Abstract
The effect of endogenous glucocorticoids on the expression of the cyclooxygenase enzyme was studied by contrasting cyclooxygenase expression and prostanoid synthesis in adrenalectomized and sham-adrenalectomized mice with or without the concurrent administration of endotoxin. Peritoneal macrophages obtained from adrenalectomized mice showed a 2- to 3-fold induction in cyclooxygenase synthesis and activity when compared to sham controls. Intravenous injection of a sublethal dose of endotoxin (5 micrograms/kg) further stimulated cyclooxygenase synthesis, resulting in a 4-fold increase in prostaglandin production. Similar cyclooxygenase induction can be achieved in macrophages obtained from normal mice but only after high doses of endotoxin (2.5 mg/kg) that are 100% lethal to adrenalectomized mice. Restoration of glucocorticoids in adrenalectomized animals with dexamethasone completely inhibited the elevated cyclooxygenase and protected these animals from endotoxin-induced death. In contrast, no signs of cyclooxygenase induction were observed in the kidneys of the adrenalectomized mice, even when treated with endotoxin. Dexamethasone did not affect the constitutive cyclooxygenase activity and prostaglandin production present in normal and adrenalectomized kidneys. These data indicate the existence of a constitutive cyclooxygenase that is normally present in most cells and tissues and is unaffected by steroids and of an inducible cyclooxygenase that is expressed only in the context of inflammation by proinflammatory cells, like macrophages, and that is under glucocorticoid regulation. Under normal physiological conditions glucocorticoids maintain tonic inhibition of inducible cyclooxygenase expression. Depletion of glucocorticoids or the presence of an inflammatory stimulus such as endotoxin causes rapid induction of this enzyme, resulting in an exacerbated inflammatory response that is often lethal.
Full text
PDF


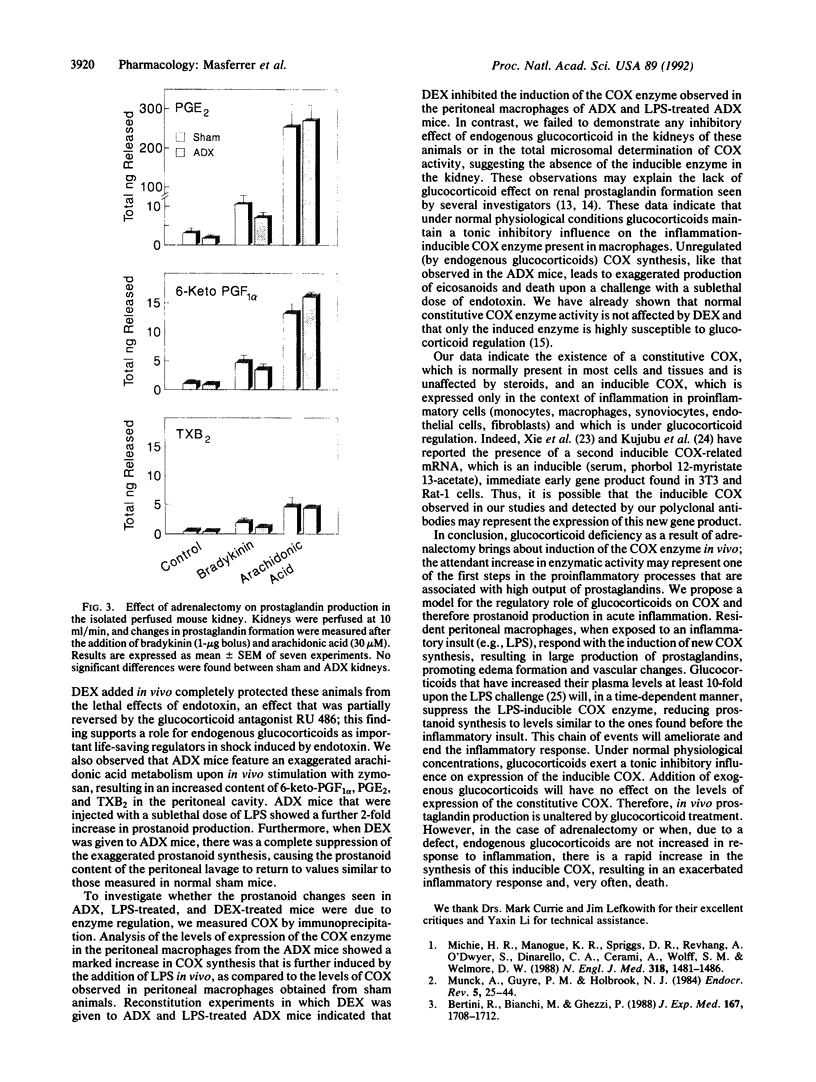

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Makheja A. N., Pash J., Verma M. Corticosteroids suppress cyclooxygenase messenger RNA levels and prostanoid synthesis in cultured vascular cells. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1159–1163. doi: 10.1016/s0006-291x(88)80995-1. [DOI] [PubMed] [Google Scholar]
- Ball H. A., Cook J. A., Wise W. C., Halushka P. V. Role of thromboxane, prostaglandins and leukotrienes in endotoxic and septic shock. Intensive Care Med. 1986;12(3):116–126. doi: 10.1007/BF00254925. [DOI] [PubMed] [Google Scholar]
- Bertini R., Bianchi M., Ghezzi P. Adrenalectomy sensitizes mice to the lethal effects of interleukin 1 and tumor necrosis factor. J Exp Med. 1988 May 1;167(5):1708–1712. doi: 10.1084/jem.167.5.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Di Rosa M., Flower R. J., Hirata F., Parente L., Russo-Marie F. Anti-phospholipase proteins. Prostaglandins. 1984 Oct;28(4):441–442. doi: 10.1016/0090-6980(84)90232-6. [DOI] [PubMed] [Google Scholar]
- Feuerstein G., Hallenbeck J. M., Vanatta B., Rabinovici R., Perera P. Y., Vogel S. N. Effect of gram-negative endotoxin on levels of serum corticosterone, TNF alpha, circulating blood cells, and the survival of rats. Circ Shock. 1990 Mar;30(3):265–278. [PubMed] [Google Scholar]
- Flower R. J., Parente L., Persico P., Salmon J. A. A comparison of the acute inflammatory response in adrenalectomised and sham-operated rats. Br J Pharmacol. 1986 Jan;87(1):57–62. doi: 10.1111/j.1476-5381.1986.tb10156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J. Y., Masferrer J. L., Seibert K., Raz A., Needleman P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem. 1990 Oct 5;265(28):16737–16740. [PubMed] [Google Scholar]
