Abstract
Background:
This study was conducted to collect informative data on the parasitic infection of wild rodents, emphasizing on finding parasites, which have medical importance to human.
Methods:
During 2012–2014, a total number of 91 wild rodents were captured from rural areas of Turkmen Sahra, Golestan Province, using handmade traps. Animals were anesthetized, surveyed for any ectoparasite and then their carcasses were carefully dissected for examination of endoparsites.
Results:
Four species of rodents including Mus musculus (52.75%), Rattus norvegicus (38.46%), Rhombomys opimus (4.40%) and Meriones libycus (4.40%) were captured. Parasitic infestation was detected in 38.5% of sampled rodents. Parasite infestation rates of sampled rodents was Hymenolepis diminuta = 7.7%, Cryptosporidium spp = 6.6%, Trichuris spp.= 5.5%, Cysticercus fasciolaris = 2.20%, Angiostrongylus spp.= 2.20%, Capillaria sp.= 1.09%, Rhipicephalus spp. = 8.70%, Nosopsyllus fasciatus = 1.09%, and Laelaps nuttalli = 3.29%. Among 10 genera/species of identified parasites, at least 8 of them were zoonotic with public health importance. L. nuttalli and N. fasciatus were the only two non-zoonotic detected parasites in this survey.
Conclusion:
Harboring a wide variety of zoonotic parasites in sampled wild rodents particularly when they live nearby villages, represents a potential risk to native inhabitants. Hence, controlling rodents’ population in residential regions and improving awareness of local people about the risk of disease transmission through rodents seems to be entirely necessary.
Keywords: Rodents, Zoonotic parasite, Non-zoonotic parasite, Iran
Introduction
Rodentia is one of the largest order of the mammals, with the highest diversity in mammals’ population (43%). Rodents cause wide variety of disorders with medical and veterinary importance (1). As rodents are highly adaptable to different types of climates, they are widely distributed in most areas in the world except Polar Regions (2).
Rodents harboring ectoparasites and endoparasites play critical role in distribution of various kinds of viruses, bacteria, rickettsia, protozoa and helminthes (3). Many of these agents can cause zoonotic diseases such as plague, tularemia, leishmaniasis, leptospirosis, toxoplasmosis and salmonellosis (4).
Some rodent species such as Rattus norvegicus play more important role in distribution of zoonotic diseases. The close association between commensal rodents and humans especially in rural areas facilitates the spread of zoonotic agents (5). Data about parasite life cycle, reservoirs dispersal and transmission models in each ecosystem are necessary for limitation of zoonotic parasites.
There are some reports on the parasite infestation of rodents in Iran (2, 3). However, regarding the vast extent of the country and variation of geographical conditions many studies are still needed to improve knowledge of parasitic fauna of rodents in different parts of Iran.
This study was conducted to collect some informative data about the parasites of wild rodents in rural areas, where rodents are in close association with human settlements, with emphasis on finding parasites, which have medical importance to human for implementation of any prevention and control measures in Turkmen Sahra.
Materials and Methods
Study area and sampling
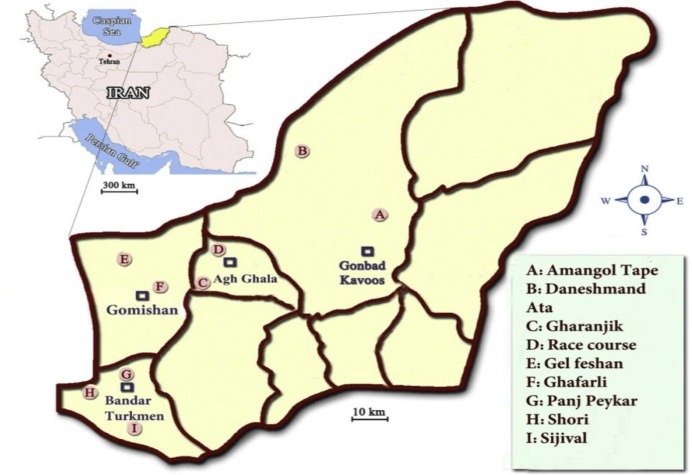
Turkmen Sahra (36° 50′ 21.48″ N, 54° 26′ 39.84″ E) is located in Golestan Province, North-East of Iran. Rodents were captured in 9 villages of 4 towns (located in Turkmen sahra) by handmade traps, and were transported to the laboratory of Gorgan University of environmental resources and natural sciences (Fig. 1).
