Abstract
Background:
We aimed to reveal the role of CD11b and hypoxia-inducible factors-1alpha (HIF-1α) expressions on monocytes and alveolar macrophages of lung tissue, and the levels of serum surfactant protein-D (SP-D) in severe malaria-associated acute lung injury (ALI).
Methods:
The C57BL/6 mice were divided into control group, renal malaria group (inoculated with 106 Plasmodium berghei ANKA), and cerebral malaria group (inoculated with 107 P. berghei ANKA). The expressions of CD11b and HIF-1α in lung tissue were observed by immunohistochemistry, and serum SP-D levels were measured by ELISA. This study was conducted from June 2014 to February 2015 in the Laboratory of Parasitology, Faculty of Medicine, Universitas Brawijaya, Malang.
Results:
The CD11b expression on pulmonary tissue of renal and cerebral malaria mice were significantly higher than control mice (P=0.002; P=0.002), as well as the HIF-1α expression on pulmonary tissue (P=0.002; P=0.002). The level of serum SP-D in renal malaria and cerebral malaria mice were significantly higher than control mice (P=0.002; P=0.002). We found a strong correlation between the expression of CD11b and HIF-1α in lung tissue (r=0.937, P=0.000), as well as between CD11b expression and serum SP-D levels (r=0.907, P=0.000) and between HIF-1α expression and serum SP-D levels (r=0.913, P=0.000).
Conclusion:
Severe malaria-associated ALI increased the expression of CD11b and HIF-1α in the lung tissue and increased serum SP-D levels of C57BL/6 mice significantly.
Keywords: Acute lung injury, Severe malaria, CD11b, HIF-1alpha, SP-D
Introduction
Severe malaria is characterized by dysfunction of vital organs, which define from clinical, or laboratory finding, and often accompanied with high degree of asexual stage of malaria parasites in the blood (1). In humans, Plasmodium falciparum is a common cause of severe malaria disease, although P. vivax and P. knowlesi can also cause severe infection (2, 3). The clinical features of severe malaria are varies and may manifest singly or simultaneously related to the target organs, such as cerebral malaria, malaria with acute renal failure (ARF) or renal malaria, and malaria with acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) (4–6).
Acute lung injury (ALI) is a common complication in severe malaria and often progress to become ARDS. Acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) can also manifest at any time regardless of the status of anti-malarial treatment that is being used. Therefore, ALI/ARDS can increase morbidity and mortality in patients with malaria (5–7). However, the pathogenesis of malaria-associated ALI/ARDS is still not clear, especially in the aspect of the host immunological response against parasite infection.
Monocytes are one of the first line defenses that play an important role to eliminate malaria parasites (8). Monocytes also involved in the initiation phase of resolution of lung tissue after the extent of ALI (9). During the occurrence of malaria with ALI/ARDS, monocytes are recruited into pulmonary tissue that mediated by the CD11b receptor (10, 11). Previous study of intravital malaria infected-mice showed that merozoites, which release directly from hepatocytes passes sinusoid barrier and were stuck in the lung microvasculature, instead of go into the blood circulation. The merozoites disintegrated and separated into pulmonary blood capillaries, and subsequently infected the erythrocytes (12).
In response to inflamed and hypoxic lung tissue, the expression and activation of hypoxia-inducible factors-1alpha (HIF-1α) on monocytes and macrophages can be increased (13). Activation of HIF-1α will increase macrophages ability in phagocytosis and production of pro-inflammatory cytokines (e.g., Interleukin-1, Tumor Necrosis Factor-α) and adhesion molecules (14). Hypoxia-inducible factors-1α can also increase the levels of vascular endothelial growth factor (VEGF), which is associated with increased capillary permeability (15, 16). Vascular endothelial growth factor (VEGF) can trigger pulmonary endothelial barrier disruption and contribute to the onset of ALI in mice models of severe malaria (17).
The expression of CD11b and CD11c on alveolar macrophages was regulated by surfactant protein D (SP-D) to prevent it from receptor degradation (18). Surfactant protein D (SP-D) is a pulmonary collecting, which acts as humoral immunity and able to regulate the inflammatory response of macrophages (19). The expression of SP-D also altered during acute and persistent hypoxia in ALI (20). Thus, SP-D may be correlated with the expression of CD11b and HIF-1α in lung macrophages of mice with severe malaria with ALI. Given the lack of standard criteria for studying the pathogenesis of severe malaria-associated ALI, the biomarker measurements can support the laboratory evidences of malaria with ALI. Related to this, serum SP-D may be used as a potent biomarker because its levels increase during ALI in humans (21, 22) and experimental animals (23, 24).
The objective of this study was to determine the immune response that occurs during severe malaria-associated ALI, especially on monocytes/alveolar macrophages, by observing the expression of CD11b and HIF-1α in lung tissue of C57BL/6 mice, as well as serum SP-D levels of C57BL/6 mice infected by P. berghei ANKA.
Materials and Methods
Experimental animals
The design of study is a randomized posttest only control group. Eighteen female C57BL/6 mice, 6–8 weeks old, were bred and maintained in a pathogen-free facility at the Eijkman Institute for Molecular Biology, Jakarta. The mice were then transferred to the Laboratory of Parasitology, Faculty of Medicine, Universitas Brawijaya, Malang, and adapted for seven days prior to the experiment. The mice were housed six animals per cage on wood shaving bedding, and were maintained under controlled temperature and humidity, with 12 h light/dark cycles. The mice were fed ad libitum and had unlimited access to water. All protocols were approved by the Health Research Ethics Committee of the Faculty of Medicine, Universitas Brawijaya (410/EC/IEC/07/2014).
