceae) were collected from forests of Sahyadri Ghats of Maharashtra (India); fruits of Vitis venifera (Venifera) and jaggery were procured from local market.
Instruments
Calibrated digital pH (HANNA) was used to check the pH of the formulation. Viscosity of formulations was determined with the help of Ostwald Viscometer. The refractive index of formulations was determined using Abbe's Refractometer. HPTLC (Anchrome Mumbai) was used for quantitative analysis of the formulation and Gallic acid.
Isolation of microorganisms
Microorganisms (MO) were isolated from Woodfordia fruticosa (Dhātakī) flowers using a streak plate technique.[13] Sabouraud dextrose agar medium was used for isolation of microorganisms. pH of this agar medium was adjusted to 5.6 ± 0.2 using acid or base.[14] Isolated microorganisms were stored on Sabouraud dextrose agar slant at 4°C. Suspension of these micro organisms in sterile water were prepared and transferred aseptically in each flask for fermentation. Numbers of cells present in suspension were counted using Neubars chamber. A cell count of 4.6 × 107 cells ml-1 was adjusted by suitable dilution.
Preparation of formulation
Traditional method
Pulverized Terminalia arjuna bark was passed through sieve no. 44. Required quantity of powder was taken in a flask and soaked in distilled water overnight. Decoction of Arjuna powder was prepared, by heating till only one fourth of volume of the water remained. It was then filtered and jaggery was added in required amount and dissolved completely. Dhātakī flowers, Mahua flowers, drākṣā in required quantity were taken, properly cleaned and then added to the flask. After addition of the ingredients the flask was tightly closed with a cotton plug and kept in a dark room for 25 days for fermentation. It was then opened and the contents were filtered through a muslin cloth. Filtrate was collected in a container and evaluated for different parameters.[15] Using similar method, Formulation A was prepared with all ingredients, namely Arjuna bark powder, Dhātakī flower, Mahua flower, Drākṣā, jaggery and water. Similarly, formulation C and formulation E were prepared, excluding Mahua and Dhātakī flowers respectively [Table 1].
Table 1.
Formulations of arjunāriṣṭa by using traditional and modern method
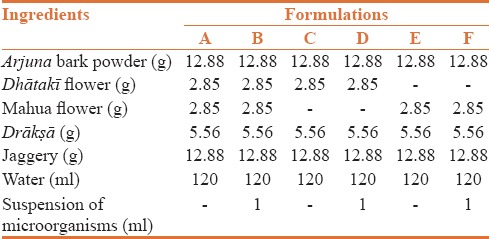
New method of Arjunāriṣṭa preparation
Arjunāriṣṭa is a fermented liquid preparation made with ingredients in the composition shown in Table 1. Pulverized Terminalia arjuna bark was passed through sieve no. 44. Required quantity of powder was taken in a flask and soaked in distilled water overnight. Decoction of Arjuna powder was prepared boiling the solution to one fourth the initial volume. It was then filtered, Jaggery and Drākṣā were added in required amount and dissolved completely. Woodfordia fruticosa (Dhātakī) and Mahua flowers were added. Autoclaving of the flask was done at 121°C for 20 minutes. 1 ml suspensions of microorganisms isolated from the flowers were then added aseptically in flask which was sealed thereafter with cotton plug and parafilm sealing. These flasks were kept at constant temperature in the incubator at 37 ± 0.5°C for 20 days. After 20 days these flasks were opened, content filtered through Muslin cloth and the filtrate was used for further evaluation studies. Using this procedure formulation B was prepared with all ingredients, namely Arjuna bark powder, Dhātakī flower, Mahua flower, Drākṣā, Jaggery, Water and suspension (isolated microorganism from dhātakī flowers). Formulation D was prepared with the ingredients, namely Arjuna bark powder, Dhātakī flower, Drākṣā, Jaggery, Water and suspension (isolated microorganism from dhātakī flowers). Formulation F was prepared using Arjuna bark powder, Mahua flower, Drākṣā, Jaggery, Water and suspension (isolated microorganism from dhātakī flowers) [Table 1].