- GELLER P., MERRILL E. R., JAWETZ E. Effects of cortisone and antibiotics on lethal action of endotoxins in mice. Proc Soc Exp Biol Med. 1954 Aug-Sep;86(4):716–719. doi: 10.3181/00379727-86-21211. [DOI] [PubMed] [Google Scholar]
- Goppelt-Struebe M., Wolter D., Resch K. Glucocorticoids inhibit prostaglandin synthesis not only at the level of phospholipase A2 but also at the level of cyclo-oxygenase/PGE isomerase. Br J Pharmacol. 1989 Dec;98(4):1287–1295. doi: 10.1111/j.1476-5381.1989.tb12676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Panczenko B., Korbut R., Grodzinska L., Ocetkiewicz A. Corticosteroids inhibit prostaglandin release from perfused mesenteric blood vessels of rabbit and from perfused lungs of sensitized guinea pig. Prostaglandins. 1975 Aug;10(2):343–355. doi: 10.1016/0090-6980(75)90053-2. [DOI] [PubMed] [Google Scholar]
- Kujubu D. A., Fletcher B. S., Varnum B. C., Lim R. W., Herschman H. R. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991 Jul 15;266(20):12866–12872. [PubMed] [Google Scholar]
- Laue L., Kawai S., Brandon D. D., Brightwell D., Barnes K., Knazek R. A., Loriaux D. L., Chrousos G. P. Receptor-mediated effects of glucocorticoids on inflammation: enhancement of the inflammatory response with a glucocorticoid antagonist. J Steroid Biochem. 1988 Jun;29(6):591–598. doi: 10.1016/0022-4731(88)90156-2. [DOI] [PubMed] [Google Scholar]
- Masferrer J. L., Zweifel B. S., Seibert K., Needleman P. Selective regulation of cellular cyclooxygenase by dexamethasone and endotoxin in mice. J Clin Invest. 1990 Oct;86(4):1375–1379. doi: 10.1172/JCI114850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Munck A., Guyre P. M., Holbrook N. J. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984 Winter;5(1):25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Nasjletti A., Erman A., Cagen L. M., Baer P. G. Plasma concentrations, renal excretion, and tissue release of prostaglandins in the rat with dexamethasone-induced hypertension. Endocrinology. 1984 Mar;114(3):1033–1040. doi: 10.1210/endo-114-3-1033. [DOI] [PubMed] [Google Scholar]
- Náray-Fejes-Tóth A., Fejes-Tóth G., Fischer C., Frölich J. C. Effect of dexamethasone on in vivo prostanoid production in the rabbit. J Clin Invest. 1984 Jul;74(1):120–123. doi: 10.1172/JCI111391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A., Wyche A., Needleman P. Temporal and pharmacological division of fibroblast cyclooxygenase expression into transcriptional and translational phases. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1657–1661. doi: 10.1073/pnas.86.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo-Marie F. Glucocorticoid control of eicosanoid synthesis. Semin Nephrol. 1990 Jul;10(4):421–429. [PubMed] [Google Scholar]
- Sebaldt R. J., Sheller J. R., Oates J. A., Roberts L. J., 2nd, FitzGerald G. A. Inhibition of eicosanoid biosynthesis by glucocorticoids in humans. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6974–6978. doi: 10.1073/pnas.87.18.6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp C. S., Wyche A., Unanue E. R., Needleman P. The functional significance of the regulation of macrophage Ia expression by endogenous arachidonate metabolites in vitro. J Immunol. 1986 Dec 15;137(12):3915–3920. [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Xie W. L., Chipman J. G., Robertson D. L., Erikson R. L., Simmons D. L. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]