CD11b and HIF-1α expression in mice lung tissue were observed by immunohistochemistry, while the serum SP-D levels were measured by enzyme-linked immunosorbent assay (ELISA). This study also observed the degree of parasitemia, serum creatinine and blood urea nitrogen (BUN) levels, kidney tissue histopathology, signs/symptom of experimental cerebral malaria (ECM), brain tissue histopathology, and lung tissue histopathology, to confirm the presence of renal and cerebral malaria-associated ALI in C57BL/6 mice.
Parasites, infection, and disease assessment
Plasmodium berghei ANKA obtained from the Eijkman Institute for Molecular Biology, Jakarta. Six C57BL/6 mice were inoculated with 106 P. berghei ANKA-infected erythrocytes intraperitoneally (i.p.) to induce malaria with acute renal failure (ARF) or renal malaria according to previous studies (25, 26), while the other six mice were inoculated with 107 P. berghei ANKA-infected erythrocytes i.p. to induce the ECM as a previous study (17). The last six mice were not inoculated with P. berghei ANKA-infected erythrocytes and were used as a control group. The parasitemia in renal and cerebral malaria groups was examined by Giemsa staining, observed under a microscope with a total magnification of 1000× (oil immersed), and was calculated based on the percentage of infected erythrocytes in 1000 erythrocytes. It was observed for 7 days for cerebral malaria group and 14 days for renal malaria group. Renal malaria was confirmed by measuring the serum creatinine and BUN, observed the histopathology of kidney tissue, and no signs/symptoms of ECM. Cerebral malaria was confirmed based on the histopathology of brain tissue and signs/symptoms of ECM, such as ruffled fur, hunching, wobbly gait, limb paralysis, convulsions, and coma, according to previous study (27). Acute lung injury was confirmed by the histopathology of lung tissue.
Histopathology
C57BL/6 mice with cerebral malaria and control mice were sacrificed on day 7, while mice with renal malaria were sacrificed on day 14 according to the standard of ethics. The brain, kidney, and lung were harvested immediately after death. These organs were fixed in formaldehyde solution (10%) for 24–48 h, dehydrated in an ethanol solution, embedded in paraffin blocks, sectioned with 4 μm thickness, stained with Hematoxylin-eosin (HE) and immunohistochemistry. The histopathology of brain, kidney, and lung tissue were observed using a light microscope (Olympus ® CX22) at a total magnification of 400× across 20 microscopic fields. The images were captured with MicroStepper® and OptiLab® camera. Renal malaria confirmed by the presence of acute tubular necrosis (ATN), which is marked by morphological changes in tubular epithelial cells (swelling, karyolysis, necrosis), hemosiderin deposits, infiltration of mononuclear (MN) cells or polymorphonuclear (PMN) cells, and peritubular hemorrhage (25, 26, 28). Cerebral malaria is indicated by the presence of hemorrhage, cortex edema, vascular congestion, MN or PMN infiltrations, and leukocytes adhesion with erythrocytes and endothelial brain tissue (17, 29). The extent of ALI was measured by semi-quantitative scoring system (modified from (30)), by assessing the presence of neutrophils in the alveolar and interstitial space, hyaline membranes, thickening of the alveolar septal, and exudates in alveolar space or lung parenchyma, with a score of 0–2 for each parameter per field in a total magnification of 400×.
Immunohistochemistry
CD11b and HIF-1α expression was observed by immunohistochemistry using anti-CD11b (101 202; Biolegend; San Diego; California; USA) and anti-HIF-1α (sc-53546; Santa Cruz Biotechnology; Santa Cruz; California; USA). CD11b and HIF-1α expressions were measured by counting the number of positive monocytes/macrophages in 20 microscopic fields with 1000× magnification. Observations made by researchers and confirmed by at least two expert analysts with double-blind examinations.
Determination of protein content
The blood was collected from cardiac puncture, and then injected to BD Vacutainer ® SST ™ plus Blood Collection Tubes (Becton, Dickinson and Company; Plymouth; UK) to obtain the serum. Serum SP-D levels was measured using a commercial ELISA kit (EEL-M0993; Elabscience; Wuhan; Hubei; China) according to manufacture protocols.
Statistical Analysis
SPSS 20.0 software (Chicago, IL, USA) was used for statistical analysis of CD11b and HIF-1α expressions and serum SP-D levels. Shapiro-Wilk test is used to confirm the distribution of data. Kruskal-Wallis test of Post Hoc Mann-Whitney U was used to evaluate the differences of CD11b and HIF-1α expressions and serum SP-D levels among groups. Spearman’s rank correlation test was used to determine the correlation between two parameters. Data that met P<0.05 was considered significant.
Results
The degree of parasitemia in renal malaria and cerebral malaria groups
The degree of parasitemia of cerebral and renal malaria groups was measured after inoculation phase until 14 days. The degree of parasitemia and the morphology of the parasites on thin blood smear are presented in Fig. 1 & 2.