Physico-chemical analysis of Arjunāriṣṭa
Physico-chemical analysis was done to ascertain the quality of the formulation. The parameters as mentioned in “Quality Standards of Indian Medicinal Plants” by Indian Council of Medical Research were followed to check the quality of the prepared formulations.[16]
Determination of organoleptic characteristics viz. odor, taste, color, clarity, pH, viscosity and refractive index of the prepared formulation of Arjunāriṣṭa was carried out. Using a calibrated pH meter the pH of the formulations was analyzed for 14 days to evaluate acidity of formulation and to study the effect of the environmental conditions on pH. Generally, the formulations having less alcohol content have higher pH. Viscosity of the Arjunāriṣṭa formulationwas determined with the help of Brookfield Viscometer. The refractive index of the prepared formulations was determined by using Abbe's refractometer.
Quantitative analysis of formulations
Determination of acid value
5g of formulation was dissolved in 25 ml of equal volume of ethanol and ether previously neutralized with 0.1 M KOH to phenolphthalein solution. To it, 1ml of phenolphthalein solution was added and titrated with 0.1 M KOH until the solution remained faint pink after shaking for 30 sec.[11]
Determination of alcohol content by distillation method
Not less than 25 ml of preparation was transferred to the distillation flask and its temperature was noted. It was diluted with equal volume of water. It was then subjected to distillation and the distillate about 2 ml less than the total volume was collected. Water was added to make up to the same volume of original test liquid and adjusted to the temperature noted before. The specific gravity of this liquid was determined and alcohol content analyzed using relative density table given in the United States Pharmacopoeia (USP).[17]
Determination of alcohol content by dichromate oxidation method
10 ml of formulation was taken and 15 ml of water was added to it and subjected to distillation to obtain 20 ml of distillate. Then 5 ml of water was added to it. 0.5 ml volume was then taken from it and 2 ml water along with 2.5 ml potassium dichromate solution were added. Absorbance of the resulting solution was measured at 590 nm. The calibration curve of alcohol determination was plotted similarly by using known concentration of ethanol as (1% to 8% v/v) From the equation of calibration curve the alcohol content of all formulations was determined.[18]
Total solid content
An evaporating dish which was previously weighed was taken and 10 g of formulation was allowed to evaporate so that only solid content remains in the dish and the rest of the fluid gets evaporated. Then it was reweighed and the solid content of formulation was calculated.[15]
Total phenolic content
Absorbance of standard tannic acid solutions are recorded on UV-visible spectrophotometer (Shimadzu-1800) at 750 nm and the standard curve was plotted. Sample of Arjunāriṣṭa prepared as per Ayurvedic Pharmacopoeia of India and processed for estimation of total phenolic content.[19]
Analysis of Arjunāriṣṭa by TLC method
The TLC - method was developed for the standardization of Arjunāriṣṭa. Different solvents such as Toluene, Benzene, Ethyle Acetate, Acetic Acid, Formic Acid, Methanol, Water, Hydrochloric Acid were screened for development of mobile phase. Solvent system was selected to give maximum number of spots. Mobile phase: Toluene: ethyl acetate: formic acid (5:5:1). Stationary phase: Precoated TLC Plate (Silica gel 60 F). Visualizing agent: UV chamber, Iodine, Folins reagent, Methanolic Ferric chloride and Anisaldehyde H2SO4. Reference Standard: Gallic acid.
Analysis of arjunāriṣṭa by high performance thin layer chromatography
HPTLC method was used to develop a standard procedure for the qualitative and quantitative evaluation of Arjunāriṣṭa formulations. Quantitative determination was carried out using Gallic acid as reference standard.
Preparation of standard gallic acid
1 mg of Gallic acid was accurately weighed and dissolved in methanol and final volume made up to 10 ml.
Preparation of sample of Arjunāriṣṭa
5 ml of formulation was taken in a petri dish and evaporated in vacuum oven at 65°C with applied vacuum 15 mm Hg. Subsequent washing using 7 ml methanol was done. This solution was filtered through 0.2 micron size filter and used for further analysis.
