Abstract
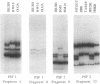
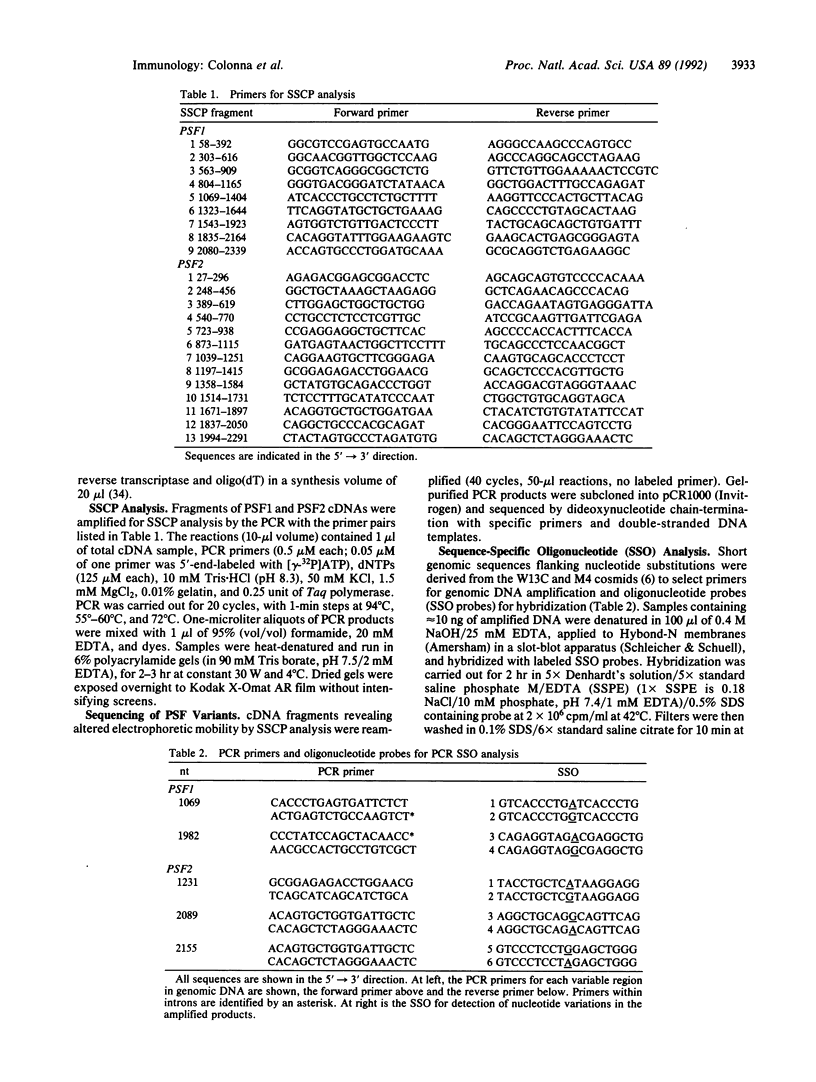
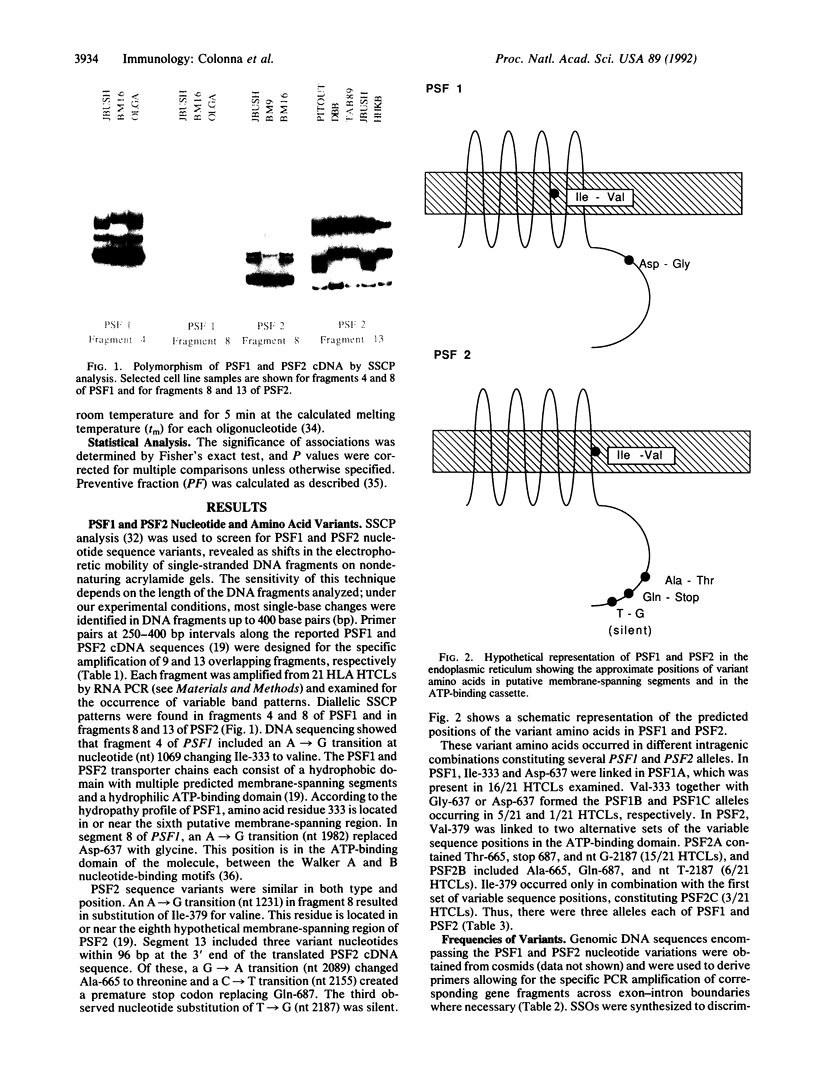
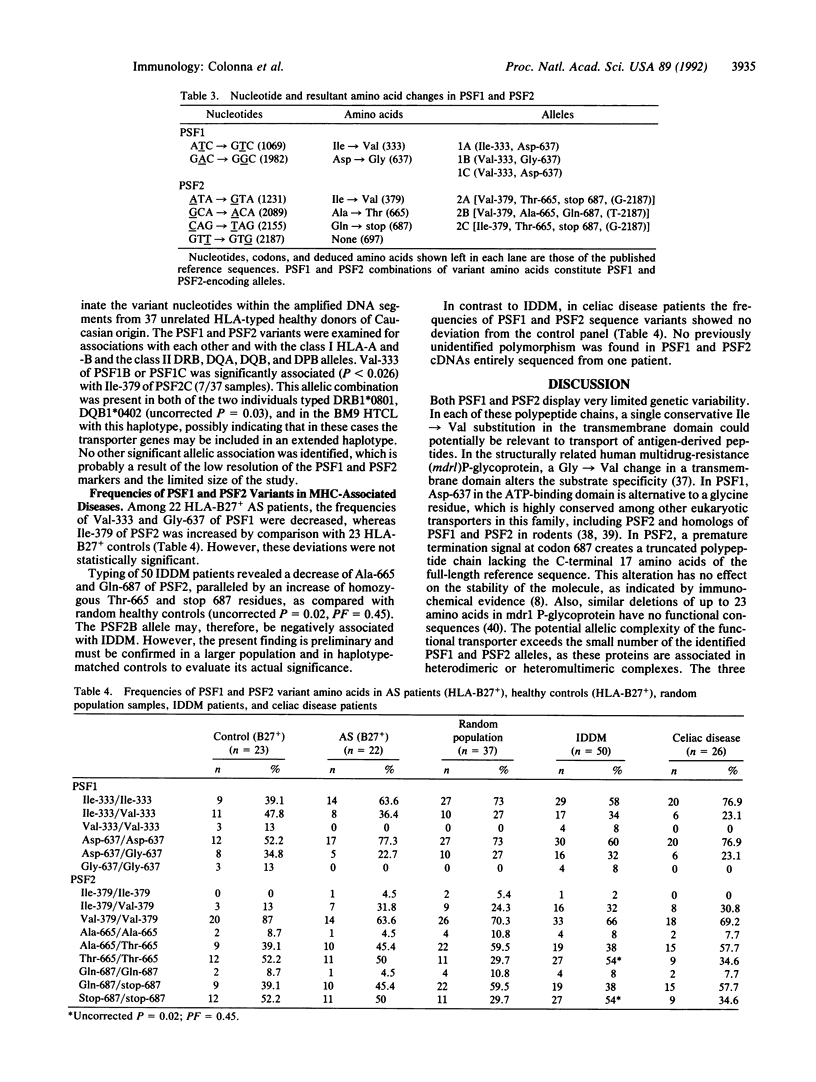
Antigen processing for presentation of peptide epitopes by major histocompatibility complex (MHC) class I molecules involves genes in the MHC class II region. Among these, PSF1 and PSF2 encode subunits of a transporter, which presumably delivers cytosolic peptides across the endoplasmic reticulum membrane to class I molecules. This close functional relationship of the transporter and class I heavy chain genes and their linkage within the MHC raise the question of whether PSF1 and PSF2, like most class I genes, are polymorphic. By single-strand conformation polymorphism analysis and DNA sequencing, a small number of amino acid sequence variants of both PSF1 and PSF2 was identified in a panel of cell lines. This limited polymorphism may contribute to a higher degree of variability at the level of the functional transporter, in which different alleles of the PSF1 and PSF2 subunits may be combined. A possible involvement of the PSF1 and PSF2 genes in susceptibility to MHC-associated diseases was examined in a preliminary assessment in patients with ankylosing spondylitis, insulin-dependent diabetes mellitus, or celiac disease.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper C. A., Fleischnick E., Awdeh Z., Katz A. J., Yunis E. J. Extended major histocompatibility complex haplotypes in patients with gluten-sensitive enteropathy. J Clin Invest. 1987 Jan;79(1):251–256. doi: 10.1172/JCI112791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahram S., Arnold D., Bresnahan M., Strominger J. L., Spies T. Two putative subunits of a peptide pump encoded in the human major histocompatibility complex class II region. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10094–10098. doi: 10.1073/pnas.88.22.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman P. J., Parham P. Structure, function, and diversity of class I major histocompatibility complex molecules. Annu Rev Biochem. 1990;59:253–288. doi: 10.1146/annurev.bi.59.070190.001345. [DOI] [PubMed] [Google Scholar]
