Abstract
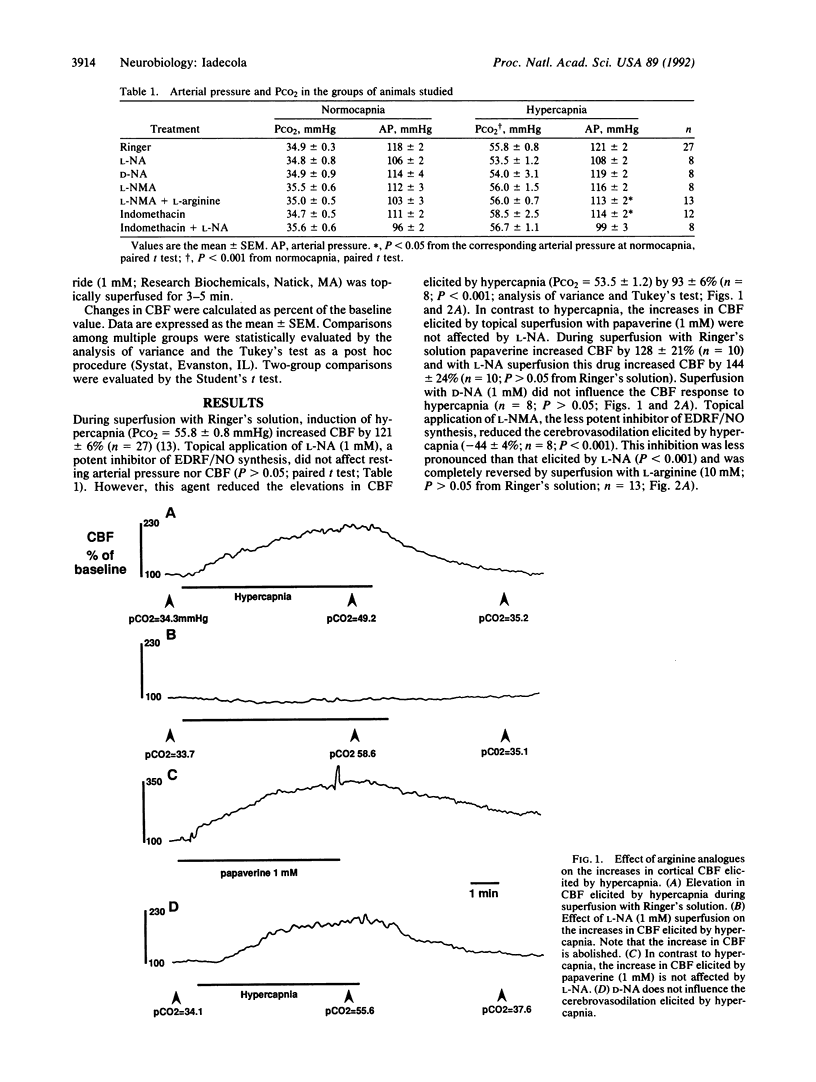
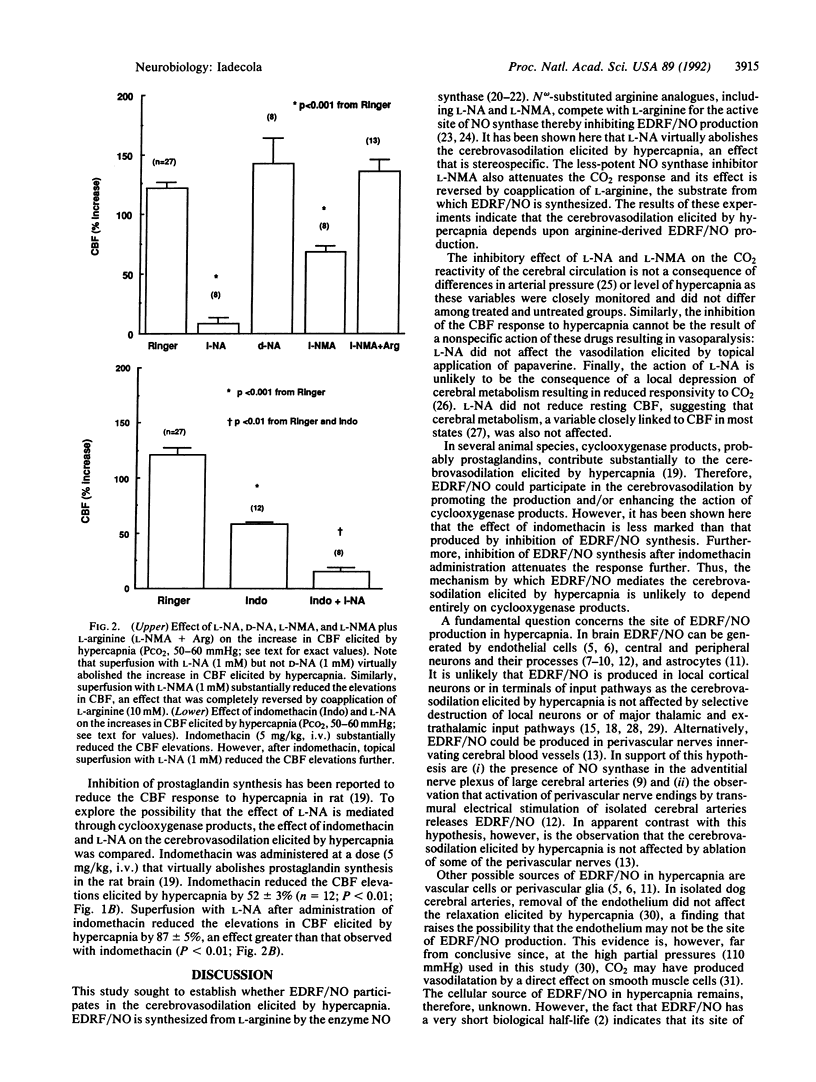
The endothelium-derived relaxing factor (EDRF), probably nitric oxide (NO) or a closely related compound (EDRF/NO), is a potent vasodilator that appears to regulate vascular tone in several vascular beds. I have investigated whether EDRF/NO is also involved in the regulation of the cerebral circulation--in particular, whether EDRF/NO participates in the increases in cerebral blood flow elicited by hypercapnia. Rats were anesthetized with halothane, 1-2% (vol/vol), paralyzed, and artificially ventilated. Arterial pressure was monitored and blood gases were controlled. Cerebral blood flow was continuously monitored through a cranial window over the sensory cortex by a laser-Doppler probe. The window was superfused with Ringer's solution (pH 7.3-7.4 at 37 degrees C). During superfusion with Ringer's solution, hypercapnia (PCO2 = 55.8 +/- 0.8 mmHg) increased cerebral blood flow by 121 +/- 6% (n = 27; P less than 0.001; analysis of variance). Topical superfusion with the NO synthase inhibitors N omega-nitro-L-arginine (1 mM) attenuated the cerebrovasodilation by 93 +/- 6% (n = 8). In contrast, the vasodilation elicited by topical papaverine (1 mM) was not affected by N omega-nitro-L-arginine (n = 10). Application of N omega-nitro-D-arginine (1 mM) did not affect the cerebrovasodilation elicited by hypercapnia (P greater than 0.05; n = 8). N omega-Methyl-L-arginine (1 mM) attenuated the cerebrovasodilation elicited by hypercapnia by 44 +/- 4% (n = 8; P less than 0.001), an effect completely reversed by coapplication of L-arginine (10 mM; P greater than 0.05; n = 13). These findings indicate that the powerful effects of CO2 on the cerebral circulation are mediated by arginine-derived EDRF/NO. EDRF/NO is an important molecular signal whose actions may also include the regulation cerebral circulation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Dwyer M. A., Bredt D. S., Snyder S. H. Nitric oxide synthase: irreversible inhibition by L-NG-nitroarginine in brain in vitro and in vivo. Biochem Biophys Res Commun. 1991 May 15;176(3):1136–1141. doi: 10.1016/0006-291x(91)90403-t. [DOI] [PubMed] [Google Scholar]