Standardization of standard gallic acid and Arjunāriṣṭa formulation
Carnage HPTLC8 system comprising of Linomat 5 as sample applicator and TLC Scanner 3 controlled by WinCATS software version 1.3.4 was used for quantitative evaluation. Stationary phase used was MERCK precoated TLC Aluminium silica gel 60 F254 plates and the mobile phase used was toluene: ethyl acetate: formic acid (5:5:1). Samples and standard were applied as 8 mm bands with 10 mm distance between the tracks. Tank saturation and plate equilibrium were carried with filter paper for 10 min. Ascending development for a distance of 80 mm in a twin trough chamber was completed in approximately 15 min. Volume of the standard was first optimized at 5 μl for fingerprinting. The volume of the sample was optimized to 5 μl for quantification. It was then simultaneously applied with different concentration of standard Gallic acid.[20]
Results
Physicochemical evaluation
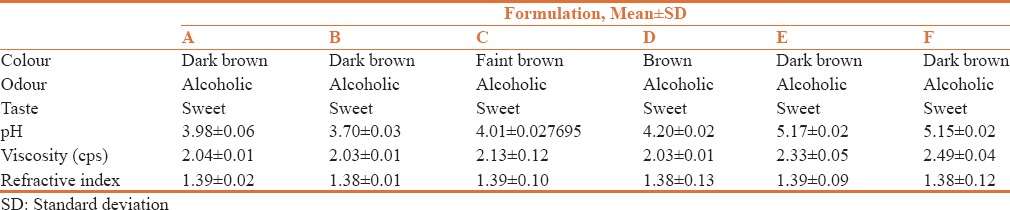
Color, odor, taste, pH, viscosity and refractive index of all formulations were determined. pH of all formulations were found to be in the range of 3.70 ± 0.03 to 5.17 ± 0.02 [Table 2]. The viscosities of formulations were found to be 1.44 ± 0.12 to 2.93 ± 0.04 cps, shown in Table 2. The range of refractive index of formulations was found to be 1.381 to 1.395, the results are shown in Table 2.
Table 2.
Physicochemical properties of Arjunāriṣṭa formulations
Quantitative analysis of formulation
Acid value
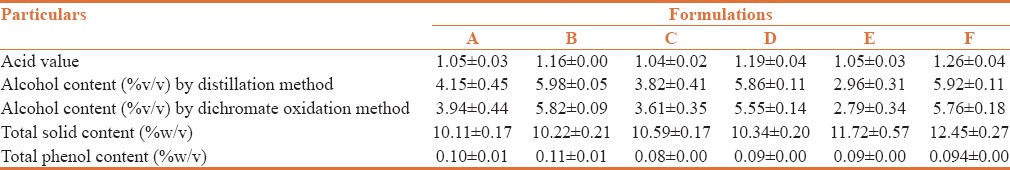
The acid value of all formulations was found to be in the range of 1.04 ± 0.02 to 1.26 ± 0.04 as shown in Table 3.
Table 3.
Quantitative analysis of Arjunāriṣṭa formulations
Determination of alcohol content
The alcohol content was determined by distillation method and dichromate technique. By distillation method - Formulation B showed a maximum (5.98 ± 0.05% v/v) and formulation E possessed a minimum (2.96 ± 0.31% v/v) alcohol [Table 3]. As per Ayurvedic Pharmacopoeia of India the limit of alcohol content for Arjunāriṣṭa is in the range 5 to 12% v/v 15. By Dichromate oxidation method - Formulation B showed a maximum (5.82 ± 0.09% v/v) and formulation E showed (2.79 ± 0.34% v/v) alcohol [Table 3].
Total solid content
Total solid content has moderate impact over viscosity. Increase in solid content increases viscosity. All evaluated formulations possessed total solid content of 10.11 ± 0.17 to 12.45 ± 0.27% w/v. Among these formulations F showed maximum and formulation A the minimum total solid content [Table 3].
Quantitative determination of phenol content by Folin-ciocalteau method
Total phenol content has an impact over the pH of the formulation. pH and total phenol content are inversely related to each other.[19] Among all the evaluated formulations, Formulation B had maximum total phenol content of (0.11 ± 0.01% w/v) and Formulation C showed a minimum total phenol content of (0.08 ± 0.00% w/v). Results are shown in Table 3.
Analysis of formulations and gallic acid (Standard) by TLC method
TLC densitometry estimation of formulations A, B, C, D, E, F, and Gallic acid (G) was conducted. TLC plates are precoated plates of silica gel 60 F (E. Merck) of a uniform thickness of 0.2 and Solvent systems – Toluene: Ethyl acetate: Formic acid (5:5:1). TLC plate and profiling of Arjunāriṣṭa formulations and Gallic acid (Standard) are shown in Figure 1a. Mobile phase and Rf value of all formulations is 0.60.
Figure 1.
