Abstract
Objective:
We aimed to investigate the frequency of Janus kinase 2 (JAK2) mutations in cases with chronic myeloproliferative disorders (CMDs), and the relationship between the presence of JAK2 mutation and leukocytosis and splenomegaly, retrospectively.
Materials and Methods:
Patients, who were diagnosed with BCR-ABL-negative CMDs according to diagnosis criteria of the World Health Organization and followed up at the hematology clinic between 2013 and 2015, were investigated in terms of the frequency of JAK2 mutation in cases with CMDs, and the relationship between the presence of JAK2 mutation and leukocytosis and splenomegaly, retrospectively.
Results:
In total, 100 patients, who were diagnosed with BCR-ABL-negative CMDs, were evaluated retrospectively. The mean age of the patients with JAK2 positivity was significantly higher compared to patients with negative. JAK2-positivity rates in the age groups were significantly different. Gender, diagnosis, splenomegaly, and leukocytosis were not statistically different for JAK2 positivity between the groups.
Conclusion:
JAK2 V617F mutation is more commonly seen in older age as a risk for complications related to CDMS. Splenomegaly and leukocytosis are not associated with JAK2 V617F mutation.
Key words: Chronic myeloproliferative disorders, Janus kinase 2 V617F mutation, leukocytosis, splenomegaly
INTRODUCTION
Chronic myeloproliferative disorders (CMDs) are associated with proliferation in one or more hematopoietic cell groups, and clonal hematopoietic disorders. CMDs are divided into four different groups in traditional classifications of Dameshek: Chronic myeloid leukemia (CML), polycythemia vera (PV), essential thrombocytosis (ET), and primary myelofibrosis (PMF).[1] CML is characterized by t (9;22) mutual replacements between chromosomes 9 and 22. The other diseases in this group, PV, ET, and PMF, are known as BCR-ABL-negative myeloproliferative disorders.[2,3] Diagnostic criteria (major) of classical CMDs have been updated with the discovery of the Janus kinase 2 (JAK2) mutation that was detected in almost all cases of PV, in approximately 50%–70% of ET and 40%–50% of PMF patients as JAK2 mutation has taken place in the criteria.[3] JAK2 is constitutively active tyrosine kinase, which activates the JAK-STAT signal transduction. JAK-STAT pathway is especially important for hematopoiesis. It allows cell proliferation, differentiation, and the regulation of apoptosis in the cell. After defining JAK2 V617F mutation in patients with CMDs in 2005, JAK2 exon 12 mutations in JAK2 V617F-negative PVR patients and thrombopoietin receptor mutation (MPLW515 L) in a minority of 5% of cases with ET and PMF were discovered.[3]
Information about the pathogenesis of BCR-ABL-negative CMDs has increased, diagnosis algorithms information has developed, and new options have emerged for advanced treatment after discovery of JAK2 V617F mutations in cases with CMDs. The frequency of the mutation was reported to be 90%–95% in PV patients, 50%–70% in ET, and 40%–50% in PMF patients. Recent studies are about the relationship between the presence of JAK2 mutation and risk factors, such as thrombosis, including age at diagnosis, presence of leukocytosis, and signs and symptoms, such as organomegaly and itching.[4,5] The presence of the JAK2 mutation in ET is related to advanced age, increased hemoglobin, increased leukocyte counts, and decreased platelet counts.[6] JAK2 has been found to be positive in almost all of the cases with PV, hemoglobin, and leukocyte levels were higher, and platelet count was lower in the heterozygous genotype group compared to homozygous genotype group.[4] It was reported that JAK2 V617F-positive PMF cases have higher leukocyte count and larger spleen size, a history of thrombosis or pruritus, greater need for cytoreductive therapies, and require less transfusion during follow-up.[4,7,8] In this study, we aimed to investigate the frequency of JAK2 mutation in cases with CMDs, and the relationship between the presence of JAK2 mutation and leukocytosis and splenomegaly, retrospectively.