Fig. 1:
Collection sites around 4 selected cities of Golestan Province, North-East of Iran
Parasitological examination
To follow ethical principles, after euthanizing trapped rodents by ether, their morphological characteristics and sex were registered. The Ethics Committee of the university approved the study.
Using valid identification keys, species of captured rodents were recognized (6). Initially ectoparasites were recovered by combining rodents’ body hair against a water filled white tray. Blood sample was taken immediately from heart of the dead rodent. Afterwards the rodents were cut open and dissected, every internal organ including the lungs, liver, intestines etc., were investigated for macroscopic parasites. All recovered macroscopic parasites were preserved in ethanol 70% for further investigation.
Fecal samples were examined as wet smear after fecal flotation with saturated sodium chloride solution. Thick and thin blood smears were prepared as well as impression smears of spleen, kidney, lung and liver. The prepared slides were stained with Geimsa and examined microscopically with suitable magnification power (100×–1000×) (7, 8). Smears were prepared from intestinal contents and stained with Modified Ziehl-Neelsen (MZN) for detection of acid-fast organisms like Cryptosporidium.
After identification of parasites, statistical analysis was performed using SPSS version 18 (Chicago, IL, USA) software.
Results
Ninety-one rodents including Mus musculus (52.74%), Rattus norvegicus (38.46%), Rhombomys opimus (4.40%) and Meriones libycus (4.40%), were captured in this study. 60.43% of sampled rodents were male and 39.57% were female. Parasite infestation, including endoparasites and ectoparasites was detected in 38.5% of sampled rodents (Table 1). Parasite infestation of male rodents (43.63%) was higher than female rodents (30.55%).
Table 1:
Detected endoparasites and ectoparasites in the sampled rodents species
| Parasite | Non-Zoonotic | Zoonotic | Infested species | Percent of infested samples (%) |
|---|---|---|---|---|
| Endoparasites | -0- | Cysticercusfasciolaris | R.norvegicus (n=2) | 2.2 |
| Hymenolepis diminuta | R. norvegicus (n=7) | 7.6 | ||
| Angiostrongylus spp | M. musculus (n= 2) | 2.2 | ||
| Capillaria sp | M. musculus (n=1) | 1.9 | ||
| Cryptosporidium spp | R. norvegicus (n=6) | 6.5 | ||
| Trichiuris spp | R.norvegicus(n= 4) | 5.4 | ||
| M. libycus (n= 1) | 4.5 | |||
| Ectoparasites | Nosopsyllusfasciatus | M musculus (n= 1) | 1.09 | |
| Rhipicephalus spp | R. norvegicus (n=7) | 8.7 | ||
| M. musculus (n=1) | ||||
| Lealaps nuttalli | M musculus (n=3) | 3.2 |
Zoonotic parasites/ Detected Zoonotic endoparasites
Totally, 25.30% (23/91) of the sampled population were infested with endoparasites which all of them have been introduced as zoonotic parasites. Detected endoparasites comprised of 5 species of helminths (Cysticercus fasciolaris 2/91 (2.2%), Hymenolepis diminuta 7/91 (7.6%) (Fig. 2), Angiostrongylus spp 2/91 (2.2%) (Fig. 3). Capillaria sp 1/91 (1.09%) (Fig. 4), Trichiuris spp 5/91 (5.4%) (Fig. 5) and 2 species of protozoan parasites (Cryptosporidium spp 6/91 (6.5%) C. fasciolaris was detected macroscopically in the liver tissues of two R. norvegicus while eggs of other identified helminthic parasites and the protozoans were observed during microscopic examination of the tissues, fecal wet and stained smears. Trichuris spp. nematode was macroscopically noted and separated from intestine of four R. norvegicus and one M. libycus, Capillaria spp. and Trichuris eggs were differentiated based on egg morphology as described by Baker (2007) (7). The larval stage of Angiostrongylus was found in the lung impression smears of two M. musculus. Cryptosporidium species were identified based on the oocyst size as oocysts of C. muris are larger than C. Parvum oocysts (7). Examination of prepared blood smears did not show any blood parasites in sampled rodents.