- Brewerton D. A., Hart F. D., Nicholls A., Caffrey M., James D. C., Sturrock R. D. Ankylosing spondylitis and HL-A 27. Lancet. 1973 Apr 28;1(7809):904–907. doi: 10.1016/s0140-6736(73)91360-3. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Guagliardi L. E. The cell biology of antigen processing and presentation. Annu Rev Immunol. 1991;9:707–744. doi: 10.1146/annurev.iy.09.040191.003423. [DOI] [PubMed] [Google Scholar]
- Brown M. G., Driscoll J., Monaco J. J. Structural and serological similarity of MHC-linked LMP and proteasome (multicatalytic proteinase) complexes. Nature. 1991 Sep 26;353(6342):355–357. doi: 10.1038/353355a0. [DOI] [PubMed] [Google Scholar]
- Cerundolo V., Alexander J., Anderson K., Lamb C., Cresswell P., McMichael A., Gotch F., Townsend A. Presentation of viral antigen controlled by a gene in the major histocompatibility complex. Nature. 1990 May 31;345(6274):449–452. doi: 10.1038/345449a0. [DOI] [PubMed] [Google Scholar]
- Choi K. H., Chen C. J., Kriegler M., Roninson I. B. An altered pattern of cross-resistance in multidrug-resistant human cells results from spontaneous mutations in the mdr1 (P-glycoprotein) gene. Cell. 1988 May 20;53(4):519–529. doi: 10.1016/0092-8674(88)90568-5. [DOI] [PubMed] [Google Scholar]
- Currier S. J., Ueda K., Willingham M. C., Pastan I., Gottesman M. M. Deletion and insertion mutants of the multidrug transporter. J Biol Chem. 1989 Aug 25;264(24):14376–14381. [PubMed] [Google Scholar]
- Deverson E. V., Gow I. R., Coadwell W. J., Monaco J. J., Butcher G. W., Howard J. C. MHC class II region encoding proteins related to the multidrug resistance family of transmembrane transporters. Nature. 1990 Dec 20;348(6303):738–741. doi: 10.1038/348738a0. [DOI] [PubMed] [Google Scholar]
- Faustman D., Li X. P., Lin H. Y., Fu Y. E., Eisenbarth G., Avruch J., Guo J. Linkage of faulty major histocompatibility complex class I to autoimmune diabetes. Science. 1991 Dec 20;254(5039):1756–1761. doi: 10.1126/science.1763324. [DOI] [PubMed] [Google Scholar]
- Glynne R., Powis S. H., Beck S., Kelly A., Kerr L. A., Trowsdale J. A proteasome-related gene between the two ABC transporter loci in the class II region of the human MHC. Nature. 1991 Sep 26;353(6342):357–360. doi: 10.1038/353357a0. [DOI] [PubMed] [Google Scholar]
