Abstract
Health-related quality of life is an important, yet neglected aspect of chronic kidney disease (CKD) care. We evaluated the quality of life and its determinants across CKD 3 to 5D using a kidney disease specific tool (Kidney Disease Quality of Life-SF™) in an underprivileged, predominantly rural population with high rates of illiteracy and unemployment. The scores of individual domains were summarized to three composite scores – physical composite summary (PCS), mental composite summary (MCS), and kidney disease component summary score (KDCS). A total number of 204 participants were recruited from nephrology outpatient clinics. About 68.1% of participants were males. The mean age of the study population was 49.14 ± 13.63 years. There was a high proportion of illiteracy (36.3%) and unemployment (80.9%). KDCS showed a significant decline (P = 0.01) from CKD 3 to CKD 5D whereas MCS and PCS showed a nonsignificant decrease. There was no difference in KDCS, PCS, or MCS scores between patients treated by hemodialysis and CAPD. Illiteracy and unemployment were associated with significantly lower KDCS, PCS, and MCS scores. Age ≥50 years was associated with poor PCS (29.49 ± 8.20 vs. 34.17 ± 9.99; P < 0.001). Hemoglobin <10 g/dL was associated with poor KDCS (58.93 ± 13.09 vs. 65.55 ± 13.38; P < 0.001) and PCS (29.56 ± 8.13 vs. 33.37 ± 9.82; P < 0.001). The presence of comorbidities such as diabetes and hypertension had no impact on the composite scores. KDCS, MCS, or PCS scores did not vary among patients having high serum phosphorus (≥4.5 mg/dL), low albumin (<3.5 g/dL), and elevated parathyroid hormone (≥150 pg/ml). On multiple linear regression analysis, the predictors of KDCS were unemployment (P < 0.001) and illiteracy (P = 0.03). Unemployment (P < 0.001) and age (P < 0.001) were predictors of PCS whereas literacy level (P < 0.001) was predictive of MCS.
Keywords: Chronic kidney disease, kidney disease component summary score, quality of life
Introduction
The prevalence of chronic kidney disease (CKD) has steadily increased over the last few decades.[1,2] Multiple factors including improvements in life expectancy and increasing prevalence of comorbid conditions such as hypertension and diabetes have contributed to this rise. CKD has impacts the health-related quality of life (HRQoL). There is minimal literature on HRQoL in CKD patients from low and middle-income countries, including India.[3,4,5,6,7,8,9,10,11,12,13] A few issues unique to the developing world which could potentially affect the HRQoL include age, economic status, literacy level, loss of employment, and gender bias. Apart from the disease and its complications, socioeconomic and cultural environment of the patients also play a major role in determining HRQoL. HRQoL is a neglected aspect of CKD care, as the available resources will be often diverted to address the general medical needs. Measurements of HRQoL would aid the physician to have a better understanding of the disease from patient's perspective and would aid in optimal supportive care. There is only limited data on HRQoL in CKD from Indian subcontinent.[4,5,6,9,10,11,12,13] Most of the Indian studies are limited to patients having advanced CKD or end stage renal disease (ESRD). Considering the social and cultural diversity in India, there can be significant differences in HRQoL and its determinants across the country. The current study intends to assess the HRQoL and its determinants in CKD 3 to 5D using a kidney disease-specific tool (Kidney Disease Quality of Life ([KDQOL]-SF™).
Materials and Methods
This study was done in a tertiary referral center in South India, which predominantly caters to patients from socially and economically backward areas. The study included adult patients with CKD stage 3 to 5D. Patients with CKD 5D required more than 3 months dialysis vintage for recruitment. All patients attending nephrology outpatient and dialysis clinics were given consecutive numbers and entered in a register. A systematic random sampling was employed to select the study participants. Patients with a history of kidney transplantation, pregnancy, receiving immunosuppression, malignancy, psychiatric illness, and significant impairment of hearing, speech, or cognitive disturbances were excluded. The Institute Ethics Committee approved the study protocol. The study was conducted from January to May 2015.
