Abstract
Background:
The present study aimed to survey antileishmanial activity of methanolic Holothuria leucospilota extract against Leishmania major promastigotes in vitro.
Methods:
Promastigotes were cultured in RPMI 1640 and after reaching the stationary phase, the study was conducted with different concentrations of the extract. Afterwards, MTT colorimetric assay for the obtaining of 50% inhibitory concentration (IC50) was utilized. Furthermore, in order to determine the possible induction of apoptosis in L. major promastigotes, flow cytometry and DNA fragmentation methods were employed using annexin-V FLUOS staining kit and DNA ladder kit, respectively.
Results:
The IC50 value of H. leucospilota extract at three time points of 24, 48, and 72 h was estimated 2000, 300 and 85 μg/ml, respectively. In addition, the extract revealed a dose and time-dependent antileishmanial activity. Furthermore, various characteristics of apoptosis appeared after L. major promastigotes treatment, which included cell shrinkage, formation of apoptotic bodies, blebbing of the cell membrane, and externalization of phosphatidylserine, although no laddering pattern was observed.
Conclusion:
The methanolic extract of H. leucospilota possesses lethal effect on L. major promastigotes and induces the apoptosis in parasites. Further studies are required to address the apoptosis mechanism in vivo.
Keywords: Leishmania major, Sea cucumber, Holothuria leucospilota, Apoptosis, MTT, Flow cytomtry, DNA Fragmentation
Introduction
Leishmaniasis is a vector-borne tropical/subtropical disease caused by the obligate intracellular parasite belonging to Leishmania genus, which, according to clinical manifestation and etiological agents, are classified into at least three categories including cutaneous leishmaniasis (CL), mucocutaneous leishmaniasis (MCL), and visceral leishmaniasis (VL) (1, 2). The infection has a wide spectrum that ranges from self-healing cutaneous ulcers, to progressive and fatal mucocutaneous and visceral leishmaniasis (2, 3). Leishmaniasis transmits to the vertebrate hosts by the mosquito bite of the infected female Phlebotomus genus in the Old World or Lutzomyia in the New World (2, 4).
According to WHO, leishmaniasis is considered a major global health problem in five continents with economic wastage and 12 million people are currently infected worldwide in about 100 countries and territories (3). In addition, it is estimated that 350 million people are at risk of infection. The annual incidence of new cases of CL and VL is approximately 0.7–1.2 million and 0.2–0.4 million, respectively (3). Despite many efforts and human advances in disease control, leishmaniasis continues to be considered a major health concern, particularly in developing countries (3, 5, 6). The majority (approximately 70–75%) of CL cases are found in ten countries worldwide annually, such as Brazil, Peru, Costa Rica, Colombia, Algeria, North Sudan, Ethiopia, Afghanistan, Syria, and Iran (3).
As of yet, there is a lack of effective vaccines against leishmaniasis. First-line drugs for the treatment of the disease are pentavalent antimonial compounds, such as meglumine antimonate (Glucantime) and sodium stibogluconate (Pentostam), which have been utilized since the 1940s until the present (7). These drugs, with interruption in phosphokinase enzyme activity, prevent the production of adenosine triphosphate (ATP). Although pentamidine, paromomycin, and amphotericin B are being used as second-choice drugs, amphotericin B is teratogen and not suitable for pregnant women (8). Pentavalent antimonial drugs have several limitations including: drug resistance, toxicity and systemic side effects, painful administration, parenteral administration, prolonged course of treatment, parasite resistance, and high cost (9). In addition, many treatment failures have been reported (10). Furthermore, damage to the heart, liver, pancreas, hematopoietic tissues, and renal failure are other probable complications (9).
Since herbal medicines and marine invertebrates contain valuable ingredients, easily available, and inexpensive, the use of such native plants and marine invertebrates could be considered rich sources of antileishmanial compounds (11–13). Approximately 80% of the world’s individuals tend to use traditional medicines to remedy their ailments (14). Therefore, there is an urgent need to discover more affordable, cheaper, more effective, and safer antileishmanial drugs. In recent years, several studies have been completed on the efficacy of different plant extracts against Leishmania spp. worldwide and, of course, in Iran, giving these compounds the ability to create new hopes for the treatment of leishmaniasis (11, 12, 15–20).