Fig. 2:
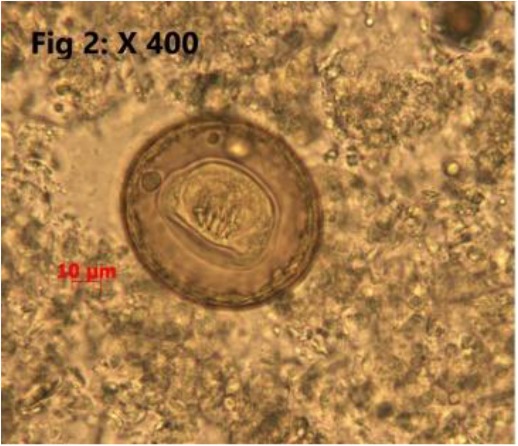
Hymenolepis diminuta
Fig. 3:
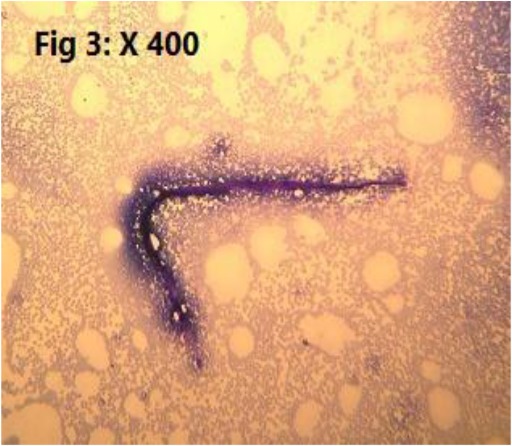
Angiostrongylus spin impression smear of Lung
Fig. 4:
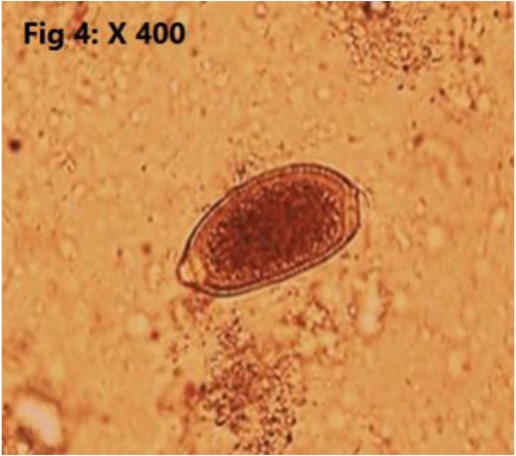
Eggs of Capillaria sp. in wet smear
Fig. 5:
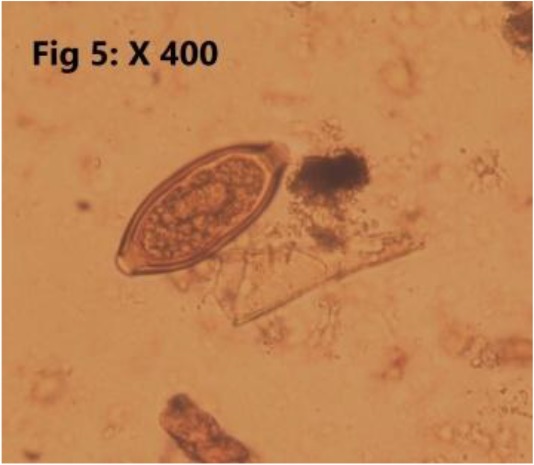
Trichiuris spp.