HRQoL was assessed with KDQOL-SF™, v. 1.3 Questionnaire from RAND Corporation (refer website: http://www.rand.org/content/dam/rand/www/external/health/surveys_tools/kdqol/kdqol13.pdf). It is a validated quality of life instrument that combines the generic SF-36 instrument with a kidney disease-specific instrument. The English questionnaire has been validated in Indian population.[9] Validation studies have been undertaken in Indian languages including Kannada, Marathi, and Hindi.[10,11,12] A study with a translated Tamil version has also been published.[13]
The SF 36 assess the HRQoL in eight domains (physical functioning, role limitations caused by physical problems, role limitations caused by emotional problems, pain, general health, energy/fatigue, emotional well-being, and social function). Results from SF 36 are further summarized into a physical composite summary (PCS) and a mental composite summary (MCS) score.
The kidney disease-specific instrument assesses the burden of kidney disease in eleven domains (symptoms/problems of kidney disease, burden of kidney disease, effects of kidney disease, work status, cognitive function, quality of social interaction, sexual function, sleep, social support, patient satisfaction, and dialysis staff encouragement). Each domain is scored on a 100-point scale, with higher scores representing better QoL. The individual scores can be averaged to a kidney disease component summary score (KDCS).[14] The tool had already been used in predialysis patients after excluding the dialysis-specific component (dialysis staff encouragement and patient satisfaction).
The questionnaire was translated to local language according to the instructions given by RAND corporation. The translation was done by professional translators. A pilot testing with 10 patients was undertaken to assess the cultural suitability, and the inputs were used for formulating the final version. The first author was trained for administering the questionnaire. Even though KDQOL-SF™ is a self-reported questionnaire, considering the high proportion of illiterate participants, the first author administered the questionnaire by an interview to all the study participants.
Statistical methods
All categorical variables were expressed as frequencies and percentages, and continuous variables were expressed as mean with standard deviation or median with range. Student's t-test was used to compare the scores between hemodialysis (HD) and CAPD. ANOVA was used to compare the scores between the different stages of CKD from 3 to 5D. Student's t-test was also used to identify the significant changes in the KDCS, PCS, and MCS scores between categorical variables. A multiple linear regression analysis was done to identify the predictors of KDCS, PCS, and MCS. P < 0.05 was considered significant. The data were analyzed using SPSS version 19 (IBM Corporation; Armonk, NY, US).
Results
Two hundred and four patients were randomly selected from the nephrology outpatient clinic during the study period. The dialysis-specific component was excluded as predialysis patients were also included in the study. The sexual function component was also excluded from final analysis because of poor response from the participants.
The mean age of the study population was 49.14 ± 13.63 years. About 68% of participants were males. About 90% of the study population were hailing from rural areas. The etiology of CKD is given in Figure 1. The most common etiology was CKD of unidentified etiology (CKD u). The baseline demographic and clinical characteristics are shown in Table 1. There was a high proportion of loss of employment resulting from disease. Prior to the diagnosis of kidney disease, 85.3% (n = 174; 134 males and 40 females) were employed; but only 19.1% were employed at the time of enrollment to the study.
Figure 1.
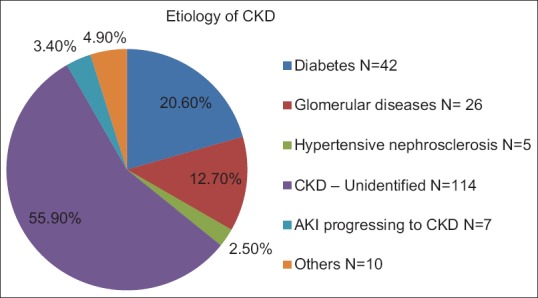
Etiology of chronic kidney disease
Table 1.