Sea cucumbers are one of the marine invertebrate animals that have their therapeutic property proven in several studies (21–23). They are from marine invertebrates which belong to Echinodermata (due to spiny-skinned) phylum and Holothuroidea class (Fig. 1) (24). For different species of sea cucumber, various properties have been mentioned such as antioxidant, anti-inflammatory, anti-histamine, anti-anaphylactic, wound healing, anti-angiogenic, anticoagulant, anti-thrombotic, anti-hypertension, antitumor and anti-cancer, antiviral, antibacterial and antifungal activities. Sea cucumbers contain various compounds with therapeutic properties and health functions that can be related to certain bioactive substances, namely triterpene glycosides (saponins), glycosaminoglycan (GAGs), chondroitin sulfates, sulfated polysaccharides, phenolics, sterols (glycosides and sulfates), lectins, cerberosides, peptides, glycosphingolipids, glycoprotein, and essential fatty acids (23). Antileishmanial effects of some marine organisms, such as marine sponges (Haliclona exigua, Sarcotragus spp. and Ircinia spinosula) have been previously proven (25, 26).
Fig. 1:

Holothuria leucospilota
Apoptosis, or programmed cell death (PCD), phenomenon regulates homeostasis and number of cells in multicellular animals physiologically. Primarily, it was believed that PCD just occurred in multicellular animals however recent studies have verified that the process of PCD also occurs in eukaryotic organisms like Leishmania, Plasmodium, Trypanosoma, Toxoplasma, Trichomonas, Blastocystis, Entamoeba and Giardia (12, 27). Consequently, if each compound and drug is able to initiate apoptosis in Leishmania parasite, it could be claimed as an effective drug against leishmaniasis (12, 16, 28). Leishmania major promastigotes, after exposure with some drugs like miltefosine (an anticancer drug), undergo PCD (29).
Concerning lack of evidence on the antileishmanial effects of sea cucumber (Holothuria leucospilota species), the current study was aimed to evaluate probable apoptosis induction by methanolic H. leucospilota (in the local language is called Khiar daryaei) extract in L. major promastigotes using flow cytometry and DNA fragmentation methods.
Materials and Methods
Materials
L. major Iranian standard strain promastigotes (MRHO/IR/75/ER) were purchased from Pasteur Institute (Tehran, Iran). In addition, all materials obtained were the following: dimethyl sulfoxide (DMSO), 4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid (HEPES), 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) colorimetric assay kit, phosphate-buffered saline (PBS), and L-glutamine were purchased from Sigma Chemical Co. (St. Louis, Missouri, USA). In addition, annexin-V FLUOS staining kit and apoptotic DNA ladder kit were purchased from Roche-applied-science (Berlin, Germany). RPMI 1640 (Roswell Park Memorial Institute), fetal bovine serum (FBS), and antibiotic (streptomycin and penicillin) were purchased from Gibco (Gibco, New York, USA). Reference drug, Glucantime® (meglumine antimoniate, 85 mg Sbv/ml; batch 020, Rhodia SA, Brazil) were purchased from Brazil, kept at 4 °C, and diluted with RPMI 1640 medium at the time of incubation.
Preparation H. leucospilota extract
H. leucospilota were collected from the coastal areas of the Persian Gulf and, in order to extraction were transferred to the Medicinal Plant and Natural Product Research Center located at Ahvaz Jundishapur University of Medical Sciences (AJUMS). Extraction from the body wall of H. leucospilota was performed using maceration method as formerly described (13).
Culture of L. major promastigotes (MRHO/IR/75/ER)
L. major promastigotes (MRHO/IR/75/ER) were routinely cultured in RPMI 1640 media (without phenol red) at 25±1 °C in 25 mM HEPES (PH 7.2) and enriched by 10% heat-inactivated FBS, antibiotics (100 μg/ml streptomycin and 100 IU/ml penicillin) and L-glutamine (2 mM) as previously described. Each flasks’ culture was examined every day in order to detect bacterial and fungal contamination. Flasks were incubated at 25±1 °C. Eventually on promastigotes that were reached at the stationary phase, next tests were performed (16, 30). All tests were repeated three times.
MTT assay
In order to obtain the 50% inhibitory concentration (IC50), 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) kit was employed. For preparation of MTT reagent, 5 mg MTT powder in 1 ml PBS sterile solution (5 mg/ml) was dissolved. 96-well microplate was used for the MTT assay (5 mg/ml, 20μl/well). Briefly after adding 2×106 promastigotes/100 μl/well, methanolic H. leucospilota extract in different increasing concentrations ranged from 0–156 mg/ml were added to treat the parasites (15, 28). Plates incubated in 25±1°C and after 24, 48 and 72 h, promastigotes viability using MTT assay were examined. Then, absorbance at 540 nm wavelength was measured using ELISA reader device. Cell viability percentage of exposed and unexposed samples acquisition was carried out by the following formula:
AT: absorbance of the exposed promastigotes
AC: absorbance of the unexposed promastigotes
AB: absorbance of the blank
Ultimately, findings expressed by IC50 (that inhibited half of the promastigotes growth) and in the experimental group were compared to positive controls (promastigotes treated with 100 μl of Glucantime® 85 mg/ml) and negative controls (promastigotes treated with 100 μl of DMSO 1% without extract) (13, 30, 31).