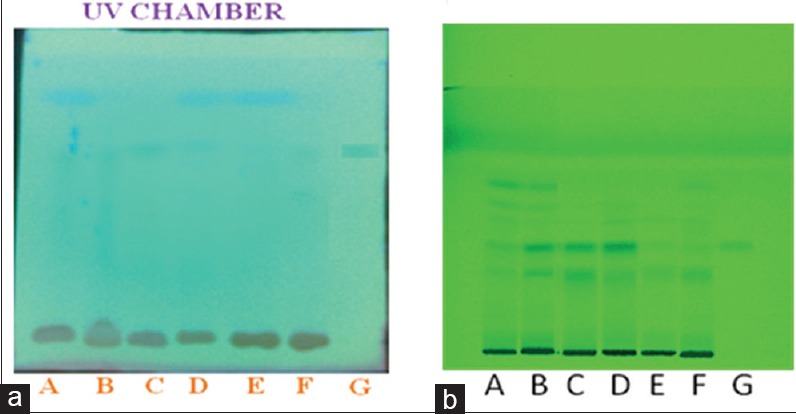
(a) TLC of Formulation and gallic acid (b) HPTLC profile of Formulations and gallic acid (g) at 254 nm
Estimation of gallic acid in formulations A, B, C, D, E and F by HPTLC
Gallic acid was found to be 0.0227, 0.0277, 0.0110, 0.0207, 0.00538 and 0.00834% w/v in Formulations A, B, C, D, E and F respectively. HPTLC chromatogram of standard Gallic acid is shown in Figure 2a. The suitability of the method was examined by estimation of Gallic acid in formulation A, B, C, D, E and F. Bands of Rf = 0.37 were observed in the densitogram for Gallic acid standard [Figure 1b] which resembles the bands of the same Rf as observed in the densitogram for formulation A, C, D, E and F [Figure 2b, d, e, f and g]. While Formulation B, showed bands of Rf = 0.36 for Gallic acid as shown in [Figure 2c].
Figure 2.
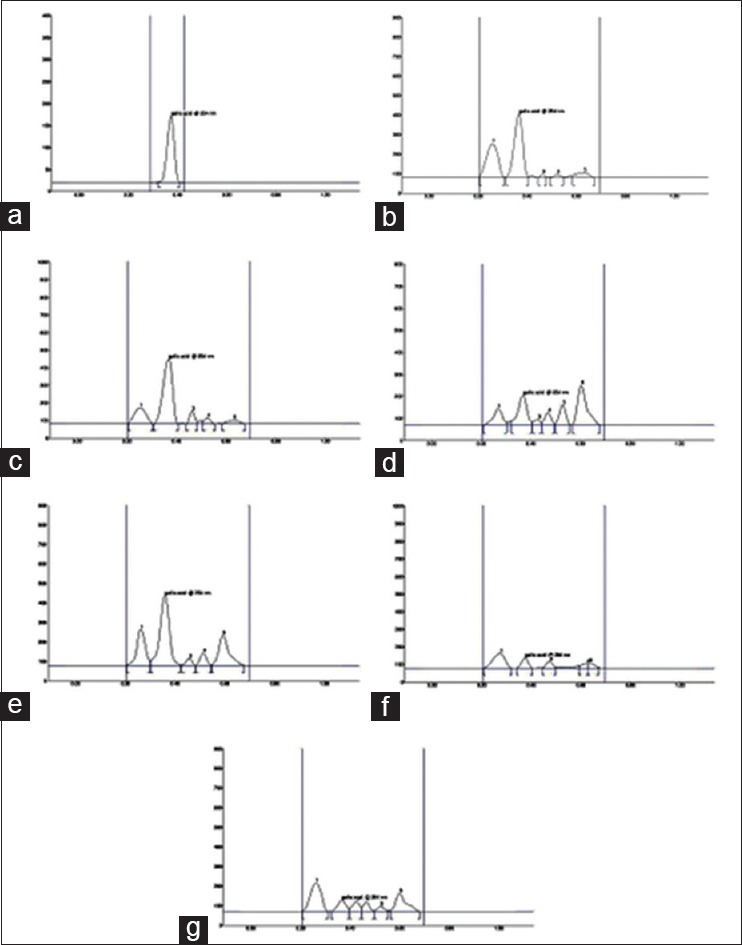
HPTLC chromatogram of (a) standard gallic acid (b) Formulation A (c) Formulation B (d) Formulation C (e) Formulation D (f) Formulation E (g) Formulation F
Discussion
It may be suggested that Brewer's yeast-induced fermentation, as employed by many manufacturers of Ayurvedic medicines to reduce production costs, is no substitute for the traditional fermentation described in the ancient Ayurvedic literature. Such practices may not produce authentic fermented Ayurvedic medicines.[21]
Fermentation processes help in rupturing the cells of the herbs and exposing their contents to biotransformation. It also creates an active transport system with dissolved constituents from the herbal material to the solvent.[22]
In the traditional technique of preparation of Arjunāriṣṭa, there are issues related to safety, quality and efficacy of the product due to different plant materials obtained from different sources. The flowers which are responsible for fermentation vary in number of microorganisms which are responsible for fermentation and thus affect alcohol production. Also, earthen pot containing all ingredients is buried underground (21-25 days) for the fermentation process, therefore uniform temperature is not maintained which adversely affects the fermentation process. As indicated in available literature, marketed formulations of Arjunāriṣṭa, have brand to brand variation in content and therefore, action. Hence, reproducibility of the results is the prime issue in the manufacture of Ayurvedic formulations.
