Abstract
We studied the role of serum hepcidin in anemia of chronic kidney disease (CKD) in a hospital-based cross-sectional study. Serum hepcidin, ferritin, and high-sensitivity C-reactive protein (hsCRP) levels were evaluated in patients of CKD. Hepcidin levels were increased in patients as compared to healthy adults. Hepcidin levels increased as CKD progressed through stage 3–5 (P trend = 0.015) but did not correlate with estimated glomerular filtration rate. Hepcidin correlated positively with ferritin (P < 0.0001) and transferrin saturation (TSAT) (P = 0.0217) and negatively with erythropoietin (EPO) levels (P = 0.0258) but did not correlate with either hsCRP or estimated glomerular filtration rate. Iron status influenced hepcidin levels of patients. Patients were divided according to iron status on the basis of TSAT and serum ferritin levels. We observed that while absolute iron deficiency (transferrin saturation <20%, ferritin <40 ng/ml) is associated with downregulation of hepcidin, hepcidin is elevated in other two categories of CKD patients (P = 0.0039). Iron status of patients also influenced interaction between hepcidin and hemoglobin (Hb). Hepcidin correlated negatively with Hb in patients with sufficient iron status (r = −0.7452, P < 0.0001) but nearly correlated positively with Hb in patients with absolute iron deficiency (r = 0.9428, P = 0.0572). Almost similar association persisted when cutoff value for serum ferritin was raised to 100 ng/ml as per NKF/KDOQI 2006 clinical practice guidelines except that no association was observed in absolute iron deficiency category. Cutoff value for hepcidin for differentiating absolute iron deficiency from other categories in our study population is ≤ 34 ng/ml (area under curve = 0.836, P < 0.0001). In conclusion, serum hepcidin level is increased in nondialysis CKD patients as compared to healthy adults possibly due to associated inflammation and decreased renal clearance. Furthermore, iron status modifies hepcidin level and its association with Hb. Raised hepcidin can predict the need for parenteral iron therapy and need for higher dose of recombinant human EPO to overcome iron-restricted erythropoiesis.
Keywords: Anemia, chronic kidney disease, hepcidin
Introduction
The global prevalence of chronic kidney disease (CKD) is 8–16%.[1] Anemia is a major complication of CKD. The major cause of anemia in CKD is erythropoietin (EPO) deficiency.[2,3] Thus, anemia management in CKD patients currently revolves around the use of erythropoiesis-stimulating agent (ESA) and supplement iron.[3] However, hyporesponsiveness and resistance to ESAs are emerging. It has been hypothesized that inflammation may play an important role in anemia of CKD.[4,5]
Although serum ferritin and transferrin saturation (TSAT) are commonly used as biomarkers for iron status in CKD patients, these markers are not sensitive enough to distinguish functional iron deficiency from iron overload.[6]
Recently, hepcidin, an acute phase reactant protein produced in the liver, is thought to be a central regulator of body iron metabolism.[7,8] Hepcidin controls the plasma iron concentration by inhibiting iron export by ferroportin from enterocytes and macrophages.[9] Hence, increased hepcidin production leads to decrease in plasma iron concentration and to iron-restricted erythropoiesis.[10] Hepcidin expression is upregulated by inflammation[11] and iron loading[12] and downregulated by erythropoietic activity.[10,13] Studies of humans with chronic infection and severe inflammatory disease have shown markedly elevated levels of hepcidin, strongly suggesting that hepcidin level plays a key role in the anemia of inflammation and reticuloendothelial blockade.[11]
Researchers have in recent past tried to look into factors influencing hepcidin and its association with hemoglobin (Hb) in CKD.[7,14]
Subjects and Methods
We did a hospital-based cross-sectional observational study conducted at Vardhaman Mahavir Medical College and Safdarjung Hospital, New Delhi. Study was conducted on 100 adult CKD patients attending the department of internal medicine and nephrology of VMMC and Safdarjung Hospital. Patients >18 years both male and female with diagnosis of nondialysis-dependent CKD (stage 3–5) with anemia were included. Whereas patients on renal replacement therapy (hemodialysis, peritoneal dialysis, and kidney transplant), parenteral iron therapy within preceding 6 months, EPO therapy in preceding 2 weeks, with evidence of active bleeding or evidence of active infection, who have received blood transfusion within past 6 months, with previously diagnosed case of nonrenal causes of anemia except iron deficiency anemia and prediagnosed comorbidities such as malignancy, end-stage liver disease, and collagen vascular disease (systemic lupus erythematosus and rheumatoid arthritis) were excluded from our study. Furthermore, 30 age-sex-matched healthy adults were taken up for a study to determine serum hepcidin levels in adult Indian population.