Detected zoonotic ectoparasites
Rhipicephalus spp. was isolated from a M. musculus (1.09%) and seven R. norvegicus (7.6%). Nosopsyllus fasciatus (Fig. 6) was detected in a M. musculus (1.09%) (Table 1).
Fig. 6:
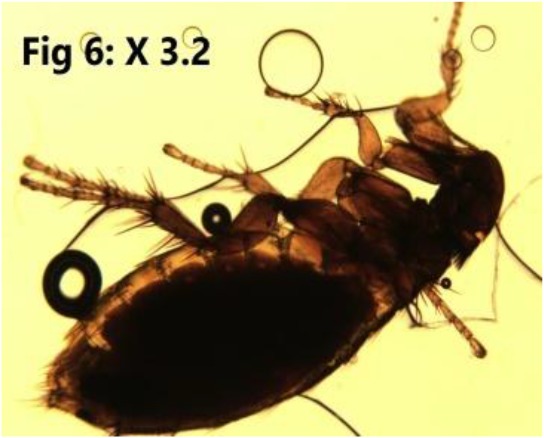
Nosopsyllus fasciatus
Non-zoonotic
Laelaps nuttalli (Fig. 7) was the only nonzoonotic ectoparasite which has been detected in three (2%) M. musculus (Table 1).
Fig. 7:
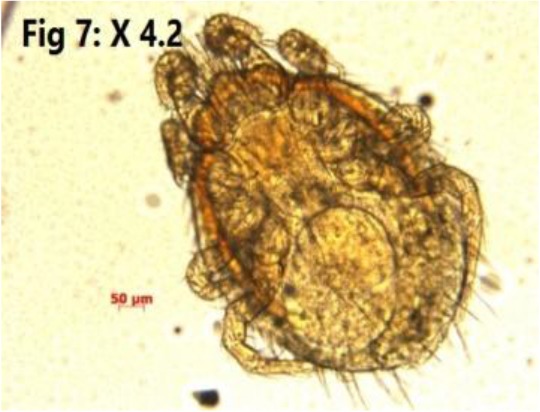
Laelaps nuttalli
Parasite infestation of sampled rodents according to the region and rodent species
Most parasite infestation of sampled rodents was detected in Bandar Turkmen (28.57%) and the lowest was found in Gonbad (5.5%). Parasite infestation of R. norvegicus (28.5%) was higher than other sampled species (Table 2).
Table 2:
Frequency of detected parasites according to the species and location
|
Host Species Location |
R. opiums No. infected/no. sampled (%) | M. musculus (Angiostrongylus spp., Capillaria sp., Rhipicephalus spp., L. nuttalli) No. infected/no. sampled (%) | M. libycus (Trichiuris spp.) No. infected/no. sampled (%) | R. norvegicus (H. diminuta, Trichiuris spp., C. fasciolaris, Cryptosporidium spp, Rhipicephalus spp.,N. fasciatus) No. infected/no. sampled (%) |
|---|---|---|---|---|
| Gomishan | - | 26.66 | -0- | 100 |
| Bandar Turkmen | - | 18.75 | - | 91.66 |
| Gonbad | -0- | -0- | 100 | 0 |
| Agh Ghala | - | - | - | 80 |
| Total frequency of parasite infection | 0/4(0) | 18.75 | 25 | 28.5 |
Discussion
In the present study, 91 rodents belonging to one subfamily and four species (M. musculus 52.74%, R. norvegicus 38.46%, R. opimus 4.40%, and M. libycus 4.40%) were sampled from Turkmen Sahra. Predominance of M. Musculus and R. norvegicus was expected regarding to their living habits, dispersal in majority of regions, and adaption to all habitats (9). In agreement with the present study, M. Musculus and R. norvegicus were predominant species (10), whereas Rasti et al. reported that Great gerbil (25.8%) and Libyan jird (24.2%) were predominant species (11). Besides, Milazzo et al. detected 73 M. musculusand 40 R. rattus in Italy (12).