Fig. 1:
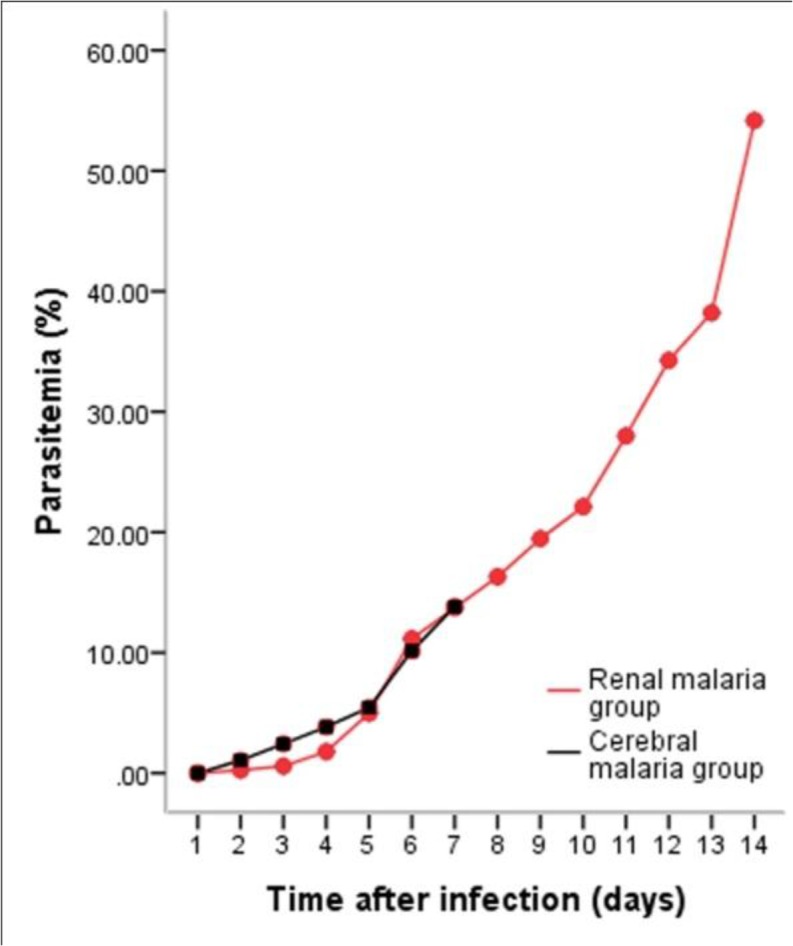
The degree of parasitemia. The mean percentage of parasitemia in renal and cerebral malaria groups. Each group consisted of six mice
Fig. 2:
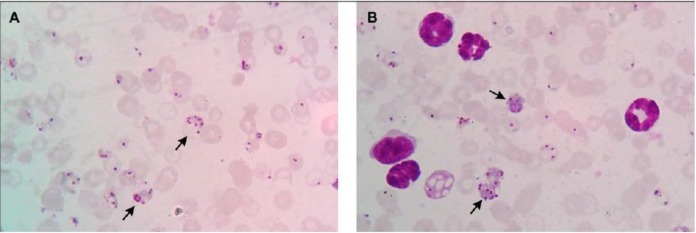
Thin blood smear of cerebral malaria and renal malaria groups. (A) Thin blood smear of cerebral malaria mice on 7 dpi, and (B) renal malaria mice on 14 dpi. Images were observed under 1000-x magnification with Giemsa staining. Schizonts are indicated by the black arrow
Renal function and kidney tissue histopathology of renal malaria group
The mean levels of serum creatinine in renal malaria group was 0.52±0.17 mg/dL, which was higher than the cerebral malaria group (0.1±0.01 mg/dL) and the control group (0.21±0.04 mg/dL), with (P=0.002; P=0.002). The mean level of BUN serum in renal malaria group was 40.59±25.5 mg/dL, significantly different from the cerebral malaria group (15.72±3.77 mg/dL) and the control group (16.64±0.73 mg/dL) with (P=0.002; P=0.002). The observation of kidney tissue of control group showed normal histology, while renal malaria group showed evidences of ATN, which were characterized by the degeneration of tubular epithelial cells (karyorrhexis, karyolysis, and loss of brush border), debris in the lumen of the tubular, peritubular hemorrhage, and dilation of the tubular lumen. There were sequestration of infected red blood cells (iRBCs) and malaria pigment in glomerular capillaries and peritubular capillaries. Vascular edema is depicted by thickening of the tunica media.
Signs/symptoms and brain tissue histopathology of cerebral malaria group
All mice with cerebral malaria showed the signs and symptoms of ECM since the 5 days post inoculation (dpi), when the mean percentage of parasitemia reached 4.98±1.30%. Other clinical signs, such as hunching and wobbly gait, generally appear at 6 dpi, while the limb paralysis observed at 7 dpi. Three of the six mice had convulsions and coma at 7 dpi, shortly prior to the surgery. The observation of brain tissue of control group showed normal histology, whereas cerebral malaria group showed evidences of ECM, which were characterized by multifocal hemorrhage, congestive blood vessels, leukocytes infiltration, and cortical edema.
Histopathology of lung tissue of control, renal malaria and cerebral malaria groups
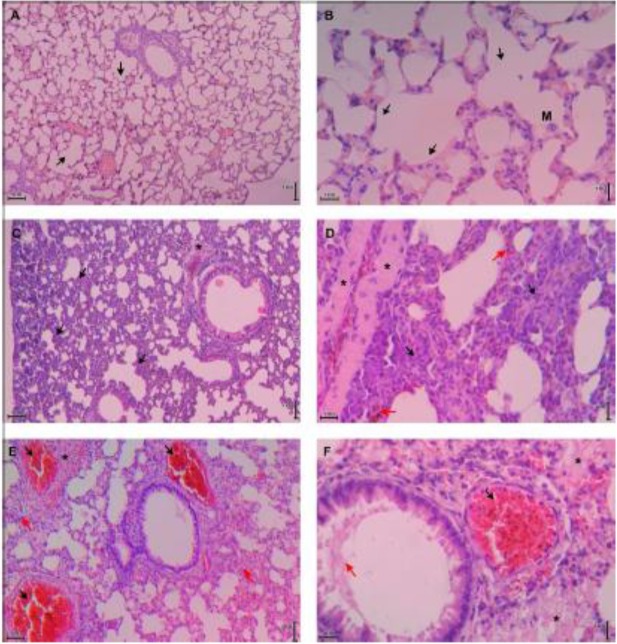
The histopathology of lung tissue was assessed by a scoring system (modified from (30)). The mean score of ALI of renal malaria and cerebral malaria group were 0.66±0.06 and 0.81±0.02. The score of renal malaria group was significantly lower compared to cerebral malaria group (P=0.001). The lung tissue histopathology of control mice showed normal histology (Fig. 3A & 3B). Lung tissue in renal malaria mice showed diffuse inflammatory cells infiltration (Fig. 3C & 3D), whereas lung tissue of mice with cerebral malaria was dominated with exudate and alveolar septal thickening (Fig. 3E & 3F). Thickening of the alveolar septal were found on all lungs of C57BL/6 mice with renal and cerebral malaria, which were rarely observed in previous studies (17, 31).