A written informed consent and Institution's Ethics Committee clearance was obtained before recruitment of the participants for the study. All the participants were subjected to detailed history, thorough physical examination and investigation. Laboratory investigation included complete blood count with peripheral smear, absolute reticulocyte count, serum iron profile, serum B12 and folic acid, serum EPO level, serum hepcidin, high-sensitivity C-reactive protein (hsCRP), kidney function test, liver function test, plasma glucose, and HbA1c. Diagnosis and staging of CKD were done as per National Kidney Foundation/Kidney Disease Outcome Quality Initiative (NKF/KDOQI) 2002[15] classification criteria and estimated glomerular filtration rate (eGFR) was calculated using 4-variable modification of diet in renal diseases equation. Anemia in CKD was defined as Hb <13 g/dl in males and <12 g/dl in females as per KDIGO 2012[16] clinical practice guidelines. The blood sample was collected by venipuncture. For complete blood count, blood was collected in ethylenediaminetetraacetic acid coated vacutainers. Serum was obtained by centrifuging the blood at 3000 rpm for 10 min after clot formation. Serum was then stored at −80°C. Blood was collected into iron free tubes for iron studies. hsCRP is measured by CRP ultra EIA kit manufactured by Xema co, Ltd., Moscow, Russia. C-reactive protein was estimated quantitatively using solid phase ultrasensitive enzyme immunoassay. This test is based on two-site sandwich enzyme immunoassay principle. The expected upper limit of hsCRP using the provided kit in healthy individuals is 5.0 mg/L.
Hepcidin-25 was estimated using Diagnosis-Related Group (DRG) hepcidin-25 (bioactive) enzyme-linked immunosorbent assay (ELISA) kit, a solid phase ELISA, based on the principle of competitive binding. The legal manufacturer of kit is DRG instruments, Germany. The expected normal values provide by the manufacturer is 10.1 ng/ml with a 97.5 percentile of 39.3 ng/ml. Normal values of hepcidin-25 in Indian population is not known, so we also took thirty healthy adults to estimate normal values of serum hepcidin in Indian population. Estimation of serum EPO was done using immunoassay method using Beckman Coulter®, Access immunoassay system, manufactured by Beckman Coulter, Inc., CA, USA. The expected normal values are 2.59–18.50 mIU/ml.
Statistical analysis
Baseline characteristics were assessed with standard descriptive statistics. eGFR was examined both on a continuous scale and also categorically using the National Kidney Foundation stage system. Categorical variables were presented in number and percentage (%), and continuous variables were presented as mean ± standard deviation and median with interquartile range (IQR) (as applicable). Normality of data was tested by Kolmogorov–Smirnov test. If the normality was rejected then nonparametric test was used. Quantitative variables were compared using independent t-test and Mann–Whitney test (for nonparametric data) between two groups. Qualitative variables were compared using Chi-square test/Fisher's exact test. Pearson correlation coefficient was used to find association between various variables. For Nonparametric data, i.e., eGFR, serum ferritin, serum hepcidin, and serum EPO, log of data was used to find out the correlation. To examine the trend of each parameter across the CKD stages, the Jonckheere–Terpstra trend test and the Cochran–Armitage test were used. Quantitative variables were correlated by the Spearman rank test.
Multivariate regression analysis was performed to examine the relationship between various parameters after adjusting for confounders. The data were entered in MS EXCEL spreadsheet, and analysis was done using Statistical Package for Social Sciences (SPSS) version 21.0, manufactured by International Business Machine (IBM). P < 0.05 was considered statistically significant.