Observation of morphological changes in treated and untreated promastigotes with methanolic H. leucospilota extract
IC50 dose related to 24 h were used to examine the morphological changes. After 72 h incubation period, promastigotes were centrifuged at low speed (1000g for 3 minutes) and suspended in PBS. Then, cells were examined using an optical microscope under ×100 magnification. Morphological changes of treated and untreated promastigotes with extract, including cell shrinkage and motility, were evaluated at different times and at least 20 microscopic fields for each sample were observed. Finally, the experimental group in terms of morphological changes and motility was compared with the control group (16, 31).
Flow cytometry analysis
In order to differentiate between viable, necrotic, and apoptotic treated or untreated promastigotes with extract, flow cytometry technique by double staining with annexin V-FLUOS and propidium iodide (PI) was employed. Annexin-V can differentiate between necrotic cells (PI positive / upper left), apoptotic cells (only annexin-V positive as eraly apoptosis / lower right - both annexin-V and PI positive as late apoptosis / upper right), and normal cells (both annexin-V and PI negative / lower left) (32). Based on the manufacturer’s instructions, treated promastigotes (2×106 parasites/ml) with IC50 value of extract and untreated promastigotes were washed with cold PBS solution twice and were centrifuged 10 minutes at 1400g. Then, they were incubated at room temperature and in dark site for 15 minutes in 100 μl of annexin-V FLUOS in the presence of PI. Finally, samples were examined by FACS Calibur flow cytometer (Becton Dickinson, USA) and were analyzed using CellQuest software. For each sample, percentage of positive cells was determined.
DNA Fragmentation technique
For qualitative measure of the total gDNA fragmentation, agarose gel electrophoresis was employed as formerly described (29). Briefly, L. major promastigotes (5×106 parasites/ml) were harvested at different growth time points. Then, in order to extract DNA from apoptosis-induced and non-induced promastigotes, apoptotic DNA ladder kit was utilized in accordance with the manufacturer’s protocol. Ten μg from each extracted DNA was electrophoresed in 1.5% agarose gel for 2 h at 80 V. Eventually, it was analyzed using Gel Doc device (Uvidoc, Gel Documentation System, Cambridge, UK).
Statistical analysis
In vitro antileishmanial activity, which expressed as IC50, was obtained using SPSS software version 19 with linear regression test (SPSS Inc., Chicago, IL, USA).
Results
Characteristics of PCD were seen after L. major promastigotes exposure with H. leucospilota extract. Some of these changes include cell shrinkage, nuclear morphology changes, blebbing of cell membrane, mitochondria function loss, formation of apoptotic masses, and phosphatidylserine (PS) externalization from inner to outer plasma membrane with maintaining membrane integrity; though no laddering pattern was observed.
Determination of the IC50
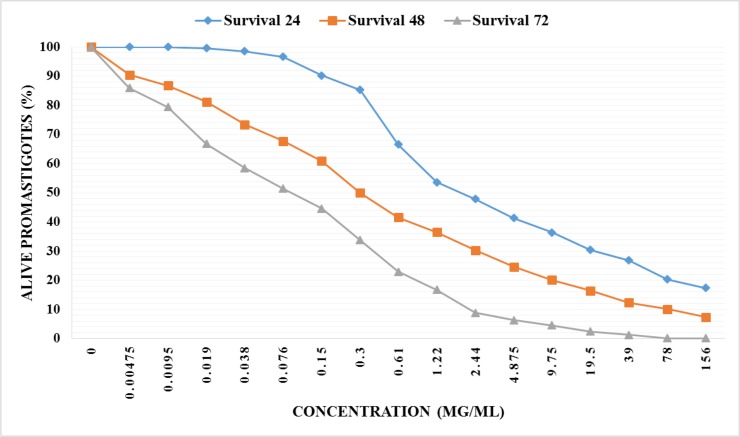
We screened the efficacy of methanolic extract of H. leucospilota body wall against L. major promastigotes. The IC50 value of extract at three time points of 24, 48, and 72 h was estimated about 2000 μg/ml, 300μg/ml, and 85μg/ml, respectively. In addition, H. leucospilota extract revealed a dose and time-dependent antileishmanial activity against parasites in vitro that is exhibited in Fig 2. In the control group, cells treated with Glucantime® in several concentrations and IC50 value at three time points of 24, 48, and 72 h was obtained 37 μg/ml, 22 μg/ml, and 14 μg/ml by MTT assay.