- Fujishima M., Scheinberg P., Busto R., Reinmuth O. M. The relation between cerebral oxygen consumption and cerebral vascular reactivity to carbon dioxide. Stroke. 1971 May-Jun;2(3):251–257. doi: 10.1161/01.str.2.3.251. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Charles S. L., Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988 Nov 24;336(6197):385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991 Feb;14(2):60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Harik S. I., Prado R., Busto R., Ginsberg M. D. Increased cerebral blood flow during hypercapnia is not affected by lesion of the nucleus locus ceruleus. Stroke. 1986 Nov-Dec;17(6):1235–1238. doi: 10.1161/01.str.17.6.1235. [DOI] [PubMed] [Google Scholar]
- Heistad D. D., Marcus M. L., Abboud F. M. Role of large arteries in regulation of cerebral blood flow in dogs. J Clin Invest. 1978 Oct;62(4):761–768. doi: 10.1172/JCI109187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C., Arneric S. P., Baker H. D., Tucker L. W., Reis D. J. Role of local neurons in cerebrocortical vasodilation elicited from cerebellum. Am J Physiol. 1987 Jun;252(6 Pt 2):R1082–R1091. doi: 10.1152/ajpregu.1987.252.6.R1082. [DOI] [PubMed] [Google Scholar]
- Iadecola C., Kraig R. P. Focal elevations in neocortical interstitial K+ produced by stimulation of the fastigial nucleus in rat. Brain Res. 1991 Nov 1;563(1-2):273–277. doi: 10.1016/0006-8993(91)91544-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C., Mraovitch S., Meeley M. P., Reis D. J. Lesions of the basal forebrain in rat selectively impair the cortical vasodilation elicited from cerebellar fastigial nucleus. Brain Res. 1983 Nov 21;279(1-2):41–52. doi: 10.1016/0006-8993(83)90161-0. [DOI] [PubMed] [Google Scholar]
- Iadecola C., Reis D. J. Continuous monitoring of cerebrocortical blood flow during stimulation of the cerebellar fastigial nucleus: a study by laser-Doppler flowmetry. J Cereb Blood Flow Metab. 1990 Sep;10(5):608–617. doi: 10.1038/jcbfm.1990.112. [DOI] [PubMed] [Google Scholar]
- Ishitsuka T., Iadecola C., Underwood M. D., Reis D. J. Lesions of nucleus tractus solitarii globally impair cerebrovascular autoregulation. Am J Physiol. 1986 Aug;251(2 Pt 2):H269–H281. doi: 10.1152/ajpheart.1986.251.2.H269. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontos H. A., Wei E. P., Marshall J. J. In vivo bioassay of endothelium-derived relaxing factor. Am J Physiol. 1988 Nov;255(5 Pt 2):H1259–H1262. doi: 10.1152/ajpheart.1988.255.5.H1259. [DOI] [PubMed] [Google Scholar]