MATERIALS AND METHODS
Patients, who were diagnosed with BCR-ABL-negative CMDs according to diagnosis criteria of the World Health Organization and followed up at the hematology clinic of Ministry of Health Istanbul Training and Research Hospital between 2013 and 2015, were investigated in terms of the frequency of JAK2 mutation in cases with CMDs, and the relationship between the presence of JAK2 mutation and leukocytosis and splenomegaly, retrospectively.[3] Splenomegaly was defined by >12 cm diameter.[9] Polymerase chain reaction-based DNA sequencing method has been performed for detection JAK2 V617F mutation in whole blood samples of the patients.
Statistical analysis
SPSS 15.0 (SPSS Inc, Chicago, IL) for Windows was used for statistical analysis. Numbers and percentages for categorical variables and minimum, maximum, mean, and standard deviation for numeric variables were given for descriptive statistics. Quantitative comparison of two groups of independent variables was performed by Mann–Whitney U-test. Rates of categorical variables between the groups were analyzed by Chi-square test. If conditions were not provided, Monte Carlo simulation was performed. Statistical significance was considered as alpha level of P < 0.05.
RESULTS
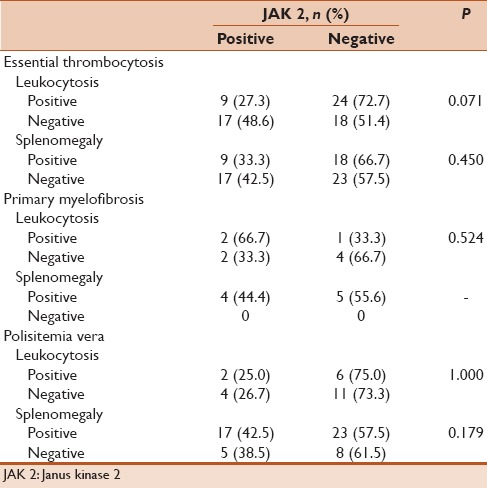
In total, 100 patients, who were diagnosed with BCR-ABL-negative CMDs, were evaluated retrospectively. The mean age was 56.8 ± 15.1 years, and 53 patients were female. Distribution of disorders was 68 with ET, 23 with PMF, and 9 with PV [Table 1]. Splenomegaly was examined in 46 patients, and 44 patients had leukocytosis. Rates of JAK2 gene positivity were 64% (n: 64). The mean age of the patients with JAK2 positivity was significantly higher compared to patients with negative (P: 0.014). JAK2-positivity rates in the age groups were significantly different (P: 0.002, Table 2). Gender (P: 0.089), diagnosis (P: 0.495), splenomegaly (P: 0.253), and leukocytosis (P = 0.233) were not statistically different for JAK2 positivity in the groups [Table 3].
Table 1.
Characteristics of the patients with chronic myeloproliferative disorders
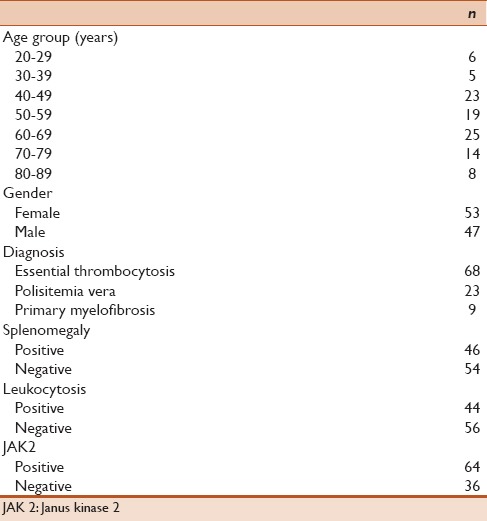
Table 2.