Fig. 3:
Histopathology of lung tissue of control, renal malaria and cerebral malaria groups. Images (A) and (B) are representatives of control mice lung tissue that show normal histology; no thickening in septal alveolar; black arrow indicated the normal alveoli; M=alveolar macrophage. Images (C) and (D) are representatives of lung tissue of renal malaria mice that show alveolar septal thickening 2–4 times than control due to infiltration of inflammatory cells (black arrow), vascular edema (*), and interstitial microhemorrhage (red arrow). Images (E) and (F) are representatives of lung tissue of cerebral malaria mice that show alveolar septal thickening more than 4 times compare to control (red arrow in E), exudates (*), thrombus with sequestration of iRBCs and hemozoin (black arrow), and hyaline membrane (red arrow in F). A, C, E = total magnification of 100×; B, D, F = total magnification of 400×. All tissues were stained with HE
CD11b expression in the lung tissue of control, renal malaria and cerebral malaria groups
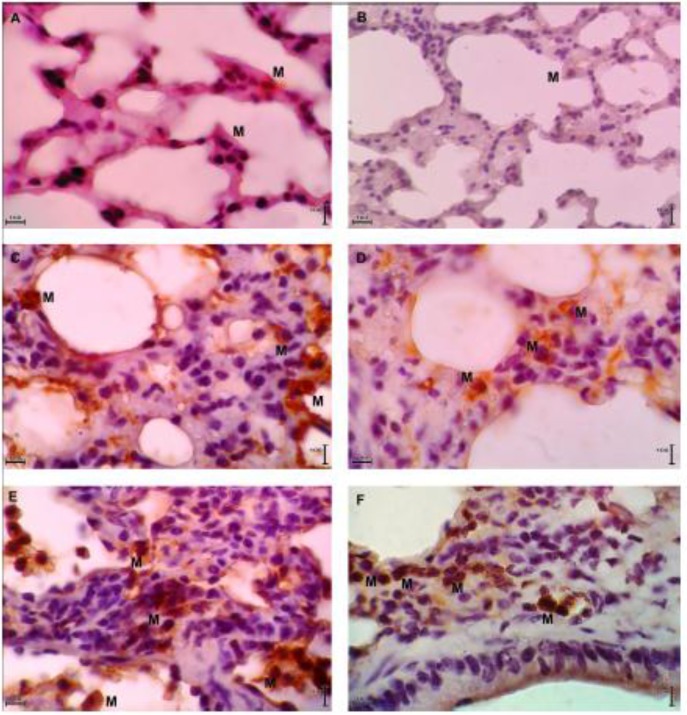
The mean number of CD11b expression in the renal malaria group was 3.32±0.19 monocytes/macrophages per field, and it was 5.68±0.45 monocytes/macrophages per field in cerebral malaria group. Both of malaria mice groups had higher expression of CD11b than control group (0.12±0.03 monocytes/macrophages per field), with P=0.002 and P=0.002. Expressions of CD11b of lung tissue of C57BL/6 mice are presented in Fig. 4 (A, C, E).
Fig. 4:
CD11b and HIF-1α expressions in lung tissue of control, renal malaria and cerebral malaria groups. Images (A), (C), (E) are representatives of CD11b expressions in lung tissue of control mice, renal malaria mice, and cerebral malaria mice, respectively. Images (B), (D), (F) are representatives of HIF-1α expressions in lung tissue of control mice, renal malaria mice, and cerebral malaria mice, respectively. All tissues were stained with immunohistochemical staining (anti-CD11b for A, C, E; anti-HIF-1α for B, D, F) and were observed at total magnification of 1000×. M = monocytes/macrophages.
HIF-1α expression in the lung tissue of control, renal malaria and cerebral malaria groups
The mean number of HIF-1α expression in the renal malaria group was 3.32±0.14 monocytes/macrophages per field, and it was 5.40±0.09 monocytes/macrophages per field in cerebral malaria group. Both of malaria mice groups had significantly higher expressions of HIF-1α than control group (0.13±0.01 monocytes/macrophages per field), with P=0.002 and P=0.002. Expressions of HIF-1α of lung tissue of C57BL/6 mice are presented in Fig. 4 (B, D, F).
Serum SP-D levels of control, renal malaria and cerebral malaria groups
The mean levels of serum SP-D in the group of renal and cerebral malaria groups (23.48±3.57 ng/mL and 57.74±4.33 ng/mL), were significantly higher than control group (1.63±0.31 ng/mL), with P=0.002 and P=0.002.
Correlation among CD11b, HIF-1α, SP-D and ALI score in renal malaria and cerebral malaria groups
Correlation between the expression of CD11b and HIF-1α in lung tissue of renal malaria and cerebral malaria groups showed significant values (r=0.937, P=0.000), as well as correlation between CD11b expression and serum SP-D levels (r=0.907, P=0.000) and correlation between HIF-1α expression and serum SP-D levels (r=0.913, P=0.000). From the results of Spearman’s rank correlation test, the mean levels of CD11b expression in lung tissue and SP-D in the serum were strongly correlated with ALI score (r=0.926, P=0.000; r=0.908, P=0.000). The Spearman’s rank correlation test also showed that the mean of HIF-1α expression in lung tissue was correlated with ALI score (r=0.864, P=0.000).