Results
Mean age of patients enrolled was 42.21 ± 14.7 years out of which 51% (n = 51) were males. About 72% (n = 72) patients in our study were hypertensives and 22% (n = 22) were diabetic. All the patients in our study were on oral iron therapy and were EPO free. Mean Hb concentration of our study population was 7.99 ± 1.42 g/dl. Normocytic normochromic anemia (63%, n = 63) followed by a microcytic hypochromic anemia (22%, n = 22) were the common types. Median serum hepcidin levels were significantly high in patients 60 (IQR 25–80) ng/ml as compared to age- and sex-matched healthy adults 19 (IQR [9–26]) ng/ml in controls (P < 0.05).
Majority of patients in our study had undetermined etiology (42%, n = 42). In the patients with identified cause, diabetic nephropathy was most common (22%, n = 22), followed by chronic glomerulonephritis (18%, n = 18), hypertensive kidney disease (12%, n = 12), and polycystic kidney disease (6%, n = 6). Causes were determined based on clinical and biochemical parameters and not based on renal biopsy.
The stage-wise clinical and laboratory profile of patients in the study are as per Table 1. About 20% (n = 20), 39% (n = 39), and 41% (n = 49) of the study population belonged to stage-3, stage-4, and stage-5 CKD, respectively. Hb level decreased, and serum hepcidin level increased significantly as CKD progressed through stage 3–5 (P trend = 0.00000, 0.01511, respectively).
Table 1.
Clinical characteristics and laboratory parameters according to chronic kidney disease stage
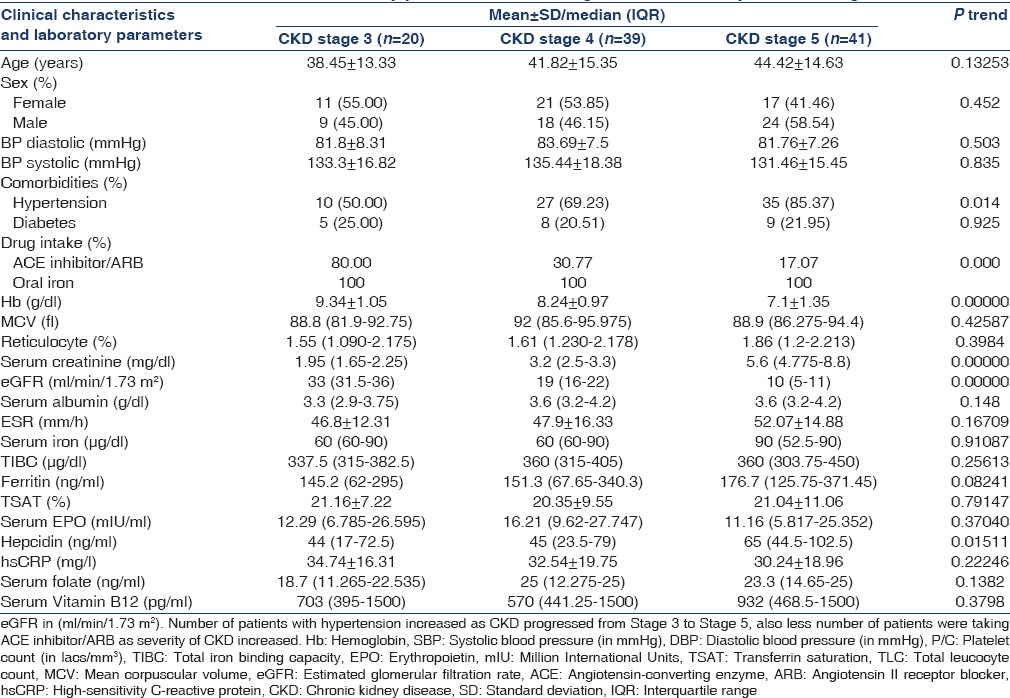
Predictors of log-normalized serum hepcidin-25 level
Factors determining hepcidin 25 were studied using univariate and multivariate regression analysis. The univariate model (not shown) showed log normalized hepcidin positively correlated with log normalized ferritin (r = 0.6712, P < 0.0001) and percentage TSAT (r = 0.224, P = 0.0249) and negatively with log normalized serum EPO levels (r = −0.208, P = 0.0377), whereas it did not correlate with either eGFR or hsCRP. On applying multiple regression analysis [Table 2], the positive correlation between hepcidin and ferritin as well as hepcidin and TSAT persisted in the multivariate model. Multiple regression analysis also showed a significant negative correlation between hepcidin and EPO as demonstrated by univariate model.