Janus kinase 2 positivity by age groups
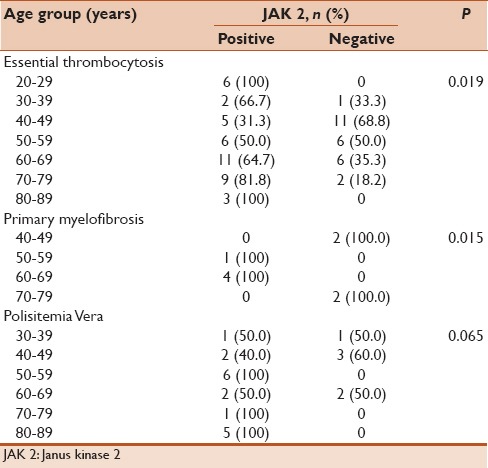
Table 3.
Rates of leukocytosis and splenomegaly by Janus kinase 2 positivity
DISCUSSION
JAK2, which is the somatic mutation of V617F and detected in different percentages in different subgroups of CMDs, is usually determined in DNA obtained from peripheral blood leukocyte by genomic polymerase chain reaction and restriction enzyme-based assay.[5] Since 2005, when screening of JAK2 mutation has begun, it was reported that the rates of correct ET diagnosis have increased by 31% and the rates of correct PV diagnosis have decreased by 21%.[10] In our study, the frequency of the JAK2 V617F mutation has been found 73% in PV patients, and 61% in ET, as those rates were found to be lower in PV patients compared to reported rates.[11] JAK2 mutation has been found in five of nine cases (55%) with PMF. Although the number of patients in this group is few and not sufficient for statistical interpretation, the presence of the mutation in the PMF group could be shown. In previous studies, the frequency of the JAK2 V617F mutation in PMF patients was reported by 50% that is consistent with our rates with 55%. In addition, the frequency of the JAK2 mutation was not significantly different in both sexes.[12] In a study of 140 cases with CMDs, the presence of JAK2 mutation found 82% (23/28) in PV cases, 53.1% (17/32) in ET patients, 40% (4/10) in PMF cases, and 60% (6/10) in other indistinguishable CMD cases.[13] In another study conducted in Taiwan, Lieu et al. screened JAK2 mutation in the 108 CMDs cases, and it was found positive in 85% (28/33) PV cases, 59% of patients (29/49) in ET patients, 33% (2/6) in PMF cases, 0/11 in MDS patients, and 0/10 in the other cases with 10 different hematological diseases.[14]
JAK2 V617F mutation is known to stimulate the proliferation causing growth factor hypersensitivity on the progenitor hematopoietic cells. A significant relationship was found between the presence of JAK2 positivity, splenomegaly, high leukocyte count, and higher hemoglobin levels; however, there was no correlation between JAK2 positivity thrombosis and hemorrhage risk.[14] A significant increase in spleen size has been observed in PMF cases that JAK2 V617F mutation is homozygote.[11] A significant increase in spleen size has been reported to be in patients with PV JAK2 V617F allele burden more than 75%.[15] A higher frequency of JAK2 mutation has been reported to be in ET patients with organomegaly.[16] There was no relation between the presence of mutations and the presence of splenomegaly in the time of diagnosis in our PV and ET patient groups. The study that Carobbio et al. evaluated the relationship between the presence of leukocytosis in ET patients and risks observed in CMDs revealed that leukocytosis is a risk factor for thrombosis.[12] It has not found a relationship between the JAK2 V617F allele burden and leukocytosis. In our study, there was no significant relationship between JAK2 mutation and the presence of leukocytes at diagnosis.