In this work, 60.43% (55 mice) of the rodents were male. The highest amount of infestation with intestinal parasites was seen in males, although no significant difference was seen between female and male mice (P>0.05). This might be owing to more activity or higher number of the males. The highest parasite infestation rate was detected in sampled rodents of Bandar Turkmen (50%) and the lowest was found in Gonbad (5.5%). Higher parasite infestation rate in Bandar Turkmen can be caused by higher pollution in shores as well as number of sampled rodents in this region compared to other regions.
In a study in Kashan, the zoonotic parasites H. nana fraterna (10.8%), H. diminuta (4.2%), and Trichuris muris (1.7%) were identified in 120 rodents including Meriones persicus, M. libycus, Gerbillus nanus, M. musculus, R. norvegicus, R. rattus and Jaculus blanfordi (11).
In a research on 90 rodents including R. norvegicus, R. rattus, and M. musculus in Ahvaz, the parasites Trypanosoma lewisi, Trichosomoides crassicauda, Gongylonema monigi, Streptopharagus kuntzi, and Rictularia ratti were identified; none of these parasites resembled the ones found in the present study (15). This might be explained by difference in geographical situations and climates of Ahvaz and Turkmen Sahra.
The most commonly recognized zoonotic parasite in the present study was H. diminuta (rat tapeworm) with the highest infestation rate in R. norvegicus. This is consistent with the results of Kia et al. in Ardebil and Rokni in Hamedan (13, 14). Several reports of infestation with this parasite exist in human populations of Iran and the highest outbreak of human infestation with this parasite was reported in rural area of Minab (15). In Tabriz, 24.47% of sampled rodents (56 M. Musculus, 38 R. norvegicus, and 26 Cricetulus migratorius) were infested by this zoonotic parasite (10). 4.2% of sampled rodents in Kashan were also infested by this parasite (11). In Mazandaran, 15% of the sampled rodents including M. musculus, R. rattus, R. norvegicus, Glisglis, Apodemus witherby, Tatera indica and Microtusaruvalis were infested with H. diminuta (16).
Infestation with C. parvum and C. muris was also detected in 6 R. norvegicus (6.6%). Different kinds of rodents, especially those in close contact with humans such as M. musculus and R. norvegicus, were identified as main sources of this parasite (17). Although this single-celled organism has not been regarded as a zoonosis from rodent origin, few cases of human infestation have been reported in recent years and it is known as a parasite causing diarrhea in humans (18,19). Infestation with different genotypes of C. parvum has been reported in M. musculus and R. norvegicus (18, 20). In Tehran, 13% of the samples in dye method and 27% of them in molecular method were infected with C. parvum (19). Therefore, it seems that more comprehensive studies via sensitive molecular methods are necessary to determine the role of rodents in dispersion and transmission of this parasite.
In the present study, the zoonotic parasite Trichuris spp. was detected and separated from intestine of four R. norvegicus and one M. libycus, but the species were not further identified. In a study on 77 R. opimus in the eastern Turkmen Sahra (Maraveh Tappeh) in 2014, infestation with the same parasite with similar frequency (5.5%) was reported. The role of climate in achieving the same results in similar regions is inevitable. It is noteworthy that the species of Hymenolepis was H. nana whereas here, it was H. diminuta (21, 22). Infestation with H. diminuta, T. muris, and C. hepatica in the species M. musculus and R. rattus has been reported in Thailand (22).