Discussion
Acute lung injury in human malaria as a result of P. falciparum infection is marked with diffuse alveolar damage (DAD), characterized by inflammatory infiltrates, close contact between iRBCs and alveolar capillaries endothelium, hyaline membranes deposition, thickening of the septa, and swelling of endothelial cells (30, 32). As long as the study of pathological hallmark correlates with malaria-associated ALI in human malaria, there are many of murine models that could provide some evidences to examine malaria-associated ALI, such as VEGF (17) and hemozoin (33). In the lung tissue of renal and cerebral malaria mice, we found alveolar epithelial damages, which were more diffuse in mice with cerebral malaria (P< 0.05). The alveolar epithelial cells of cerebral group were destroyed because of accumulation of inflammatory cells. We also found other aspects that met the criteria of DAD, such as vascular edema, infiltration of MN and PMN in the interstitial and alveolar space, hyaline membrane, hemorrhage (thromboembolism formation), and thickening of the alveolar septal (Fig. 3C–F). The mean of total ALI score in the lung tissue of cerebral malaria group was significantly higher than the group of renal malaria (P< 0.05), which may indicate that cerebral malaria mice had more severe lung injury than renal malaria mice.
Based on the lung histopathology features, lung injuries that occur in both of malaria group were still in exudative phase. It was also supported with no finding of alveolar epithelial cells or type II pneumocytes hyperplasia, and there were no fibrosis formation in interstitial area, which is the hallmark of ALI in the proliferative phase. This condition was similar with previous studies (17, 31, 32). Nonetheless, the thickening of the alveolar septa was observed in all C57BL/6 mice with renal malaria and cerebral malaria.
This feature was not found in most lungs of C57BL/6 mice with malaria, but observed in DBA and Balb/c mice with cerebral malaria or malaria-associated ALI/ARDS (17, 32). In this study, thickening of the alveolar septal in cerebral malaria mice was more clear (4× normal septal), compared to renal malaria mice (2–4× normal septal). Thickening of the alveolar septal is caused by congestion of blood vessels and leukocytes infiltration, especially monocytes/macrophages and neutrophils. Monocytes recruitment into several organs is mediated by CD11b/CD18 and endothelial receptor (intercellular adhesion molecule-1 [ICAM-1]). CD11b and ICAM-1 interaction will activate endothelial and facilitate monocytes migration into the extravascular compartment (10).
In the lungs, monocytes will differentiate into alveolar macrophage, which is followed with CD11b receptor pattern changes (18). The change of monocytes receptor pattern will give a difference functional aspect (34, 35). According to this, the higher mean number of CD11b expression in mice with cerebral malaria to renal malaria may indicate different response to malaria parasites in the lung. This result may relate to the parasitic inoculation doses, which was higher in cerebral malaria mice (107) compared to renal group (106). Furthermore, lungs of cerebral malaria mice were observed at 7-day post infection, whereas 14 day post infection for renal malaria mice. In this case, the different time of observation may affect the quantity and quality of CD11b expressions, but it remains to be further investigated. Thus, the lower mean number of CD11b expression in renal malaria group compared to cerebral malaria group may be caused by the increasing number of monocytes that have differentiated into macrophages, or increasing of CD11b expressed-monocytes/macropaghes apoptosis during 7 to 14 day post infection.
The role of monocytes/macrophages as a protective or pathological agent in malaria still becomes polemic (8). In this study, we did not elaborate the role of CD11b in severe malaria-associated ALI. But in previous study (36), monocytes that differentiate into alveolar macrophages were proposed as protective agent, since the administration of anti-CD11b diminished the monocytes homing and decreased parasite elimination in the lung. However, monocytes and macrophages also have capabilities to damage the lung tissue, due to excessive cytokine production such TNF-α, IL-1β, IL-12, and IL-18, as response to hemozoin and glycosylphosphatidylinositol (33).
Related to that issue, the role of monocytes/macrophages CD11b in human malaria may be different from murine malaria, since human malaria is not always characterized by adhesion of leukocyte to the vascular bed (37). There is a possibility that increased CD11b may lead to an improved potential for phagocytosis of opsonic iRBCs, as CD11b can play role in complement-mediated phagocytosis (38). Therefore, a more comprehensive study of monocytes CD11b role in malaria-associated ALI remains needed.
The mean number of HIF-1α expression on monocytes/macrophages lung tissue of cerebral malaria mice was significantly higher compared to renal group (P< 0.05). As we know, this is the first study that quantifies the HIF-1α expression in the lung of malaria mice. HIF-1α is a transcription factor that responds to hypoxia and plays important role in the pathogenesis of ALI (39). The level of HIF-1α was also increased in murine cerebral malaria and contributed to the pathogenesis of severe malaria (40). Increased of monocytes/macrophages HIF-1α expression may reflect hypoxia caused by capillary congestion, leukocytes accumulation, and sequestration of iRBCs. It also may indicate enhancement of monocytes/macrophage immune responses (13), because HIF-1α stabilization lead to phagocytosis increasing in monocytes/macrophages (14).
Reflect to human malaria, the previous study about HIF-1α expression is observed in placental hypoxia during placental malaria in African women. This study found higher HIF-1α expression in the syncytiotrophoblast of all malaria cases versus asymptomatic, particularly with higher intensity and consistency in HIF-1α expression of monocytes and neutrophils in women with intervillositis malaria. The higher HIF-1α expressions in placental are expected to be a result of local inflammation or the presence of iRBCs, which may lead to malaria-associated low birth weight (LBW) (41).
In the previous studies (23, 24), the rising of serum SP-D level can be caused by instillation of keratinocyte growth factor (KGF), bleomycin, HCl, lipopolysaccharides (LPS), and injection of paraquat plus oxygen for fibrosis mice model. However, it is not known whether severe malaria infection will give raise to the increasing of serum SP-D levels or not. Our results showed that serum SP-D levels of both cerebral and renal malaria groups were higher compared to normal group (P< 0.05), which were increase 35-fold and 14-fold, respectively.