Baseline demographic and clinical characteristics of study population
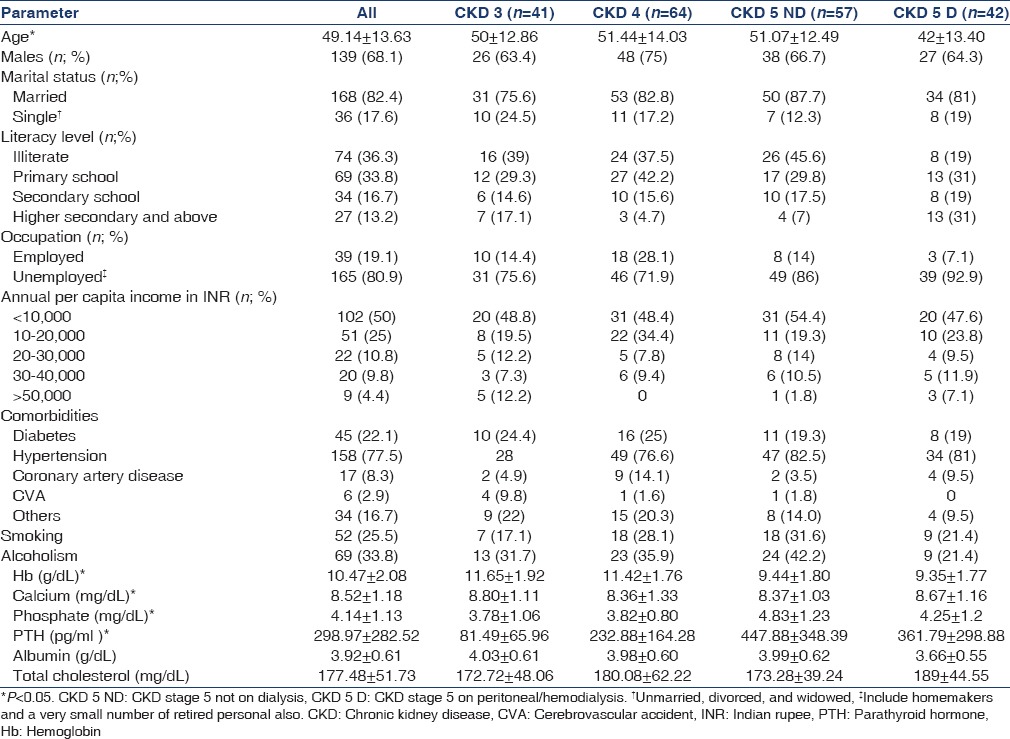
Measurements of HRQoL with the summary scores of the entire study population and across different categories of CKD are shown in Table 2. A significant decline was noticed in 5/8 kidney disease-specific domains from CKD 3 to 5D. The domains under SF 36 also showed decreasing scores, but only energy/fatigue and social function showed a significant difference with increasing severity of CKD. There was no difference in the composite scores between patients on hemodialysis and peritoneal dialysis [Table 3]. HD patients had lower scores for general health, emotional status, and disease burden.
Table 2.
Health-related quality of life in the study population
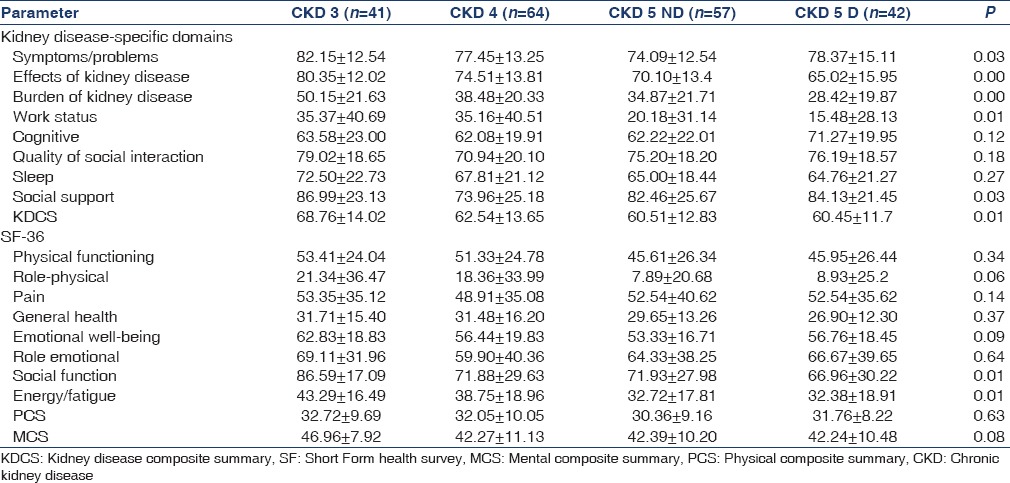
Table 3.