The eggs of Capillaria sp. were detected in the feces of sampled M. musculus. This parasite is abundantly identified in rodents in Iran. For instance, Capillaria spp was identified in 13% of M. Musculus and R. norvegicus in Kermanshah (2013) (23).
Infestation with external zoonotic parasites, Rhipicephalus spp. was detected in one M. musculus and seven R. norvegicus. Besides, Nosopsyllus fasciatus was detected in one M. musculus. Rhipicephalus spp. takes part in transmission of zoonotic diseases such as Crimean Congo Haemorrhagic Fever (CCHF), Babesiamicroti, Q fever and some cases of Rickettsiosis (25). Similarly, two zoonotic parasites N. fasciatus (47.22%) and R. sanguineus (13.88%) were detected in rodents of Tabriz (10). Additionally, infestation with N. fasciatus, a potential vector of plagues and rat typhus, was found in the rodents caught in Mazandaran Province (26, 27).
Lealaps nuttali was the only non-zoonotic ectoparasites detected in three M. Musculus. In Thailand, Laelaps nuttali and Laelaps echidninus were identified in rodents (Bandicota indica, Bandicota savilei, Rattus losea, R. rattus, Rattus exulans, R. norvegicus, Menetes berdmorei and Tamiops mcclellandii) (22). In Bandar Abbas, L. nuttali was the only non-zoonotic parasite in rodents and only M. Musculus was infested with this parasite (27). Infection of M. Musculus with L. nuttali indicates the susceptibility of this species to this parasite.
Conclusion
Considering the infestation of sampled rodents with various zoonotic parasites, especially H. diminuta and Cryptosporidium spp, it is strongly recommended to take measures in order to control rodent populations in the mentioned regions of current study and make local people aware of the risk of disease transmission to humans through rodents.
Acknowledgments
The authors would like to thank Dr. Hosein Varasteh Moradi for his useful cooperation, Mr Hamed Mirrezaei for laboratory support and Mr. Mohammad Hosein Ayoubi Joshaghani and Mr. Erfan Asgari for edition of the paper. This study was financially supported by Gorgan University of Agricultural Sciences & Natural Resources. The authors declare that there is no conflict of interests.
References
- 1. Azizi K, Davari B, Kalantari M, Fekri S. Gerbillid Rodents Fauna (Muridae: Gerbillinae) and detection of reservoir hosts(s) of Zoonotic Cutaneous Leishmaniasis using a Nested-PCR technique in Jask City in Hormozgan Province. Sci JKurdistan Univ Med Sci. 2011; 16( 2): 66– 76. [Google Scholar]
- 2. Khaghani R. The economic and health impact of rodent in urban zone and harbors and their control method. J Artesh Univ Med Sci. 2007; 4( 4): 1071– 1078. [Google Scholar]
- 3. Rasti S, Moubedi I, Dehghani R, Dorodgar A. A case report on Meggitina gerbilli in Iran. Feyz. 2001; 5( 17): 88– 92. [Google Scholar]
- 4. Dehghani R. Health pests and Safe Control Methods of Them: 1st ed: Publications of Farmaneshand Kashan University of Medical Sciences; 2011 . . p. 303 –386.