Higher leukocytes and platelet counts have been reported to be in PV patients with JAK2 mutation compared to those without JAK2 mutation, and elevated hemoglobin values were found in patients with JAK2 negative. Higher leukocyte counts, elevated hemoglobin values and thromboembolic events, have been reported to in ET cases with JAK2 mutation.[17] Ha JS et al. revealed that there was no relationship between the presence of leukocytosis and JAK2 positivity.[16] Likewise, in our study, there was no significant relationship between cases with leukocytosis and those without leukocytosis in terms of frequency of JAK2 in three groups. In a study evaluating the relationship between the presence of JAK2 mutation and thrombosis, hemorrhage, and some clinical signs and symptoms in 157 cases with CMDs between 2003 and 2013, both arterial and venous thrombosis risks were the most common in PV group (29%), followed by in the ET group (14%), and 0% in PMF group; however, bleeding was mostly observed in PMF group (17%), followed by 11% in ET, and 7% in PV, respectively.[18] In addition, it was reported that JAK2 V617F mutation is more frequently higher in older age, and those patients have a higher risk of thrombosis.[19] In another study that evaluated 135 cases with CMDs, JAK2 was found positive in 95 cases (95/135) and levels of JAK2 V617F mRNA were found higher in patients above 60 years of age compared to patients younger than 60 years of age.[20] JAK2 positivity was found to be higher in older ages, but there was no significant relation between JAK2 positivity and leukocytosis in our study. A study that contains a larger patient group is needed for an accurate conclusion.
Limitations of our study are the small sample size that was evaluated from a single center, retrospectively. The JAK2 mutation rates can vary in different provinces depending on the number of cases. A prospective, multicenter study with a big sample size may present the exact mutation rates. The relationship between thrombosis/hemorrhage complications and JAK2 positive/negativity could not be evaluated; as another limitation of our study is that relevant data of patients could not obtain.
CONCLUSION
JAK2 V617F mutation is more common in ET and PMF cases and the elderly cases. It poses a risk factor for developing complication in cases with CDMs. The presence of JAK2 mutation is not related to gender, splenomegaly, and higher leukocyte count.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951;6:372–5. [PubMed] [Google Scholar]
- 2.Faderl S, Kantarjian HM, Talpaz M. Chronic myelogenous leukemia: Update on biology and treatment. Oncology (Williston Park) 1999;13:169–80. [PubMed] [Google Scholar]
- 3.Tefferi A, Thiele J, Orazi A, Kvasnicka HM, Barbui T, Hanson CA, et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: Recommendations from an ad hoc international expert panel. Blood. 2007;110:1092–7. doi: 10.1182/blood-2007-04-083501. [DOI] [PubMed] [Google Scholar]
- 4.Tefferi A, Lasho TL, Schwager SM, Steensma DP, Mesa RA, Li CY, et al. The JAK2(V617F) tyrosine kinase mutation in myelofibrosis with myeloid metaplasia: Lineage specificity and clinical correlates. Br J Haematol. 2005;131:320–8. doi: 10.1111/j.1365-2141.2005.05776.x. [DOI] [PubMed] [Google Scholar]
- 5.Wu X, Lee JW, Peker D, Spitzer SG, Laser J, Reddy VV, et al. Is low positive JAK2 V617F mutation test result clinically significant? Multi-institutional study. Appl Immunohistochem Mol Morphol. 2016;24:589–94. doi: 10.1097/PAI.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 6.Tefferi A, Strand JJ, Lasho TL, Knudson RA, Finke CM, Gangat N, et al. Bone marrow JAK2V617F allele burden and clinical correlates in polycythemia vera. Leukemia. 2007;21:2074–5. doi: 10.1038/sj.leu.2404724. [DOI] [PubMed] [Google Scholar]