Table 2.
Predictors of log normalized serum hepcidin-25 level (multivariate model)
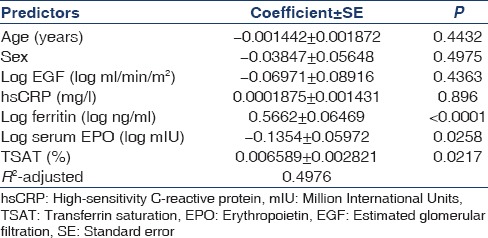
Predictors of hemoglobin in study population
Univariate analysis showed that Hb was negatively and significantly associated with log normalized ferritin (r = −0.3694, P = 0.0002) and log normalized hepcidin (r = −0.4764, P < 0.0001) and positively and significantly associated with eGFR (r = 0.553, P < 0.0001). However, multiple regression analysis [Table 3] showed that Hb was only significantly associated with log eGFR and not with hepcidin or ferritin as suggested in univariate model.
Table 3.
Predictors of hemoglobin in study population (multivariate model)
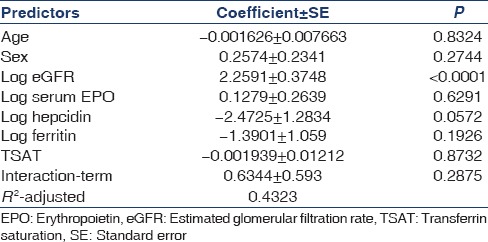
Effect of iron status on hepcidin level and association of hepcidin with hemoglobin in the study population
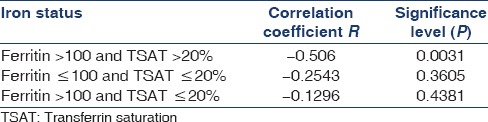
To define the iron status in the study population, patients were divided into categories based on % TSAT (%TSAT) and ferritin together as follows: Absolute iron deficiency, Functional iron deficiency, and Normal iron status. Cutoff value for %TSAT and Ferritin were 20% and 40 ng/ml, respectively, and was based on a recent study by Mercadal et al.[14] Normal iron status, Functional iron deficiency, and Absolute iron deficiency were defined as %TSAT ≥20 and Ferritin ≥40, %TSAT <20 and Ferritin ≥40, and %TSAT <20 and Ferritin <40, respectively. Serum hepcidin levels were significantly higher in patients with normal iron status followed by functional iron deficiency as compared to absolute iron deficiency (70 vs. 45 vs. 17.5, P = 0.0039) [Table 4]. Serum hepcidin was downregulated in patients with absolute iron deficiency. Furthermore, in our study, iron status modified association between hepcidin and Hb [Table 5]. There was significant negative correlation between hepcidin and Hb in patients with normal iron status (r = −0.7452, P < 0.0001) and near positive correlation in patients with absolute iron deficiency (r = 0.9428, P = 0.0572). Almost similar association persisted when cutoff value for serum ferritin was raised to 100 ng/ml as per NKF/KDOQI 2006[17] clinical practice guidelines for starting iron therapy in nondialysis CKD patients except that no correlation was found in the absolute iron deficiency category [Tables 6 and 7]. The serum hepcidin levels were significantly higher in patients with normal iron status followed by functional iron deficiency as compared to absolute iron deficiency (79 vs. 60 vs. 23, P < 0.0001). Furthermore, significant negative correlation was observed between hepcidin and Hb in patients with sufficient iron store (P < 0.0001).
Table 4.
Median hepcidin in (ng/ml) and number of patients (in parenthesis) according to iron status (Mercadel et al.)