To overcome the aforesaid problem, an attempt has been made to employ a modern technique to use isolated microorganisms from the Woodfordia fruticosa (Dhātakī) flower which are responsible for fermentation. These isolated microbes were introduced into the mixture containing ingredients of Arjunāriṣṭa and further kept in an incubator at 37°C. The number of days required for fermentation were reduced from 25 to 20 days. The fixed amount of suspension of microorganism was found to generate approximately the same amount of alcohol which causes extraction of active constituents from Arjuna bark and also act as a preservative.
In the present approach, the basic principles of Ayurveda with regard to the preparation method remain the same. But with an improvement in the quality, safety and efficacy of the product. The alcohol content was determined by distillation method and dichromate technique.
We have prepared three formulations (formulation A, C and E) to assess the effect of flowers Woodfordia fruticosa (Dhātakī) and Mahua on traditional fermentation process. It was found that microorganisms from Woodfordia fruticosa (Dhātakī) flower have a major role to play in the fermentation process. Three formulations were prepared by modern technique (formulation B, D and F). It does not show marked variation in alcohol production. HPTLC study revealed that gallic acid content in the formulations was in the range of 0.01-0.02.
Woodfordia fruticosa (Dhātakī) and Mahua flowers have a number of essential constituents which contain different antioxidant compounds required for its action. Therefore, they were included in the present method of preparation to be true to the basic principles of Ayurveda.
Conclusion
Scientific explorations in the area of Ayurvedic drug manufacturing along with customization of present technologies to be true to the principles of Ayurveda would greatly facilitate standardization of drug manufacture. In the present study, we have tried to implement current evidence based knowledge for modernization of a traditional Ariṣṭa formulation.
One of the characteristics of Ayurvedic herbal medicinal preparations is that single herbs or as collections of herbs in composite formulae and are extracted using boiling water during the decoction process. This may be the main reason why quality control of such herbal drugs is more difficult than that of biomedicines. Fermentation period as per traditional method is around 25 days. By adopting the proposed modern technique this period was reduced to 20 days without compromising with the quality of the product. A defined procedure for fermented formulation, will ensure reproducibility and facilitate easier quality assessment. Preparation of Ariṣṭa by using isolated microorganisms on the basis of their fermenting capabilities can help generate approximately same quantities of alcohol in formulation. Formulations prepared by using modern techniques (Formulation B, D and F) were found to contain approximately same quantity of self generated alcohol as compared to formulations prepared by traditional methods (Formulation A, C and E), which may be attributed to use of isolated microorganism from Dhātakī flowers. This method can also ensure prevention of the growth of unwanted microbes. It may lead to an extraction to the same extent in all batches and will improve the efficacy and safety of the product.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sayyad SF, Randive DS, Jagtap SM, Chaudhari SR, Panda BP. Preparation and evaluation of fermented Ayurvedic formulation: Arjunarishta. J Appl Pharm Sci. 2012;2:122–4. [Google Scholar]
- 2.Thatte UM, Dahanukar SA. Ayurveda and contemporary scientific thought. Trends Pharmacol Sci. 1986;7:247–55. [Google Scholar]
- 3.Bouldin AS, Smith MC, Garner DD, Szeinbach SL, Frate DA, Croom EM. Pharmacy and herbal medicine in the US. Soc Sci Med. 1999;49:279–89. doi: 10.1016/s0277-9536(99)00118-5. [DOI] [PubMed] [Google Scholar]