Health-related quality of life in hemodialysis versus continuous ambulatory peritoneal dialysis
Effect of selected clinical and social parameters on kidney disease component summary, physical composite summary, and mental composite summary scores
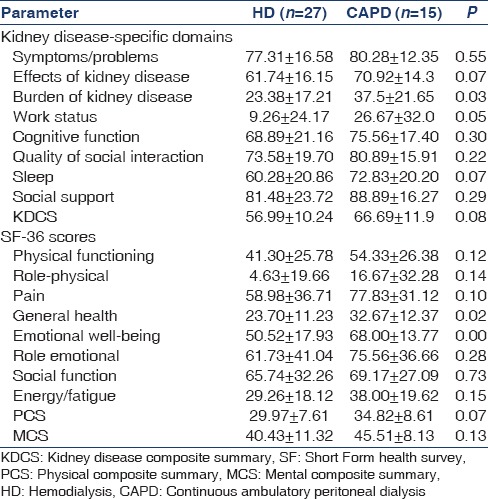
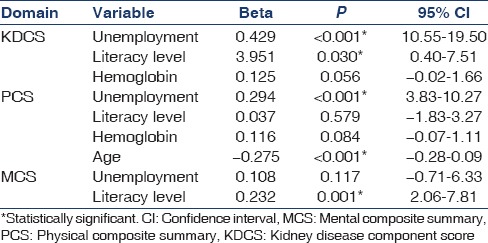
The impact of selected social and clinical parameters (age, gender, occupation, literacy rate, marital status, hemoglobin, albumin, phosphorus, and parathyroid hormone) on the composite scores is given in Table 4. Illiterate and unemployed persons had significantly lower KDCS, PCS, and MCS scores whereas gender and marital status had no impact. Female gender was associated with lower scores in only two individual domains – burden of kidney disease (33.27 ± 22.26 vs 39.83 ± 21.56.; P = 0.046) and physical function (43.08 ± 22. 87 vs. 51.83 ± 26.19; P = 0.02). The presence of comorbidities such as diabetes and hypertension had no impact on the composite scores. On multiple linear regression analysis, the predictors of KDCS were unemployment (P < 0.001) and illiteracy (P = 0.03). Unemployment (P < 0.001) and age (P < 0.001) were predictors of PCS whereas literacy level (P < 0.001) was predictive of MCS [Table 5]. Age was analyzed as a continuous variable whereas others were categorical.
Table 4.
Impact on social and clinical parameters on kidney disease component score, physical composite summary, and mental composite summary scores
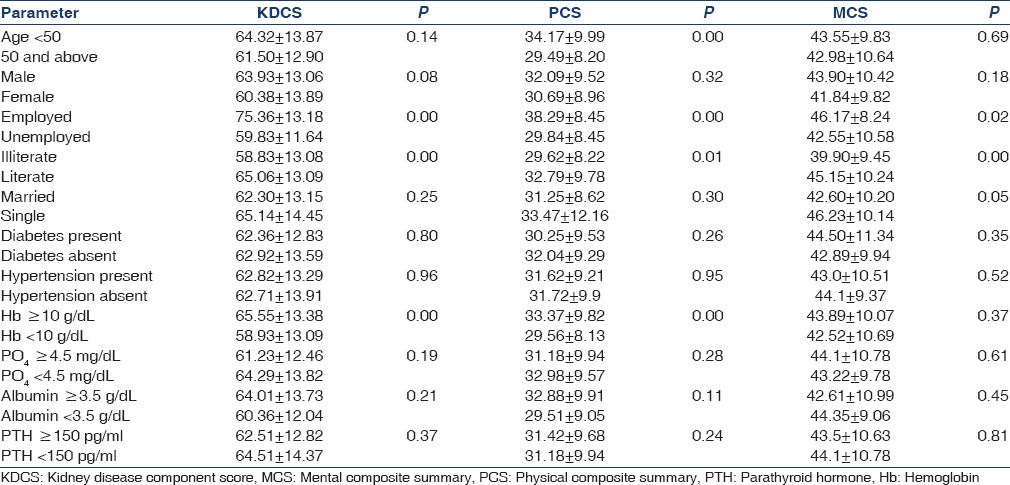
Table 5.
Results of multiple linear regression analysis
Discussion
There is only limited information on HRQoL in Indian patients with CKD; most of the data is restricted to patients with ESRD. This study attempted to find out the quality of life and the determinants across CKD 3 to 5D in an underprivileged, predominantly rural population with high rates of illiteracy and unemployment.