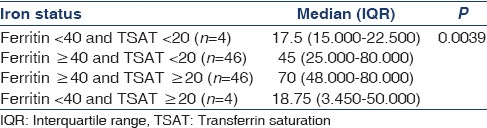
Table 5.
Correlation between log hepcidin and hemoglobin according to iron status categories (Mercadel et al.)

Table 6.
Median hepcidin in (ng/ml) and number of patients (in parenthesis) according to iron status (Kidney Disease Outcome Quality Initiative 2006)

Table 7.
Correlation between log hepcidin and hemoglobin according to iron status categories (Kidney Disease Outcome Quality Initiative 2006)
We also determined cutoff value for hepcidin to differentiate between absolute iron deficiency from other types of iron status in our study population. Taking serum ferritin <40 ng/ml as gold standard for absolute iron deficiency, we constructed receiver operating characteristic (ROC) curve and calculated area under curve (AUC). Cutoff value for serum hepcidin was ≤34 ng/ml (AUC = 0.836, P < 0.0001). Nondialysis CKD patients with serum hepcidin >34 ng/ml have very low probability of having absolute iron deficiency [Figure 1].
Figure 1.
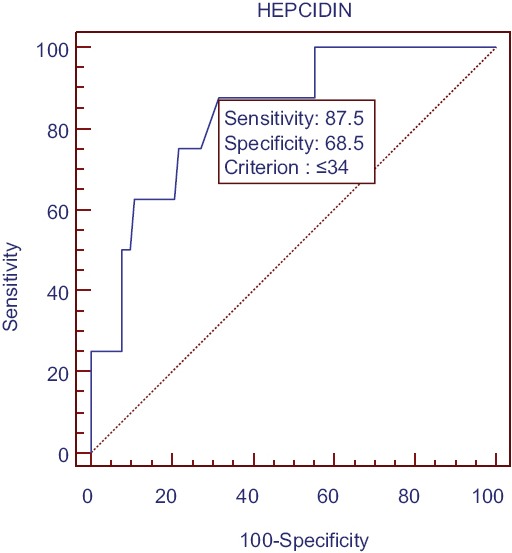
Receiver operating characteristic curve for calculation of hepcidin cutoff P < 0.0001
Discussion
Differentiating EPO hypo- or unresponsiveness due to iron-restricted erythropoiesis resulting from inflammation from absolute iron deficiency is one of the major concerns in the management of anemia in CKD patients. Hepcidin, an acute phase reactant is currently thought to be the major regulator of iron homeostasis, and according to several recent studies have the potential to provide information beyond iron profile. In our study, we assessed the role of serum hepcidin in anemia of CKD. Furthermore, we tried to find out whether iron status of the patient modifies the association between hepcidin and Hb. Median serum hepcidin level was significantly higher in patients as compared to age- and sex-matched healthy adults in controls. Hepcidin levels followed an increasing trend through stages 3–5 which was significant.
On multivariate analysis, no association was found between serum hepcidin-25 level and eGFR. Our observation is similar to two earlier studied.[7,18] However, several earlier studies have also demonstrated association between hepcidin and GFR.[14,19,20] These contradictory results could be due to difference in methods used to assay hepcidin. Hepcidin measurement is based on either mass-spectrometry or antibody-mediated hepcidin detection. Competitive radioimmunoassays and ELISA tests have been developed for antibody-based methods. It is reported that hepcidin level measured by various methods vary considerably[21] and that immunochemical assays lack the sensitivity to distinguish hepcidin-25 from hepcidin-20 and -22.[22] Furthermore, difference in sample size and inherent difference in study population might have contributed to the difference. Furthermore, hepcidin correlated positively with ferritin and %TSAT and negatively with EPO level in our study population. Positive correlation of hepcidin and ferritin has been shown by previous other studies.[7,14,18,19] The positive correlation between hepcidin and TSAT and negative correlation between hepcidin and EPO in our study is in accordance with the study by Mercadal et al.[14]
In our study, only eGFR was found to be a significant predictor of Hb level. Hb level decreased as CKD progressed through stage 3–5. No association was found between Hb and hepcidin as well as Hb and ferritin. Two earlier studies[18,20] also did not found any association between hepcidin and Hb. However, in a recent study by Uehata et al.,[7] there was a negative correlation between Hb and hepcidin and log-normalized hepcidin was a significant predictor of Hb concentration in the study population. These conflicting results may be attributed to difference in iron status of the population studied, difference in inflammatory state or sample size.