Fig. 2:
The viability of L. major promastigotes in the presence of various concentrations of methanolic exctract of H. leucospilota which was assessed by MTT
Morphological alterations in exposed and unexposed promastigotes
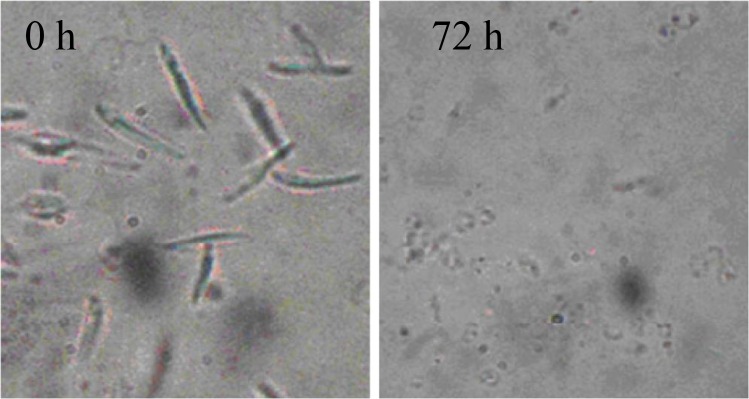
Observation using optical microscope under ×100 magnification showed that IC50 dose of H. leucospilota extract (2000 μg/ml) leads to obvious alterations in treated promastigotes including cell shrinkage, cytoplasmic condensation, and immobility. Furthermore, the average number of promastigotes at the initial treatment (0 h) was approximately 2×106 cells/ml, so that the decreasing trend in number of live cells was continuous in other intervals up to 72 h. On the other hand, in the control group no visible changes were seen in cells morphology, as well as multiplication of promastigotes. Morphological alterations in exposed and unexposed promastigotes at time points are illustrated in Fig. 3.
Fig. 3:
Analysis of morphology in light microscopy (magnification, x 100) of L .major promastigotes following treatment with H. leucospilota extracts A. L. major 0 hours after treatment and B. L. major 72 hours after treatment
Identification of phosphatidylserine located at outer membrane in apoptotic cells by flow cytometry technique
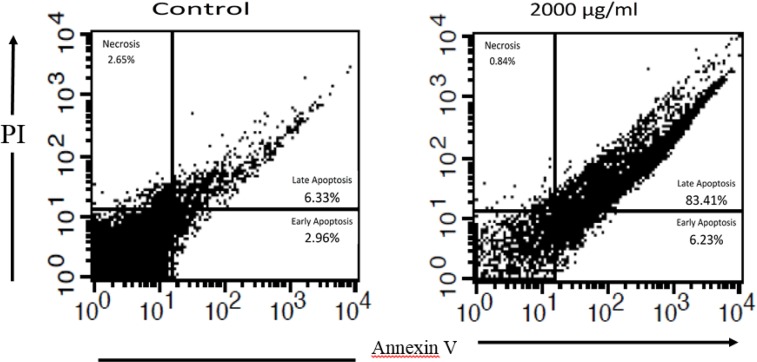
In order to differentiate between necrosis and apoptosis, annexin-V FLUOS staining kit was employed and obtained findings analyzed using flow cytometry method. After 72 h incubation of exposed L. major promastigotes with IC50 dose (2000 μg/ml), the status of cells was assessed. The percentage of promastigotes in early and late phases of apoptosis in the experimental group was 6.23% and 83.41%, respectively. However, in the control group, the status of the cells was 2.96% and 6.33%, respectively. More details are illustrated in Fig. 4.