The etiologic spectrum of CKD in the current study is different, with more than half of the population having CKD u. A high prevalence of CKD u varying from 57.4% to 66.7% has been reported in predominantly agricultural communities from various countries including India, Srilanka, Egypt, and Central America.[15] The proportion of CKD u in the current study stands much higher than what is reported from the CKD registry of India.[16]
The mean age of the study population is similar to previous studies from India.[2,4,5,6] We observed a significant decline in KDCS with increasing severity of CKD. Among individual domains, work status scores were affected to a greater degree. Eighty five percent of the study population had prior employment, but at the time of recruitment, only one-fifth were employed. This might be secondary to the fact that majority of the patients (65%) were daily laborers involved in agricultural/construction activities that require hard physical labor. The physical effects of kidney disease might negatively affect performance at work, which eventually leads to loss of employment.
There is only limited information on HRQoL and its determinants in predialysis patients. There is conflicting data on the effect of decreasing renal function on KDCS, PCS, and MCS assessed by KDQOL. We observed that KDCS declined with worsening renal function whereas the PCS and MCS showed a nonsignificant declining trend. A study from North America reported a contrasting picture with all three composite scores showing a progressive decline with advancing kidney disease.[8] On the other hand, a Canadian study reported no differences in HRQoL between predialysis and ESRD patients, but they excluded patients with early CKD.[17] The same study reported that existential well-being showed a moderate association with HRQOL.[17] A German study reported no significant differences in MCS across the entire spectrum of CKD.[18] A study from Brazil reported findings similar to our study. Even though CKD patients had lower KDCS, MCS, and PCS when compared to the general population, the values did not vary between the different stages of CKD[19]. Literature from India using KDQOL is limited to patients with ESRD. The nonsignificant trends in PCS and MCS could result from the comparatively younger age of study population where the tolerance to nonspecific factors might be higher. As the general living standards are poor, the focus might be entirely on the effects of disease per se which would account for the decline in KDCS with progressive disease. The MCS was higher when compared to PCS, reflecting the psychologic adaptation to chronic illness. MCS and PCS could be affected by multiple factors other than the medical disease. Apart from the physical, clinical, and functional parameters, factors such as the sociocultural environment, economic status, emotional status, accessibility to medical care, and spiritual attitudes possibly play a significant role in an individual's perception of life and disease.[17,18,19,20] These parameters could not be assessed with the current tool for HRQOL.
There was no difference in the KDCS, PCS, or MCS between patients on CAPD and HD. Even though the composite scores were comparable, HD patients had more burden of disease, poor general health, and lower emotional well-being. The impact of dialysis modality on HRQoL is inconclusive. As CAPD is associated with better patient autonomy, it would provide better emotional well-being. Frequent hospital visits and cannulations, complications associated with dialysis, and empiric dialysis prescriptions might account for the higher disease burden and poor emotional status in HD patients. An Indian study reported better physical and emotional well-being for patients on CAPD.[21] The comparatively lesser number of CAPD patients in the current study would have affected the results.
We tried to analyze the impact of selected demographic and clinical variables on KDCS, PCS, and MCS. We observed that socioeconomic factors had a greater impact on HRQoL than the clinical variables. Unemployment and illiteracy were associated with significantly poor KDCS, MCS, and PCS. A similar observation was made by Cruz et al. in a CKD cohort that had a similar etiologic profile and socioeconomic status as in the current study.[19]
Increasing age, presence of comorbidities, and lower levels of hemoglobin have been reported to be associated with poor PCS and KDCS scores.[4,12,14,16,22,23] We found that lower hemoglobin levels were associated with poor KDCS and PCS whereas older age was associated with poor PCS. The presence of comorbidities had no impact on HRQoL. The relatively younger age and comparatively lesser proportion of patients with comorbid conditions such as diabetes and coronary artery disease would account for this finding. Even though hypertension was present in three-fourth of the study population, it could be easily controlled with drugs. We did not attempt to assess the impact of income on HRQoL as the per capita income was much lower compared to the national average. We did not try to analyze the effect of alcoholism and smoking on HRQoL. Majority of patients were male and they had quit smoking and drinking by the time they were diagnosed to have CKD.