On the basis of serum ferritin and %TSAT patients in the study population were divided into different groups, i.e., normal iron status, functional iron deficiency, and absolute iron deficiency. Cutoff values for %TSAT and ferritin were based on a recent study conducted by Mercadal et al.[14] Two indices were used to divide study population into various iron status categories as described earlier. We found that median hepcidin levels were significantly higher in patients with normal iron status, followed by relative iron deficiency as compared to absolute iron deficiency. This finding was in accordance with study conducted by Mercadal et al.[14] showing that hepcidin was raised in patients with anemia in CKD with normal iron status and functional iron deficiency but was downregulated in patients with absolute iron deficiency. Furthermore, in our study, relation between hepcidin and Hb varied according to iron status. There was a significant negative correlation between hepcidin and Hb in patients with normal iron status and near positive correlation in patients with absolute iron deficiency. Only 4% patients in the study population had absolute iron deficiency, and this may be responsible for lack of absolute significance in this category. Above association almost persisted when cutoff value for serum ferritin was raised to 100 ng/ml except that there was no correlation between hepcidin and Hb in absolute iron deficiency group. Our findings are quite similar to the study conducted by Mercadal et al.[14] who showed that in absolute iron deficiency group Hb decreased with hepcidin. On the contrary, in patients with functional iron deficiency, anemia is related to increased hepcidin level. In this study, using ferritin instead of combined iron marker produced similar results for the relation between hepcidin and Hb. Furthermore, in another study by Ueheta et al.,[7] hepcidin was negatively correlated with Hb in high ferritin (≥91 ng/ml) group indicating that ferritin modifies association between hepcidin and Hb. Reason for negative association between hepcidin and Hb in patients with sufficient iron store is multifactorial. Raised hepcidin level inhibits iron absorption from gut and iron recycling from macrophages leading to limited iron availability for erythropoiesis. As erythropoiesis is suppressed in CKD due to decreased EPO level, hepcidin production is also not suppressed. Furthermore, as CKD is currently is being considered as an inflammatory state per se, leads to upregulation of hepcidin. However, no apparent correlation was found in our study between inflammatory marker hsCRP and hepcidin possibly due to exclusion of patients with active infection in this study. Furthermore, in the study by Ueheta et al.,[7] no apparent correlation was observed between hepcidin and inflammatory markers.
Although we have estimated cutoff value for hepcidin in our study population to differentiate absolute iron deficiency from other categories, it may have limitation due to diurnal variation and high intrapatient coefficient of variation for hepcidin.[23]
However, our study has potential limitations due to its cross-sectional design, small sample size, and hepcidin measurement method.