- Lifson J. D., Rubinstein E. H., Scremin O. U., Sonnenschein R. R. Cerebrovascular reactivity to CO2: modulation by arterial pressure. Experientia. 1985 Apr 15;41(4):467–468. doi: 10.1007/BF01966150. [DOI] [PubMed] [Google Scholar]
- Lou H. C., Edvinsson L., MacKenzie E. T. The concept of coupling blood flow to brain function: revision required? Ann Neurol. 1987 Sep;22(3):289–297. doi: 10.1002/ana.410220302. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Murphy S., Minor R. L., Jr, Welk G., Harrison D. G. Evidence for an astrocyte-derived vasorelaxing factor with properties similar to nitric oxide. J Neurochem. 1990 Jul;55(1):349–351. doi: 10.1111/j.1471-4159.1990.tb08860.x. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Persson M. G., Wiklund N. P., Gustafsson L. E. Nitric oxide requirement for vasomotor nerve-induced vasodilatation and modulation of resting blood flow in muscle microcirculation. Acta Physiol Scand. 1991 Jan;141(1):49–56. doi: 10.1111/j.1748-1716.1991.tb09043.x. [DOI] [PubMed] [Google Scholar]
- Pickard J. D. Role of prostaglandins and arachidonic acid derivatives in the coupling of cerebral blood flow to cerebral metabolism. J Cereb Blood Flow Metab. 1981;1(4):361–384. doi: 10.1038/jcbfm.1981.41. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum W. I. Endothelial dependent relaxation demonstrated in vivo in cerebral arterioles. Stroke. 1986 May-Jun;17(3):494–497. doi: 10.1161/01.str.17.3.494. [DOI] [PubMed] [Google Scholar]
- Sakuma I., Stuehr D. J., Gross S. S., Nathan C., Levi R. Identification of arginine as a precursor of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8664–8667. doi: 10.1073/pnas.85.22.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. H., Bredt D. S. Nitric oxide as a neuronal messenger. Trends Pharmacol Sci. 1991 Apr;12(4):125–128. doi: 10.1016/0165-6147(91)90526-x. [DOI] [PubMed] [Google Scholar]
- Toda N., Hatano Y., Mori K. Mechanisms underlying response to hypercapnia and bicarbonate of isolated dog cerebral arteries. Am J Physiol. 1989 Jul;257(1 Pt 2):H141–H146. doi: 10.1152/ajpheart.1989.257.1.H141. [DOI] [PubMed] [Google Scholar]
- Toda N., Okamura T. Mechanism underlying the response to vasodilator nerve stimulation in isolated dog and monkey cerebral arteries. Am J Physiol. 1990 Nov;259(5 Pt 2):H1511–H1517. doi: 10.1152/ajpheart.1990.259.5.H1511. [DOI] [PubMed] [Google Scholar]