- 7.Krebs DL, Hilton DJ. SOCS proteins: Negative regulators of cytokine signaling. Stem Cells. 2001;19:378–87. doi: 10.1634/stemcells.19-5-378. [DOI] [PubMed] [Google Scholar]
- 8.Barosi G, Bergamaschi G, Marchetti M, Vannucchi AM, Guglielmelli P, Antonioli E, et al. JAK2 V617F mutational status predicts progression to large splenomegaly and leukemic transformation in primary myelofibrosis. Blood. 2007;110:4030–6. doi: 10.1182/blood-2007-07-099184. [DOI] [PubMed] [Google Scholar]
- 9.Eichhorst BF, Busch R, Hopfinger G, Pasold R, Hensel M, Steinbrecher C, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood. 2006;107:885–91. doi: 10.1182/blood-2005-06-2395. [DOI] [PubMed] [Google Scholar]
- 10.Deadmond MA, Smith-Gagen JA. Changing incidence of myeloproliferative neoplasms: Trends and subgroup risk profiles in the USA, 1973-2011. J Cancer Res Clin Oncol. 2015;141:2131–8. doi: 10.1007/s00432-015-1983-5. [DOI] [PubMed] [Google Scholar]
- 11.Tefferi A, Lasho TL, Schwager SM, Strand JS, Elliott M, Mesa R, et al. The clinical phenotype of wild-type, heterozygous, and homozygous JAK2V617F in polycythemia vera. Cancer. 2006;106:631–5. doi: 10.1002/cncr.21645. [DOI] [PubMed] [Google Scholar]
- 12.Carobbio A, Finazzi G, Guerini V, Spinelli O, Delaini F, Marchioli R, et al. Leukocytosis is a risk factor for thrombosis in essential thrombocythemia: Interaction with treatment, standard risk factors, and Jak2 mutation status. Blood. 2007;109:2310–3. doi: 10.1182/blood-2006-09-046342. [DOI] [PubMed] [Google Scholar]
- 13.Zhang SP, Li H, Lai RS. Detection of JAK2 V617F mutation increases the diagnosis of myeloproliferative neoplasms. Oncol Lett. 2015;9:735–8. doi: 10.3892/ol.2014.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lieu CH, Wu HS, Hon YC, Tsai WH, Yang CF, Wang CC, et al. Prevalence of the JAK2-V617F mutation in Taiwanese patients with chronic myeloproliferative disorders. Intern Med J. 2008;38:422–6. doi: 10.1111/j.1445-5994.2007.01589.x. [DOI] [PubMed] [Google Scholar]
- 15.Vannucchi AM, Antonioli E, Guglielmelli P, Longo G, Pancrazzi A, Ponziani V, et al. Prospective identification of high-risk polycythemia vera patients based on JAK2(V617F) allele burden. Leukemia. 2007;21:1952–9. doi: 10.1038/sj.leu.2404854. [DOI] [PubMed] [Google Scholar]
- 16.Ha JS, Kim YK, Jung SI, Jung HR, Chung IS. Correlations between Janus kinase 2 V617F allele burdens and clinicohematologic parameters in myeloproliferative neoplasms. Ann Lab Med. 2012;32:385–91. doi: 10.3343/alm.2012.32.6.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouyang Y, Qiao C, Wang J, Xiao L, Zhang S. Analysis of CALR, JAK2 and MPL gene mutations in BCR-ABL negative myeloproliferative neoplasms. Zhonghua Yi Xue Za Zhi. 2015;95:1369–73. [PubMed] [Google Scholar]
- 18.Duangnapasatit B, Rattarittamrong E, Rattanathammethee T, Hantrakool S, Chai-Adisaksopha C, Tantiworawit A, et al. Clinical manifestations and risk factors for complications of Philadelphia chromosome-negative myeloproliferative neoplasms. Asian Pac J Cancer Prev. 2015;16:5013–8. doi: 10.7314/apjcp.2015.16.12.5013. [DOI] [PubMed] [Google Scholar]
- 19.Tevet M, Ionescu R, Dragan C, Lupu AR. Influence of the JAK2 V617F mutation and inherited thrombophilia on the thrombotic risk among patients with myeloproliferative disorders. Maedica (Buchar) 2015;10:27–32. [PMC free article] [PubMed] [Google Scholar]
- 20.Chao HY, Shen YM, Zhang R, Feng YF, Cen JN, Yao L, et al. A quantitative assay for JAK2 mutation in 135 patients with chronic myeloproliferative neoplasms. Zhonghua Xue Ye Xue Za Zhi. 2009;30:321–5. [PubMed] [Google Scholar]