Increased levels of serum SP-D has long been hypothesized because of an alveolar-endothelial barrier disruption, since most of SP-D protein is synthesized by lung epithelial cells (42). The leakage of SP-D into the circulation is also facilitated by the post-translational modification, which resulted in smaller subunit SP-D protein (43). However, the source of serum SP-D is still unidentified until now (42, 43). Our study also could not identify the origin of protein SP-D that detected in serum, yet. In twins, 91% variations in the serum SP-D levels is regulated by genes (44), and upregulation of genes expression can be triggered by illness, microbes exposure, and lung injury (42).
Surfactant protein-D can also be detected in a number of extrapulmonary tissues (45, 46). Moreover, malaria infections are systemic and can infiltrate nearly all vital organs, so it may be able to trigger the synthesis of SP-D outside the lungs. Still there is no information whether extrapulmonary SP-D can be secreted into the circulation or not (42), or only act as local anti-inflammatory protein. Therefore, the use of SP-D as a seromarker needs to be considerate in severe malaria-associated ALI.
Spearman’s rank correlation test also showed that the mean levels of serum SP-D is correlated with ALI score (r=0.908, P=0.000). It means the diagnosis of ALI could be decided based on the levels of serum SP-D. Serum SPD is potential biomarker since its levels increased during lung injury in mice (24). Serum SP-D also has a high specificity for pulmonary disease and may refer to the lung epithelial disruption because most of the proteins is synthesized by the type II pneumocytes and Clara cells (22).
In this study, the expression of CD11b strongly correlate with HIF-1α (r=0.937, P< 0.05). That very strong correlation may be related to hypoxia during the lung injury. Hypoxia can induce the expression of IFN-γ that facilitates phagocytosis of macrophages by increasing the expression of CD11b/CD18 (47). In the previous study (48), myeloids cells adhesion is mediated by HIF-1-dependent induction of β2 integrin gene expression during hypoxia. Increased CD18 expression on myeloid cells occurs due to binding of HIF-1α in the CD18 gene. As CD11b/CD18 composed Mac-1 (CR-3) (49), it is possible that HIF-1α increasing affects CD11b expression on monocytes/macrophages directly.
The expression of CD11b also very strongly correlated with serum SP-D levels (r=0.907, P< 0.05). Surfactant protein-D in the bronchoalveolar lavage (BAL) and lung tissue can regulate the expression of CD11b on the surface of alveolar macrophages, thus SP-D deficiency can lead to decreased CD11b expression on alveolar macrophages (18). In mice model of acute lung injury by LPS aerosols inhalation, SP-D levels in both BAL and serum responded to pro-inflammatory stimuli and increased during acute inflammation (24). According to that previous study, increased CD11b expression may be caused by elevated levels of SP-D in BAL. In addition, increased CD11b expression in mice lung tissue may also be caused by other factors, such as increased recruitment of monocytes into the lung tissue (36), hypoxia (47), increased CD11b expression by alveolar macrophages de novo (50). Therefore, we proposed that the correlation of CD11b expression in lung tissue and serum SP-D levels were related to the alveolar epithelial disruption, which caused by a large number of monocytes recruitment that express CD11b.
Serum SP-D levels are also highly correlated with HIF-1α expression in lung tissue (r=0.913, P< 0.05). Under acute hypoxia, HIF-1α can increased the expression of SP-D in alveolar cells (20), but it is not known whether HIF-1α expression in monocytes/macrophages can be associated with levels of SP-D in lung or even in serum, directly. In mice with severe malaria, increased levels of VEGF in serum or plasma has been triggered the extent of lung injury, because VEGF is a highly potent protein to increased vascular permeability (17, 40). Because of vascular permeability increasing, plasma proteins (including SP-D) may leak into the circulation. VEGF genes also regulated by HIF-1α (51), as a result HIF-1α may also responsible to barrier disruption and plasma leakage. Thus, increased HIF-1α expression in monocytes/macrophages was a response to inflammation and infection in the lungs, which cause disruption of the alveolar epithelium integrity indirectly, resulting in increased levels of serum SP-D of C57BL/6 mice with severe malaria-associated ALI.
Conclusion
Renal malaria and cerebral malaria-associated ALI increase the expression of CD11b and HIF-1α on monocytes/macrophages in the lung tissue, and serum SP-D levels of C57BL/6 mice. A very strong correlation among CD11b, HIF-1α, and SP-D may suggest that monocytes/macrophages played important role to eliminate parasites, and may implicated in alveolar-endothelial barrier disruption. SP-D may be served as a potential biomarker candidate for the occurrence of ALI during severe malaria infections. Taken as a whole, these data support the use of murine models to study severe malaria-associated ALI, in spite of the fact that human malaria-associated ALI requires further study.
Acknowledgments
The authors thank to Dra. Rintis Noviyanti, PhD from the Malaria Pathogenesis Laboratory of Eijkman Institute for C57BL/6 mice and P. berghei ANKA parasite. This work was supported by the Research Development Unit of the Faculty of Medicine, Universitas Brawijaya, Malang, and East Java, Indonesia. The authors declare that there is no conflict of interest.