Fig. 4:
Flow cytometry analysis: Apoptosis occurrence in L. major promastigotes after treatment with H. leucospilota extract (2000 μg/ml) for 72 h
DNA fragmentation in exposed an un-exposed promastigotes using DNA ladder assay
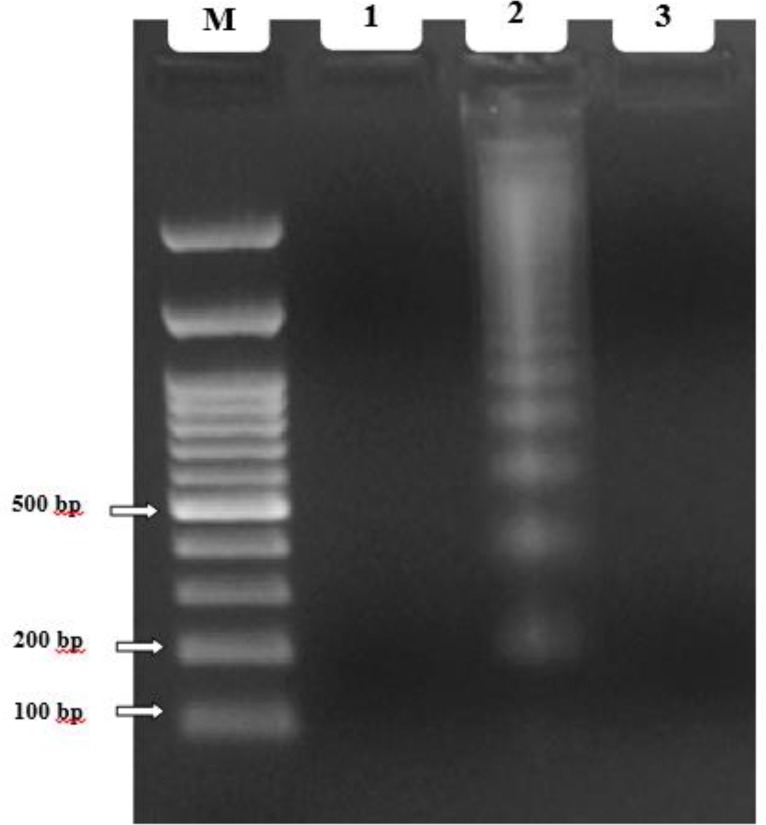
A lack of laddering pattern was observed after 72 h treatment with IC50 dose (2000 μg/ml) (Fig. 5). The major limitation of this technique is the need for a large number of cells to undergo apoptosis in order to obtain appropriate DNA to produce the laddering pattern (low sensitivity). DNA laddering is not a quantitative technique; demonstrating only apoptosis in a cell population, but does not assess the degree of apoptosis of individual cells (33). According to the aforementioned reasons, our findings could be justified with low amount of extracted DNA and low sensitivity of this method.
Fig. 5:
DNA ladder assay: Lane M. DNA size marker; Lane 1. Unexposed L. major promastigotes (DNA fragmentation did not occur); Lane 2. Positive apoptotic cell (positive control); Lane 3. Exposed L. major promastigotes (DNA fragmentation did not occur)
Discussion
Leishmaniasis is considered as one of the major public health problems worldwide, particularly in tropical and subtropical zones (3, 5). Nowadays, pentavalent antimonial compounds are the most important drugs being used for the treatment of leishmaniasis (7).
Certain reported disadvantages of these compounds include: painful of administration, various systemic side effects, expensive cost, long course of treatment, Leishmania parasite resistance, and failure treatment; thus, researchers are constantly looking for cheaper, safer, and more effective drugs (9).
In this study, MTT assay, a colorimetric method that tetrazolium salt (yellow) converted to insoluble formazan (purple) by viable cells, was used. These reduction reactions catalyze by mitochondrial succinate dehydrogenase enzyme of parasite, which is used as an indicator of promastigotes growth and viability against drug responses; thus, there is a directly proportional between the amounts of formazan produced with cell viability (13, 33). MTT in comparison with direct counting method that is non-precision, time-consuming, difficult and unreliable, have many advantages such as ease of use, being sensitive, low expense, reliability, and reproducibility. Also, due to the lack of use of radioisotope and radioactive materials, it is a safe and secure method (34).
Our results demonstrated that L. major promastigotes were sensitive to methanolic extract of H. leucospilota at different concentrations in time and in dose-dependent manner. Apoptosis occurs in Leishmania promastigotes after exposure with some chemical compounds, plant extracts, and drugs (12, 16, 28, 29). In addition, the induction of PCD by sea cucumbers was previously verified in different cells (35, 36); therefore, we aimed to evaluate the apoptosis induction in L. major promastigotes. Our findings are the first report of apoptosis induction by methanolic H. leucospilota extract against L. major promastigots. During apoptosis, many alterations occurs in three important stages; they are: cell membrane changes, cytoplasmic or mitochondrial pathway, and cell nucleus changes (27).
Fatty acids of sea cucumber, containing arachidonic acid, docosahexaenoic acid, and eicosapentaenoic acid, appear to have a potential role in wound healing and tissue repairing (reduction of wound healing period by helping to enhancement of tissue regeneration) (23). Lipid fractions of sea cucumber were introduced as responsible for wound healing and tissue repairing (37). In addition, sea cucumber has a potential role in the coagulation process, which can lead to wound healing. “For this reason, many parts of the world utilize sea cucumbers as a traditional treatment for burns and cuts” (37–39).Further studies are required to address wound healing properties of sea cucumber on cutaneous ulcers caused by Leishmania species in vivo, in future.
Sea cucumber proteins are rich of glutamate, glycine, and arginine. Glutamic acid and glycine are key elements for glutathione synthesis in cells and can provoke the proliferation and activation of immune system cells like NK cells. In addition, glycine can provoke the production of B cell antibodies and IL-2 release, which helps to increase immunity. Arginine also enhances the cellular immunity by T-cell activation (40). For these reasons, sea cucumber and their possession of these amino acid ingredients could contribute to the immunomodulatory activity.