- Horn G. T., Bugawan T. L., Long C. M., Erlich H. A. Allelic sequence variation of the HLA-DQ loci: relationship to serology and to insulin-dependent diabetes susceptibility. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6012–6016. doi: 10.1073/pnas.85.16.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A., Powis S. H., Glynne R., Radley E., Beck S., Trowsdale J. Second proteasome-related gene in the human MHC class II region. Nature. 1991 Oct 17;353(6345):667–668. doi: 10.1038/353667a0. [DOI] [PubMed] [Google Scholar]
- Livingstone A. M., Powis S. J., Diamond A. G., Butcher G. W., Howard J. C. A trans-acting major histocompatibility complex-linked gene whose alleles determine gain and loss changes in the antigenic structure of a classical class I molecule. J Exp Med. 1989 Sep 1;170(3):777–795. doi: 10.1084/jem.170.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez C. K., Monaco J. J. Homology of proteasome subunits to a major histocompatibility complex-linked LMP gene. Nature. 1991 Oct 17;353(6345):664–667. doi: 10.1038/353664a0. [DOI] [PubMed] [Google Scholar]
- Mellins E., Kempin S., Smith L., Monji T., Pious D. A gene required for class II-restricted antigen presentation maps to the major histocompatibility complex. J Exp Med. 1991 Dec 1;174(6):1607–1615. doi: 10.1084/jem.174.6.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellins E., Smith L., Arp B., Cotner T., Celis E., Pious D. Defective processing and presentation of exogenous antigens in mutants with normal HLA class II genes. Nature. 1990 Jan 4;343(6253):71–74. doi: 10.1038/343071a0. [DOI] [PubMed] [Google Scholar]
- Monaco J. J., Cho S., Attaya M. Transport protein genes in the murine MHC: possible implications for antigen processing. Science. 1990 Dec 21;250(4988):1723–1726. doi: 10.1126/science.2270487. [DOI] [PubMed] [Google Scholar]
- Monaco J. J., McDevitt H. O. H-2-linked low-molecular weight polypeptide antigens assemble into an unusual macromolecular complex. 1984 Jun 28-Jul 4Nature. 309(5971):797–799. doi: 10.1038/309797a0. [DOI] [PubMed] [Google Scholar]