References
- 1. World Health Organization Management of severe malaria: A practical handbook. Geneva: World Health Organization; 2012. [Google Scholar]
- 2. Cox-Singh J, Davis TME, Lee K-S, Shamsul SSG, Matusop A, Ratnam S, et al. Plasmodium knowlesi malaria in humans is widely distributed and potentially life-threatening. Clin Infect Dis. 2008; 46: 165– 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andrade BB, Reis-Filho A, Souza-Neto SM, Clarêncio J, Camargo LMA, Barral A, et al. Severe Plasmodium vivax malaria exhibits marked inflammatory imbalance. Malar J. 2010; 9: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mohan A, Sharma SK, Bollineni S. Acute lung injury and acute respiratory distress syndrome in malaria. J Vector Borne Dis. 2008; 45: 179– 93. [PubMed] [Google Scholar]
- 5. Taylor WR, Hanson J, Turner GD, White NJ, Dondorp AM. Respiratory manifestations of malaria. Chest. 2012; 142: 492– 505. [DOI] [PubMed] [Google Scholar]
- 6. Van den Steen PE, Deroost K, Deckers J, Van Herck E, Struyf S, Opdenakker G. Pathogenesis of malaria-associated acute respiratory distress syndrome. Trends Parasitol. 2013; 29: 346– 58. [DOI] [PubMed] [Google Scholar]
- 7. Sarkar S, Saha K, Das CS. Three cases of ARDS: An emerging complication of Plasmodium vivax malaria. Lung India. 2010; 27: 154– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chua CLL, Brown G, Hamilton JA, Rogerson S, Boeuf P. Monocytes and macrophages in malaria: protection or pathology? Trends Parasitol. 2013; 29: 26– 34. [DOI] [PubMed] [Google Scholar]
- 9. Johnston LK, Rims CR, Gill SE, McGuire JK, Manicone AM. Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am J Respir Cell Mol Biol. 2012; 47: 417– 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meerschaert J, Furie MB. The adhesion molecules used by monocytes for migration across endothelium include CD11a/CD18, CD11b/CD18, and VLA-4 on monocytes and ICAM-1, VCAM-1, and other ligands on endothelium. J Immunol. 1995; 154: 4099– 112. [PubMed] [Google Scholar]
- 11. Wang Y, Roller J, Menger MD, Thorlacius H. Sepsis-induced leukocyte adhesion in the pulmonary microvasculature in vivo is mediated by CD11a and CD11b. Eur J Pharmacol. 2013; 702: 135– 41. [DOI] [PubMed] [Google Scholar]
- 12. Baer K, Klotz C, Kappe SHI, Schnieder T, Frevert U. Release of hepatic Plasmodium yoelii merozoites into the pulmonary microvasculature. PLoS Pathog. 2007; 3: e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harris AJ, Thompson AAR, Whyte MKB, Walmsley SR. HIF-mediated innate immune responses: Cell signaling and therapeutic implications. Hypoxia. 2014; 2: 47– 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anand RJ, Gribar SC, Li J, Kohler JW, Branca MF, Dubowski T, et al. Hypoxia causes an increase in phagocytosis by macrophages in a HIF-1α-dependent manner. J Leukoc Biol. 2007; 82: 1257– 65. [DOI] [PubMed] [Google Scholar]
- 15. Becker PM, Alcasabas A, Yu AY, Semenza GL, Bunton TE. Oxygen-independent upregulation of vascular endothelial growth factor and vascular barrier dysfunction during ventilated pulmonary ischemia in isolated ferret lungs. Am J Respir Cell Mol Biol. 2000; 22: 272– 9. [DOI] [PubMed] [Google Scholar]
- 16. Lee SH, Lee SH, Kim CH, Yang KS, Lee EJ, Min KH, et al. Increased expression of vascular endothelial growth factor and hypoxia inducible factor-1α in lung tissue of patients with chronic bronchitis. Clin Biochem. 2014; 47: 552– 9. [DOI] [PubMed] [Google Scholar]
- 17. Epiphanio S, Campos MG, Pamplona A, Carapau D, Pena AC, Ataíde R, et al. VEGF promotes malaria-associated acute lung injury in mice. PLoS Pathog. 2010; 6: e1000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Senft AP, Korfhagen TR, Whitsett JA, LeVine AM. Surfactant protein D regulates the cell surface expression of alveolar macrophage β2-integrins. Am J Physiol Lung Cell Mol Physiol. 2007; 292: L469– L75. [DOI] [PubMed] [Google Scholar]
- 19. McCormack FX, Whitsett JA. The pulmonary collectins, SP-A and SP-D, orchestrate innate immunity in the lung. J Clin Invest. 2002; 109: 707– 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sakamoto K, Hashimoto N, Kondoh Y, Imaizumi K, Aoyama D, Kohnoh T, et al. Differential modulation of surfactant protein D under acute and persistent hypoxia in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2012; 303: L43– L53. [DOI] [PubMed] [Google Scholar]
- 21. Eisner MD, Parsons P, Matthay MA, Ware L, Greene K., Acute Respiratory Distress Syndrome Network Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax. 2003; 58: 983– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ware LB, Koyama T, Billheimer DD, Wu W, Bernard GR, Thompson BT, et al. Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest. 2010; 137: 288– 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pan T, Nielsen LD, Allen MJ, Shannon KM, Shannon JM, Selman M, et al. Serum SP-D is a marker of lung injury in rats. Am J Physiol Lung Cell Mol Physiol. 2002; 282: L824– L32. [DOI] [PubMed] [Google Scholar]
- 24. Gaunsbaek MQ, Rasmussen KJ, Beers MF, Atochina-Vasserman EN, Hansen S. Lung surfactant protein D (SP-D) response and regulation during acute and chronic lung injury. Lung. 2013; 191: 295– 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Elias RM, Correa-Costa M, Barreto CR, Silva RC, Hayashida CY, Castoldi Â, et al. Oxidative stress and modification of renal vascular permeability are associated with acute kidney injury during P. berghei ANKA Infection. PLoS ONE. 2012; 7: e44004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abreu TP, Silva LSS, Takiya CM, Souza MC, Henriques MG, Pinheiro AAS, et al. Mice rescued from severe malaria are protected against renal injury during a second kidney insult. PLoS One. 2014; 9: e93634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Oca M, Engwerda C, Haque A. Plasmodium berghei ANKA (PbA) infection of C57BL/6J mice: a model of severe malaria. In: Allen IC, editor. Mouse Models of Innate Immunity. Humana Press; 2013. p. 203– 13. [DOI] [PubMed] [Google Scholar]