Sea cucumber by apoptosis induction in cancerous cells, like colon cancer, eliminates them (35, 36, 41). Antifungal and antitumor activity of sea cucumbers have attributed to the presence of triterpene compounds (42). Antioxidant and anticancer properties of sea cucumbers ascribed to existence of phenols and flavonoids (21). The anticancer activity of triterpene glycoside isolated from sea cucumber (Mensamaria intercedens species) by Zou et al. was assessed and triterpene compounds of sea cucumbers are liable for cytotoxicity against human tumor cell lines (43). Researchers obtained extract of three species of sea cucumbers (H. scabra, H. leucospilota, Stichopus chloronotus) and surveyed the growth of two human cancer cells with the findings illustrating the antiproliferative activity in cell lines (21). Other researches, after treatment of aqueous extract of sea cucumber with human colon adenocarcinoma Caco-2 cells, have shown some morphological alternations and apopotosis event confirmed using PS translocation (41), which is in accordance with current investigation. Thus, since H. leucospilota possesses phenols, flavonoids, and triterpene glycoside compounds (23), the findings of the present study of the apoptosis phenomenon in L. major promastigotes can be justified due to the presence of this bioactive ingredient.
Recently, the effectiveness of methanol extract and n-butanol fraction of sea cucumber (Actinopyga lecanora species) has been reported against L. donovani, both in vitro and in vivo, considered as causative agent of VL. One hundred μg/ml of methanol extract leads to 88.5% and 72.4% inhibition of promastigotes and amastigotes, respectively. Antileishmanial effects of both methanolic extract and n-butanol fraction against L. donovani-infected hamsters leads to reduction of the parasite burden (44).
Conclusion
Natural compounds formerly have been used in traditional medicine, nowadays have created new hopes for the treatment of leishmaniasis. Methanolic extract of H. leucospilota possesses lethal effects on L. major promastigotes and leads to the inducing of the apoptosis in parasites. Further studies, especially in vivo, are required to identify the exact mechanism effect of this extract in order to explore and reach drug or suitable medicinal compounds for the treatment of leishmaniasis. Furthermore, we recommend in future studies that different fractions of H. leucospilota be purified initially and then examined.
Acknowledgments
The authors would like to thank the Research Deputy of Ahvaz Jundishapur University of Medical Sciences (code no. CMRC-125). This article was extracted from MSc thesis of Masoud Foroutan-Rad, MSc student of Medical Parasitology. The authors declare no conflict of interests.
References
- 1. Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004; 27( 5): 305– 18. [DOI] [PubMed] [Google Scholar]
- 2. Akhoundi M, Kuhls K, Cannet A, Votypka J, Marty P, Delaunay P, et al. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Negl Trop Dis. 2016; 10( 3): e0004349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012; 7( 5): e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karimi A, Hanafi-Bojd AA, Yaghoobi-Ershadi MR, Akhavan AA, Ghezelbash Z. Spatial and temporal distributions of phlebotomine sand flies (Diptera: Psychodidae), vectors of leishmaniasis, in Iran. Acta Trop. 2014; 132: 131– 9. [DOI] [PubMed] [Google Scholar]
- 5. Postigo JA. Leishmaniasis in the World Health Organization Eastern Mediterranean Region. Int J Antimicrob Agents. 2010; 36 Suppl 1: S62– 5. [DOI] [PubMed] [Google Scholar]
- 6. Khademvatan S, Salmanzadeh S, Foroutan-Rad M, Bigdeli S, Hedayati-Rad F, Saki J, et al. Spatial distribution and epidemiological features of cutaneous leishmaniasis in southwest of Iran. Alexandria J Med. 2016 . . 10.1016/j.ajme.2016.03.001. [DOI]
- 7. Singh N, Kumar M, Singh RK. Leishmaniasis: current status of available drugs and new potential drug targets. Asian Pac J Trop Med. 2012; 5( 6): 485– 97. [DOI] [PubMed] [Google Scholar]