- Monaco J. J., McDevitt H. O. Identification of a fourth class of proteins linked to the murine major histocompatibility complex. Proc Natl Acad Sci U S A. 1982 May;79(9):3001–3005. doi: 10.1073/pnas.79.9.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco J. J., McDevitt H. O. The LMP antigens: a stable MHC-controlled multisubunit protein complex. Hum Immunol. 1986 Apr;15(4):416–426. doi: 10.1016/0198-8859(86)90019-4. [DOI] [PubMed] [Google Scholar]
- Morel P. A., Dorman J. S., Todd J. A., McDevitt H. O., Trucco M. Aspartic acid at position 57 of the HLA-DQ beta chain protects against type I diabetes: a family study. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8111–8115. doi: 10.1073/pnas.85.21.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepom G. T., Erlich H. MHC class-II molecules and autoimmunity. Annu Rev Immunol. 1991;9:493–525. doi: 10.1146/annurev.iy.09.040191.002425. [DOI] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Ortiz-Navarrete V., Seelig A., Gernold M., Frentzel S., Kloetzel P. M., Hämmerling G. J. Subunit of the '20S' proteasome (multicatalytic proteinase) encoded by the major histocompatibility complex. Nature. 1991 Oct 17;353(6345):662–664. doi: 10.1038/353662a0. [DOI] [PubMed] [Google Scholar]
- Raum D., Awdeh Z., Yunis E. J., Alper C. A., Gabbay K. H. Extended major histocompatibility complex haplotypes in type I diabetes mellitus. J Clin Invest. 1984 Aug;74(2):449–454. doi: 10.1172/JCI111441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendel D. J. On the peptide model of allorecognition: cytotoxic T lymphocytes recognize an alloantigen encoded by two HLA-linked genes. Hum Immunol. 1990 Mar;27(3):229–239. doi: 10.1016/0198-8859(90)90053-r. [DOI] [PubMed] [Google Scholar]
- Schlosstein L., Terasaki P. I., Bluestone R., Pearson C. M. High association of an HL-A antigen, W27, with ankylosing spondylitis. N Engl J Med. 1973 Apr 5;288(14):704–706. doi: 10.1056/NEJM197304052881403. [DOI] [PubMed] [Google Scholar]
- Sollid L. M., Markussen G., Ek J., Gjerde H., Vartdal F., Thorsby E. Evidence for a primary association of celiac disease to a particular HLA-DQ alpha/beta heterodimer. J Exp Med. 1989 Jan 1;169(1):345–350. doi: 10.1084/jem.169.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies T., Bresnahan M., Bahram S., Arnold D., Blanck G., Mellins E., Pious D., DeMars R. A gene in the human major histocompatibility complex class II region controlling the class I antigen presentation pathway. Nature. 1990 Dec 20;348(6303):744–747. doi: 10.1038/348744a0. [DOI] [PubMed] [Google Scholar]