Female gender is considered to be associated with poor HRQOL.[4,8,17,18,24] We did not find a significant difference in the composite scores stratified by gender. This finding is rather surprising considering the fact that the study was conducted in a rural population where gender inequality is rampant. Females had inferior scores in individual domains with significantly lower scores in disease burden, cognitive function, and physical function. In the current study, males outnumbered females and the sample size might not be sufficient enough to detect a significant difference.
In our study, socioeconomic parameters emerged as a major determinant of HRQOL. Apart from anemia, other disease-related comorbidities had no major impact on QOL. Understanding the social and cultural environment of the patient is absolutely essential for optimal health care delivery. Patients with chronic medical illness need a holistic approach with a thorough understanding of the individual's perception to the disease. The policy makers should focus on measures such as education, social security, and vocational rehabilitation.
The results of the current study should be interpreted with the following limitations. Even though we could identify an association between certain parameters and HRQoL, a causal relationship could not be ascertained due to the observational nature of the study. Further longitudinal studies are required to determine a causal relationship. The impact of parameters such as gender and dialysis modality could not be determined correctly because of the relatively small numbers of participants. Dialysis prescription was empirical, and adequacy was not measured. There might be residual confounding factors such as accessibility to health care, pill burden, compliance with therapy, capacity to cope with stress, malnutrition, social, cultural, and religious practices which could affect HRQoL, for which data were not collected.
Conclusion
The results of the current study indicates that HRQoL declines with advanced stages of CKD. The kidney disease-specific domains are affected to a greater extent. Socioeconomic parameters have a major impact on HRQoL in underprivileged populations. Steps such as anemia correction and patient-centered health education could potentially improve the quality of life. Employment loss resulting from disease could be tackled by strengthening the social security measures and job retraining to suit the physical effects of the disease. Understanding the sociocultural environment of the patient is extremely important for effective health care delivery. A better understanding of HRQoL and its determinants would help to formulate individualized treatment strategies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Singh AK, Farag YM, Mittal BV, Subramanian KK, Reddy SR, Acharya VN, et al. Epidemiology and risk factors of chronic kidney disease in India – Results from the SEEK (Screening and Early Evaluation of Kidney Disease) study. BMC Nephrol. 2013;14:114. doi: 10.1186/1471-2369-14-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anand S, Shivashankar R, Ali MK, Kondal D, Binukumar B, Montez-Rath ME, et al. Prevalence of chronic kidney disease in two major Indian cities and projections for associated cardiovascular disease. Kidney Int. 2015;88:178–85. doi: 10.1038/ki.2015.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awuah KT, Finkelstein SH, Finkelstein FO. Quality of life of chronic kidney disease patients in developing countries. Kidney Int Suppl. 2013;3:227–9. doi: 10.1038/kisup.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veerappan I, Arvind RM, Ilayabharthi V. Predictors of quality of life of hemodialysis patients in India. Indian J Nephrol. 2012;22:18–25. doi: 10.4103/0971-4065.91185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abraham S, Ramachandran A. Estimation of quality of life in haemodialysis patients. Indian J Pharm Sci. 2012;74:583–7. doi: 10.4103/0250-474X.110624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abraham S, Venu A, Ramachandran A, Chandran PM, Raman S. Assessment of quality of life in patients on hemodialysis and the impact of counseling. Saudi J Kidney Dis Transpl. 2012;23:953–7. doi: 10.4103/1319-2442.100875. [DOI] [PubMed] [Google Scholar]