Conclusion
Our study highlights that median hepcidin value is elevated in nondialysis CKD patients as compared to healthy adults possibly due to increased inflammation and decreased clearance of hepcidin. Furthermore, iron status modifies serum hepcidin level and its association with Hb. Increased hepcidin level leads to iron-restricted erythropoiesis and recombinant human EPO (rhEPO) resistance by inhibiting iron absorption from gut and iron recycling from macrophages. Hence, elevated hepcidin can predict need for parenteral iron to overcome hepcidin-mediated iron-restricted erythropoiesis and need for relatively higher rhEPO doses to suppress hepcidin. However, deciding cutoff value for hepcidin from single estimation to differentiate between absolute iron deficiency and functional iron deficiency is a major issue due to diurnal variation and high intrapatient coefficient of variation for hepcidin. As hepcidin is also a potential target for future therapy in management of anemia of CKD unresponsive or hyporesponsive to ESAs further longitudinal studies involving a large number of CKD patients are needed to solve these issues.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: Global dimension and perspectives. Lancet. 2013;382:260–72. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 2.Aggawal HK, Jain D, Verma K. Efficacy and safety of continuous erythropoietin receptor activator (CERA) Int J Pharm Pharm Sci. 2011;3:292–6. [Google Scholar]
- 3.Bargman JM, Skorecki K. Chronic kidney disease. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, editors. Harrison's Principles of Internal Medicine. 18th ed. New York: The McGraw Hill Companies, Inc; 2012. [Google Scholar]
- 4.De Francisco AL, Stenvinkel P, Vaulont S. Inflammation and its impact on anaemia in chronic kidney disease: From haemoglobin variability to hyporesponsiveness. NDT Plus. 2009;2(Suppl 1):i18–26. doi: 10.1093/ndtplus/sfn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson D. Clinical factors influencing sensitivity and response to epoetin. Nephrol Dial Transplant. 2002;17(Suppl 1):53–9. doi: 10.1093/ndt/17.suppl_1.53. [DOI] [PubMed] [Google Scholar]
- 6.Young B, Zaritsky J. Hepcidin for clinicians. Clin J Am Soc Nephrol. 2009;4:1384–7. doi: 10.2215/CJN.02190309. [DOI] [PubMed] [Google Scholar]
- 7.Uehata T, Tomosugi N, Shoji T, Sakaguchi Y, Suzuki A, Kaneko T, et al. Serum hepcidin-25 levels and anemia in non-dialysis chronic kidney disease patients: A cross-sectional study. Nephrol Dial Transplant. 2012;27:1076–83. doi: 10.1093/ndt/gfr431. [DOI] [PubMed] [Google Scholar]
- 8.Kemna EH, Tjalsma H, Willems HL, Swinkels DW. Hepcidin: From discovery to differential diagnosis. Haematologica. 2008;93:90–7. doi: 10.3324/haematol.11705. [DOI] [PubMed] [Google Scholar]
- 9.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–3. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 10.Ganz T. Molecular control of iron transport. J Am Soc Nephrol. 2007;18:394–400. doi: 10.1681/ASN.2006070802. [DOI] [PubMed] [Google Scholar]
- 11.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–6. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–9. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 13.Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108:3730–5. doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mercadal L, Metzger M, Haymann JP, Thervet E, Boffa JJ, Flamant M, et al. The relation of hepcidin to iron disorders, inflammation and hemoglobin in chronic kidney disease. PLoS One. 2014;9:e99781. doi: 10.1371/journal.pone.0099781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 16.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279–335. [Google Scholar]
- 17.KDOQI; National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis. 2006;47(5 Suppl 3):S11–145. doi: 10.1053/j.ajkd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Peters HP, Laarakkers CM, Swinkels DW, Wetzels JF. Serum hepcidin-25 levels in patients with chronic kidney disease are independent of glomerular filtration rate. Nephrol Dial Transplant. 2010;25:848–53. doi: 10.1093/ndt/gfp546. [DOI] [PubMed] [Google Scholar]
- 19.Ashby DR, Gale DP, Busbridge M, Murphy KG, Duncan ND, Cairns TD, et al. Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease. Kidney Int. 2009;75:976–81. doi: 10.1038/ki.2009.21. [DOI] [PubMed] [Google Scholar]
- 20.Zaritsky J, Young B, Wang HJ, Westerman M, Olbina G, Nemeth E, et al. Hepcidin – A potential novel biomarker for iron status in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1051–6. doi: 10.2215/CJN.05931108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macdougall IC, Malyszko J, Hider RC, Bansal SS. Current status of the measurement of blood hepcidin levels in chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:1681–9. doi: 10.2215/CJN.05990809. [DOI] [PubMed] [Google Scholar]
- 22.Kroot JJ, Kemna EH, Bansal SS, Busbridge M, Campostrini N, Girelli D, et al. Results of the first international round robin for the quantification of urinary and plasma hepcidin assays: Need for standardization. Haematologica. 2009;94:1748–52. doi: 10.3324/haematol.2009.010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ford BA, Eby CS, Scott MG, Coyne DW. Intra-individual variability in serum hepcidin precludes its use as a marker of iron status in hemodialysis patients. Kidney Int. 2010;78:769–73. doi: 10.1038/ki.2010.254. [DOI] [PubMed] [Google Scholar]