- 8. Tiuman TS, Santos AO, Ueda-Nakamura T, Filho BP, Nakamura CV. Recent advances in leishmaniasis treatment. Int J Infect Dis. 2011; 15( 8): e525– 32. [DOI] [PubMed] [Google Scholar]
- 9. Croft SL, Seifert K, Yardley V. Current scenario of drug development for leishmaniasis. Indian J Med Res. 2006; 123( 3): 399– 410. [PubMed] [Google Scholar]
- 10. Sundar S, More DK, Singh MK, Singh VP, Sharma S, Makharia A, et al. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin Infect Dis. 2000; 31( 4): 1104– 7. [DOI] [PubMed] [Google Scholar]
- 11. Albakhit S, Khademvatan S, Doudi M, Foroutan-Rad M. Antileishmanial activity of date (Phoenix dactylifera L) fruit and pit extracts in vitro. J Evid Based Complementary Altern Med. 2016 . . 10.1177/2156587216651031. [DOI] [PubMed]
- 12. Foroutan-Rad M, Hazrati Tappeh K, Khademvatan S. Antileishmanial and immunomodulatory activity of Allium sativum (Garlic): a review. J Evid Based Complementary Altern Med. 2015 . . 10.1177/2156587215623126. [DOI] [PMC free article] [PubMed]
- 13. Khademvatan S, Eskandari A, Saki J, Foroutan-Rad M. Cytotoxic activity of Holothuria leucospilota extract against Leishmania infantum in vitro. Adv Pharmacol Sci. 2016; 2016: 8195381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. WHO . A report of the consultation meeting on traditional and modern medicine: harmonizing two approaches, 22–26 November 1999. Beijing, China, West Pacific Region. 2000 . . [Google Scholar]
- 15. Saki J, Khademvatan S, Pazyar N, Eskandari A, Tamoradi A, Nazari P. In vitro activity of Cordia myxa Mucilage extract against Leishmania major and L. infantum promastigotes. Jundishapur J Microbiol. 2015; 8( 3): e19640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yousefi E, Eskandari A, Gharavi MJ, Khademvatan S. In vitro activity and cytotoxicity of Crocus sativus extract against Leihmania major (MRHO/IR/75/ER). Infect Disord Drug Targets. 2014; 14( 1): 56– 60. [DOI] [PubMed] [Google Scholar]
- 17. Sadeghi-Nejad B, Saki J. Effect of aqueous Allium cepa and Ixora brachiata root extract on Leishmania major promastigotes. Jundishapur J Nat Pharm Prod. 2014; 9( 2): e15442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Allahdin S, Khademvatan S, Hashemitabar M, Eskandari A. In vitro activity of Camellia sinensis extracts against L. major and L. infantum promastigotes using the colorometric MTT assay. Urmia Med J. 2014; 25( 10): 893– 900. [Google Scholar]
- 19. Sadeghi-Nejad B, Saki J, Khademvatan S, Nanaei S. In vitro antileishmanial activity of the medicinal plant - Satureja khuzestanica Jamzad. J Med Plant Res. 2011; 5( 24): 5912– 5. [Google Scholar]
- 20. Harsha NS, Kumar G, Al-wesali MS, Rasool ST, Ibrahim FAS. Exploring the anti-leishmanial activity of Phoenix dactylifera (Date palm). Pharmacologyonline. 2009; 3: 791– 9. [Google Scholar]
- 21. Althunibat OY, Hashim RB, Taher M, Daud JM, Ikeda M-A, Zali B. In vitro antioxidant and antiproliferative activities of three Malaysian sea cucumber species. Eur J Sci Res. 2009; 37( 3): 376– 87. [Google Scholar]
- 22. Adibpour N, Nasr F, Nematpour F, Shakouri A, Ameri A. Antibacterial and antifungal activity of Holothuria leucospilota isolated from Persian Gulf and Oman Sea. Jundishapur J Microbiol. 2014; 7( 1): e8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bordbar S, Anwar F, Saari N. High-value components and bioactives from sea cucumbers for functional foods--a review. Mar Drugs. 2011; 9( 10): 1761– 805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kerr AM, Kim J. Phylogeny of Holothuroidea (Echinodermata) inferred from morphology. Zool J Linn Soc. 2001; 133( 1): 63– 81. [Google Scholar]
- 25. Dube A, Singh N, Saxena A, Lakshmi V. Antileishmanial potential of a marine sponge, Haliclona exigua (Kirkpatrick) against experimental visceral leishmaniasis. Parasitol Res. 2007; 101( 2): 317– 24. [DOI] [PubMed] [Google Scholar]
- 26. Ben Kahla-Nakbi A, Haouas N, El Ouaer A, Guerbej H, Ben Mustapha K, Babba H. Screening of antileishmanial activity from marine sponge extracts collected off the Tunisian coast. Parasitol Res. 2010; 106( 6): 1281– 6. [DOI] [PubMed] [Google Scholar]