- Spies T., Cerundolo V., Colonna M., Cresswell P., Townsend A., DeMars R. Presentation of viral antigen by MHC class I molecules is dependent on a putative peptide transporter heterodimer. Nature. 1992 Feb 13;355(6361):644–646. doi: 10.1038/355644a0. [DOI] [PubMed] [Google Scholar]
- Spies T., DeMars R. Restored expression of major histocompatibility class I molecules by gene transfer of a putative peptide transporter. Nature. 1991 May 23;351(6324):323–324. doi: 10.1038/351323a0. [DOI] [PubMed] [Google Scholar]
- Sterkers G., Zeliszewski D., Chaussée A. M., Deschamps I., Font M. P., Freidel C., Hors J., Betuel H., Dausset J., Levy J. P. HLA-DQ rather than HLA-DR region might be involved in dominant nonsusceptibility to diabetes. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6473–6477. doi: 10.1073/pnas.85.17.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svejgaard A., Platz P., Ryder L. P. HLA and disease 1982--a survey. Immunol Rev. 1983;70:193–218. doi: 10.1111/j.1600-065x.1983.tb00715.x. [DOI] [PubMed] [Google Scholar]
- Todd J. A., Bell J. I., McDevitt H. O. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987 Oct 15;329(6140):599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- Todd J. A., Mijovic C., Fletcher J., Jenkins D., Bradwell A. R., Barnett A. H. Identification of susceptibility loci for insulin-dependent diabetes mellitus by trans-racial gene mapping. Nature. 1989 Apr 13;338(6216):587–589. doi: 10.1038/338587a0. [DOI] [PubMed] [Google Scholar]
- Tosi R., Vismara D., Tanigaki N., Ferrara G. B., Cicimarra F., Buffolano W., Follo D., Auricchio S. Evidence that celiac disease is primarily associated with a DC locus allelic specificity. Clin Immunol Immunopathol. 1983 Sep;28(3):395–404. doi: 10.1016/0090-1229(83)90106-x. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Trowsdale J., Hanson I., Mockridge I., Beck S., Townsend A., Kelly A. Sequences encoded in the class II region of the MHC related to the 'ABC' superfamily of transporters. Nature. 1990 Dec 20;348(6303):741–744. doi: 10.1038/348741a0. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]