- 7.Abd ElHafeez S, Sallam SA, Gad ZM, Zoccali C, Torino C, Tripepi G, et al. Cultural adaptation and validation of the “Kidney Disease and Quality of Life – Short Form (KDQOL-SF™) version 1.3” questionnaire in Egypt. BMC Nephrol. 2012;13:170. doi: 10.1186/1471-2369-13-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mujais SK, Story K, Brouillette J, Takano T, Soroka S, Franek C, et al. Health-related quality of life in CKD Patients: Correlates and evolution over time. Clin J Am Soc Nephrol. 2009;4:1293–301. doi: 10.2215/CJN.05541008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuriokose AV, Kenchaappa V, Hiremath S, Varghese B. Assessment of Quality of Life of Chronic Renal Failure Patients in India. Quality Issues and Insights in the 21st: Century. 2012;(1):20–28. [Google Scholar]
- 10.Joshi V, Mulay A, Dighe T, Jeloka T, Biwalkar V. Validity of marathi translated kidney disease quality of life short form (KDQOL-SF)™. J Evid Based Med Healthc. 2015;2:409–20. [Google Scholar]
- 11.Mateti UV, Nagappa AN, Attur RP, Nagaraju SP, Mayya SS, Balkrishnan R. Cross-cultural adaptation, validation and reliability of the South Indian (Kannada) version of the kidney disease and quality of life (KDQOL-36) instrument. Saudi J Kidney Dis Transpl. 2015;26:1246–52. doi: 10.4103/1319-2442.168662. [DOI] [PubMed] [Google Scholar]
- 12.Patil PP, Pawar S, Thakurdesai P. Translation and Validation of Kidney Disease and Quality of Life (KDQOL-SFTM 1.2) Instrument to Measure Health Related Quality of Life of Indian Patients with Kidney Disease. Value in Health. 2014;17:A813. doi: 10.1016/j.jval.2014.08.563. [DOI] [PubMed] [Google Scholar]
- 13.Murali R, Sathyanarayana D, Muthusethupathy M. Assessment of quality of life in chronic kidney disease patients using the kidney disease quality of life-short form™ questionnaire in indian population: A community based study. Asian J Pharm Clin Res. 2015;8:271–4. [Google Scholar]
- 14.Mapes DL, Bragg-Gresham JL, Bommer J, Fukuhara S, McKevitt P, Wikström B, et al. Health-related quality of life in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2004;44(5 Suppl 2):54–60. doi: 10.1053/j.ajkd.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Almaguer M, Herrera R, Orantes CM. Chronic kidney disease of unknown etiology in agricultural communities. MEDICC Rev. 2014;16:9–15. doi: 10.37757/MR2014.V16.N2.3. [DOI] [PubMed] [Google Scholar]
- 16.Rajapurkar MM, John GT, Kirpalani AL, Abraham G, Agarwal SK, Almeida AF, et al. What do we know about chronic kidney disease in India: First report of the Indian CKD registry. BMC Nephrol. 2012;13:10. doi: 10.1186/1471-2369-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davison SN, Jhangri GS. Existential and religious dimensions of spirituality and their relationship with health-related quality of life in chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:1969–76. doi: 10.2215/CJN.01890310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seidel UK, Gronewold J, Volsek M, Todica O, Kribben A, Bruck H, et al. Physical, cognitive and emotional factors contributing to quality of life, functional health and participation in community dwelling in chronic kidney disease. PLoS One. 2014;9:e91176. doi: 10.1371/journal.pone.0091176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruz MC, Andrade C, Urrutia M, Draibe S, Nogueira-Martins LA, Sesso Rde C. Quality of life in patients with chronic kidney disease. Clinics (Sao Paulo) 2011;66:991–5. doi: 10.1590/S1807-59322011000600012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdel-Kader K, Unruh ML, Weisbord SD. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol. 2009;4:1057–64. doi: 10.2215/CJN.00430109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makkar V, Kumar M, Mahajan R, Khaira NS. Comparison of outcomes and quality of life between hemodialysis and peritoneal dialysis patients in Indian ESRD population. J Clin Diagn Res. 2015;9:OC28–31. doi: 10.7860/JCDR/2015/11472.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finkelstein FO, Story K, Firanek C, Mendelssohn D, Barre P, Takano T, et al. Health-related quality of life and hemoglobin levels in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:33–8. doi: 10.2215/CJN.00630208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen RA, Chin H, Blalock S, Joy MS. Predialysis chronic kidney disease: Evaluation of quality of life in clinic patients receiving comprehensive anemia care. Res Social Adm Pharm. 2009;5:143–53. doi: 10.1016/j.sapharm.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fructuoso M, Castro R, Oliveira L, Prata C, Morgado T. Quality of life in chronic kidney disease. Nefrologia. 2011;31:91–6. doi: 10.3265/Nefrologia.pre2010.Jul.10483. [DOI] [PubMed] [Google Scholar]