- 28. Nguansangiam S, Day NPJ, Hien TT, Mai NTH, Chaisri U, Riganti M, et al. A quantitative ultrastructural study of renal pathology in fatal Plasmodium falciparum malaria. Trop Med Int Health. 2007; 12: 1037– 50. [DOI] [PubMed] [Google Scholar]
- 29. Martins YC, Smith MJ, Pelajo-Machado M, Werneck GL, Lenzi HL, Daniel-Ribeiro CT, et al. Characterization of cerebral malaria in the outbred Swiss Webster mouse infected by Plasmodium berghei ANKA. Int J Exp Pathol. 2009; 90: 119– 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, et al. An official american thoracic society workshop report: Features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011; 44: 725– 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Souza MC, Silva JD, Pádua TA, Capelozzi VL, Rocco PRM, Henriques MdG. Early and late acute lung injury and their association with distal organ damage in murine malaria. Respir Physiol Neurobiol. 2013; 186: 65– 72. [DOI] [PubMed] [Google Scholar]
- 32. Aitken EH, Negri EM, Barboza R, Lima MRI, Álvarez JM, Marinho CRF, et al. Ultrastructure of the lung in a murine model of malaria-associated acute lung injury/acute respiratory distress syndrome. Malar J. 2014; 13: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deroost K, Tyberghein A, Lays N, Noppen S, Schwarzer E, Vanstreels E, et al. Hemozoin induces lung inflammation and correlates with malaria-associated acute respiratory distress syndrome. Am J Respir Cell Mol Biol. 2013; 48: 589– 600. [DOI] [PubMed] [Google Scholar]
- 34. Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005; 5: 953– 64. [DOI] [PubMed] [Google Scholar]
- 35. Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006; 211: 609– 18. [DOI] [PubMed] [Google Scholar]
- 36. Lagasse HAD, Scott A. Lung macrophages control malaria-induced pulmonary inflammation [abstract]. J Immunol. 2011;186 Abstr Suppl:56.17. [Google Scholar]
- 37. Turner GD, Morisson H, Jones M, Davis TM, Looareesuwan S, Buley ID, et al. An immunohistochemical study of the pathology of fatal malaria. Am J Pathol. 1994; 145: 1057– 69. [PMC free article] [PubMed] [Google Scholar]
- 38. McGilvray ID, Serghides L, Kapus A, Rotstein OD, Kain KC. Nonopsonic monocyte/macrophage phagocytosis of Plasmodium falciparum–parasitized erythrocytes: a role for CD36 in malarial clearance. Blood. 2000; 96: 3231– 40. [PubMed] [Google Scholar]
- 39. Shimoda LA, Semenza GL. HIF and the lung: Role of hypoxia-inducible factors in pulmonary development and disease. Am J Respir Crit Care Med. 2011; 183: 152– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hempel C, Hoyer N, Kildemoes A, Jendresen CB, Kurtzhals JAL. Systemic and cerebral vascular endothelial growth factor levels increase in murine cerebral malaria along with increased calpain and caspase activity and can be reduced by erythropoietin treatment. Front Immunol. 2014; 5: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boeuf P, Tan A, Romagosa C, Radford J, Mwapasa V, Molyneux ME, et al. Placental hypoxia during placental malaria. J Infect Dis. 2008; 197: 757– 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sørensen GL, Hjelmborg JvB, Kyvik KO, Fenger M, Høj A, Bendixen C, et al. Genetic and environmental influences of surfactant protein D serum levels. Am J Physiol Lung Cell Mol Physiol. 2006; 290: L1010– L7. [DOI] [PubMed] [Google Scholar]
- 43. Winkler C, Atochina-Vasserman EN, Holz O, Beers MF, Erpenbeck VJ, Krug N, et al. Comprehensive characterisation of pulmonary and serum surfactant protein D in COPD. Respir Res. 2011; 12: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Husby S, Herskind AM, Jensenius JC, Holmskov U. Heritability estimates for the constitutional levels of the collectins mannan-binding lectin and lung surfactant protein D. A study of unselected like-sexed mono- and dizygotic twins at the age of 6–9 years. Immunology. 2002; 106: 389– 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Madsen J, Kliem A, Tornøe I, Skjødt K, Koch C, Holmskov U. Localization of lung surfactant protein D on mucosal surfaces in human tissues. J Immunol. 2000; 164: 5866– 70. [DOI] [PubMed] [Google Scholar]
- 46. Akiyama J, Hoffman A, Brown C, Allen L, Edmondson J, Poulain F, et al. Tissue distribution of surfactant proteins A and D in the mouse. J Histochem Cytochem. 2002; 50: 993– 6. [DOI] [PubMed] [Google Scholar]
- 47. Acosta-Iborra B, Elorza A, Olazabal IM, Martín-Cofreces NB, Martin-Puig S, Miró M, et al. Macrophage oxygen sensing modulates antigen presentation and phagocytic functions involving IFN-gamma production through the HIF-1 alpha transcription factor. J Immunol. 2009; 182: 3155– 64. [DOI] [PubMed] [Google Scholar]
- 48. Kong T, Eltzschig HK, Karhausen J, Colgan SP, Shelley CS. Leukocyte adhesion during hypoxia is mediated by HIF-1-dependent induction of β(2) integrin gene expression. Proc Natl Acad Sci U S A. 2004; 101: 10440– 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tan SM. The leucocyte beta2 (CD18) integrins: the structure, functional regulation and signalling properties. Biosci Rep. 2012; 32: 241– 69. [DOI] [PubMed] [Google Scholar]
- 50. Strauss-Ayali D, Conrad SM, Mosser DM. Monocyte subpopulations and their differentiation patterns during infection. J Leukoc Biol. 2007; 82: 244– 52. [DOI] [PubMed] [Google Scholar]
- 51. Bruick RK. Oxygen sensing in the hypoxic response pathway: Regulation of the hypoxia-inducible transcription factor. Genes Dev. 2003; 17: 2614– 23. [DOI] [PubMed] [Google Scholar]