- 4.Sekar S, Mariappan S. Traditionally fermented biomedicine, arrests and asavas from Ayurveda. Indian J Tradit Knowl. 2008;7:548–56. [Google Scholar]
- 5.Mule S, Khale A. Asavarishtas through improved fermentation technology. Int J Pharm Sci Res. 2011;2:1421–5. [Google Scholar]
- 6.Prabhu SK, Samanta MK. Formulation and evaluation of sugar free Ashwagandharishta for diabetic population through biomedical fermentation – A holistic approach. Int J Pharm Chem Sci. 2015;4:216–21. [Google Scholar]
- 7.Vyas M, Shukla VJ, Patgiri BJ, Prajapati PK. A unique concentrated and fermented dosage form i. e. Pravahi Kwatha. Int J Pharm Bio Arch. 2010;1:287–90. [Google Scholar]
- 8.Kalaiselvan V, Kalpeshkumar Shah A, Babulal Patel F, Narendrabhai Shah C, Kalaivani M, Rajasekaran A. Quality assessment of different marketed brands of Dasamoolaristam, an Ayurvedic formulation. Int J Ayurveda Res. 2010;1:10–3. doi: 10.4103/0974-7788.59937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroes BH, van den Berg AJ, Abeysekera AM, de Silva KT, Labadie RP. Fermentation in traditional medicine: The impact of Woodfordia fruticosa flowers on the immunomodulatory activity, and the alcohol and sugar contents of Nimba arishta. J Ethnopharmacol. 1993;40:117–25. doi: 10.1016/0378-8741(93)90056-b. [DOI] [PubMed] [Google Scholar]
- 10.Mishra AK, Gupta A, Gupta V, Sannd R, Bansal P. Asava and Aristha: An ayurvedic medicine – An overview. Int J Pharm Bio Arch. 2010;1:24–30. [Google Scholar]
- 11.Weerasooriya WM, Liyange JA, Pandya SS. Quantitative parameters of different brands of Asava and Arishta used in ayurvedic medicine: An assessment. Indian J Pharmacol. 2006;38:365. [Google Scholar]
- 12.Lal UR, Tripathi SM, Jachak SM, Bhutani KK, Sing IP. HPLC analysis and standardization of Arjunarishta – An ayurvedic cardioprotective formulation. Sci Pharm. 2009;77:605–16. [Google Scholar]
- 13.Kokare C. Pharmaceutical Microbiology. 3rd ed. Nashik: Career Publication; 2010. pp. 79–81. [Google Scholar]
- 14.Anonymous, Indian Pharmacopoeia. Vol. 1. Ghaziabad: Government of India Ministry of Health and Family Welfare, the Indian Pharmacopoeia Commission; p. 37. [Google Scholar]
- 15.Anonymous, The Ayurvedic Pharmacopoeia of India. Vol. 2. New Delhi: Government of India, Ministry of Health and Family Welfare, Department of Indian Systems of Medicine and Homeopathy, Controller of Publications; 2001. pp. 47–221. [Google Scholar]
- 16.Quality Standards of Indian Medicinal Plants. Vol. 2. Indian Council of Medicinal Research (ICMR) New Delhi; 2005. p. 198. [Google Scholar]
- 17.United States Pharmacopoeia and National Formulary USP-NF the Official Compendia of Standards. 2009;1:224–5. 954. [Google Scholar]
- 18.Seo HB, Kim HJ, Lee OK, Ha JH, Lee HY, Jung KH. Measurement of ethanol concentration using solvent extraction and dichromate oxidation and its application to bioethanol production process. J Ind Microbiol Biotechnol. 2009;36:285–92. doi: 10.1007/s10295-008-0497-4. [DOI] [PubMed] [Google Scholar]
- 19.Rajalakshamy MR, Sindu A. Priliminary phytochemical screening and antioxidant activity of an ayurvedic formulation: Balarishtam. Int J Res Ayur Pharm. 2011;2:1645–7. [Google Scholar]
- 20.Shanbhag D, Khandagale A. Screening and standardization of Terminalia arjuna used as medicine in homeopathy using HPTLC method. Int J Anal Bioanal Chem. 2011;1:57–60. [Google Scholar]
- 21.Naveen Chandra D, Preethidan DS, Sabu A, Haridas M. Traditional fermentation of Ayurvedic medicine yields higher proinflammatory enzyme inhibition compared to wine-model product. Front Life Sci. 2015;8:160–4. DOI: 10.1080/21553769.2015.1005245. [Google Scholar]
- 22.Sabu A, Haridas M. Traditional fermentation of Ayurvedic medicine yields higher proinflammatory enzyme inhibition compared to wine-model product. Front Life Sci. 2015;8:160–4. DOI: 10.1080/21553769.2015.1005245. [Google Scholar]