- 27. Jimenez-Ruiz A, Alzate JF, Macleod ET, Luder CG, Fasel N, Hurd H. Apoptotic markers in protozoan parasites. Parasit Vectors. 2010; 3: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khademvatan S, Saki J, Gharavi MJ, Rahim F. Allium sativum extract induces apoptosis in Leishmania major (MRHO/IR/75/ER) promastigotes. J Med Plant Res. 2011; 5( 16): 3725– 32. [Google Scholar]
- 29. Khademvatan S, Gharavi MJ, Rahim F, Saki J. Miltefosine-induced apoptotic cell death on Leishmania major and L. tropica strains. Korean J Parasitol. 2011; 49( 1): 17– 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Feily A, Saki J, Maraghi S, Moosavi Z, Khademvatan S, Siahpoosh A. In vitro activity of green tea extract against Leishmania major promastigotes. Int J Clin Pharmacol Ther. 2012; 50( 3): 233– 6. [DOI] [PubMed] [Google Scholar]
- 31. Khademvatan S, Adibpour N, Eskandari A, Rezaee S, Hashemitabar M, Rahim F. In silico and in vitro comparative activity of novel experimental derivatives against Leishmania major and Leishmania infantum promastigotes. Exp Parasitol. 2013; 135( 2): 208– 16. [DOI] [PubMed] [Google Scholar]
- 32. Ghaffarifar F, Esavand Heydari F, Dalimi A, Hassan ZM, Delavari M, Mikaeiloo H. Evaluation of apoptotic and antileishmanial activities of Artemisinin on promastigotes and BALB/C mice infected with Leishmania major. Iran J Parasitol. 2015; 10( 2): 258– 67. [PMC free article] [PubMed] [Google Scholar]
- 33. Huerta S, Goulet EJ, Huerta-Yepez S, Livingston EH. Screening and detection of apoptosis. J Surg Res. 2007; 139( 1): 143– 56. [DOI] [PubMed] [Google Scholar]
- 34. Fumarola L, Spinelli R, Brandonisio O. In vitro assays for evaluation of drug activity against Leishmania spp. Res Microbiol. 2004; 155( 4): 224– 30. [DOI] [PubMed] [Google Scholar]
- 35. Sugawara T, Zaima N, Yamamoto A, Sakai S, Noguchi R, Hirata T. Isolation of sphingoid bases of sea cucumber cerebrosides and their cytotoxicity against human colon cancer cells. Biosci Biotechnol Biochem. 2006; 70( 12): 2906– 12. [DOI] [PubMed] [Google Scholar]
- 36. Janakiram NB, Mohammed A, Zhang Y, Choi CI, Woodward C, Collin P, et al. Chemopreventive effects of Frondanol A5, a Cucumaria frondosa extract, against rat colon carcinogenesis and inhibition of human colon cancer cell growth. Cancer Prev Res (Phila). 2010; 3( 1): 82– 91. [DOI] [PubMed] [Google Scholar]
- 37. Fredalina BD, Ridzwan BH, Abidin AA, Kaswandi MA, Zaiton H, Zali I, et al. Fatty acid compositions in local sea cucumber, Stichopus chloronotus, for wound healing. Gen Pharmacol. 1999; 33( 4): 337– 40. [DOI] [PubMed] [Google Scholar]
- 38. Yaacob H, Kim K, Shahimi M, Jamalulail S. Water extract of Stichopus sp. improves wound healing. J Perubatan Univ Kebangsaan Malays. 1994; 16: 19– 29. [Google Scholar]
- 39. San Miguel-Ruiz JE, Garcia-Arraras JE. Common cellular events occur during wound healing and organ regeneration in the sea cucumber Holothuria glaberrima. BMC Dev Biol. 2007; 7( 1): 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhao Q, Wang J-f, Xue Y, Wang Y, Gao S, Lei M, et al. Comparative study on the bioactive components and immune function of three species of sea cucumber. J Fishery Sci China. 2008; 1: 022. [Google Scholar]
- 41. Ogushi M, Yoshie-Stark Y, Suzuki T. Apoptosis-inducing activity of hot water extracts from the sea cucumber in human colon tumor cells. Food Sci Technol Res. 2006; 12( 4): 290– 4. [Google Scholar]
- 42. Kalinin VI, Aminin DL, Avilov SA, Silchenko AS, Stonik VA. Triterpene glycosides from sea cucucmbers (holothurioidea, echinodermata). Biological activities and functions. Stud Nat Prod Chem. 2008; 35: 135– 96. [Google Scholar]
- 43. Zou Z-R, Yi Y-H, Wu H-M, Wu J-H, Liaw CC, Lee K-H. Intercedensides AC, three new cytotoxic triterpene glycosides from the sea cucumber Mensamaria intercedens Lampert. J Nat Prod. 2003; 66( 8): 1055– 60. [DOI] [PubMed] [Google Scholar]
- 44. Singh N, Kumar R, Gupta S, Dube A, Lakshmi V. Antileishmanial activity in vitro and in vivo of constituents of sea cucumber Actinopyga lecanora. Parasitol Res. 2008; 103( 2): 351– 4. [DOI] [PubMed] [Google Scholar]