- 5. Hamedi E, Heydari M, Soleymani A. Intestinal parasites and blood Rattus norvegicus Bandar Abbas. Bimonthly Journal of Hormozgan University of Medical 2003 . ; 7 ( 3 ): 123 –127. [In Persian] [Google Scholar]
- 6. Eatemad A. Mammals of Iran The first volume of rodents and their diagnostic. Tehran. Publication of the National Association of Protection of Natural Resources and Human Environment. 1978. 288 [In Persian] [Google Scholar]
- 7. David G, Baker (Editor). Flynn’s Parasites of Laboratory Animals, 2nd Edition 840 pages, Wiley-Blackwell ; . 2007 . . [Google Scholar]
- 8. Taylor MA, Coop RL, Wall RL. Veterinary Parasitology, 3rd Edition 600 pages, Wiley-Blackwell ; . 2007 . . [Google Scholar]
- 9. Sedaghat MM, Salahi Moghaddam A. Mapping the distribution of the important rodents reservoir in Iran. J Artesh Univ Med Sci. 2010; 8( 3): 210– 223. [Google Scholar]
- 10. Moghadam GH, Jamali R, Niyaz Pour F, Nematolahi A. Study of helminth parasites and rodents ectoparasites the city of Tabriz. Journal of Veterinary Research (Tehran University) 2006 . ; 61 ( 3 ): 265 –268. [In Persian] [Google Scholar]
- 11. Rasti S, Moubedi I, Dehghani R, Dorodgar A, Arbabi M. Epidemiology and domestic rat intestinal parasites in Kashan city. Bimonthly Journal of Kashan University of Medical Sciences 1998 . ; 20 : 102 –108. [In Persian] [Google Scholar]
- 12. Milazzo C, Cagnin M, Bella CD, Geraci F., Ribas A. Helminth Fauna of Commensal Rodents, Mus musculus (Linnaeus, 1758) and Rattus rattus (Linnaeus, 1758) (Rodentia, Muridae) in Sicily (Italy). Rev. Ibero-Latinoam. Parasitol. 2010; 69 ( 2): 194– 198. [Google Scholar]
- 13. Kia EB, Shahryary-Rad E, Mohebali M, Mahmoudi M, Mobedi I, et al. Endoparasites of Rodents and Their Zoonotic Importance in Germi, Dashte–Mogan, Ardabil Province, Iran. Iran J Parasitol. 2010; 5( 4): 15– 20. [PMC free article] [PubMed] [Google Scholar]
- 14. Rokni MB. The present status of human helminthic diseases in Iran. Ann Trop Med Parasitol. 2008; 102( 4): 283– 295. [DOI] [PubMed] [Google Scholar]
- 15. Kia EB, Homayouni MM, Farahnak A, Mohebali M, Shojai S. Study of Endoparasites of Rodents and their Zoonotic Importance In Ahvaz, South West Iran. Iran J Public Health. 2001; 30( 1–2): 49– 52. [Google Scholar]
- 16. Gholami Sh, Motevalli-Haghi F, Moabedi I, Shahabi S. Study of helmintic intestinal parasites in the rodents from the rural and central regions of Mazandaran province in the years 1997 to 1999.. Journal of Mazandaran University of Medical Sciences. 2002 . ; 12 ( 35 ): 67 –73[In Persian] [Google Scholar]
- 17. Bahrami F, Sadraei J, Frozandeh M. Molecular Characterization of Cryptosporidium spp. in WildRats of Tehran, Iran Using 18s rRNA Gene and PCR RFLP Method. Jundishapur J Microbiol. 2012; 5( 3): 486– 90. [Google Scholar]
- 18. Kváč M, Kestřánová M, Květoňová D, Kotková M, Ortega Y, et al. Cryptosporidium tyzzeri and Cryptosporidium muris originated from wild West-European house mice (Mus musculus domesticus) and East-European house mice (Mus musculus musculus) are non-infectious for pigs. Exp Parasitol. 2012; 131( 1): 107– 110. [DOI] [PubMed] [Google Scholar]
- 19. Feng Y, Yang W, Ryan U, Zhang L, Kváč M, et al. Development of a multilocus sequence tool for typing Cryptosporidium muris and Cryptosporidium andersoni. J Clin Microbiol. 2011; 49( 1): 34– 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ng-Hublin JS, Singleton GR, Ryan U. Molecular characterization of Cryptosporidium spp. from wild rats and mice from rural communities in the Philippines. Infect Genet Evol. 2013; 16: 5– 12. [DOI] [PubMed] [Google Scholar]
- 21. Kamranrashani B, Kia EB, Mobedi I, Mohebali M, Zarei Z, et al. Helminth Parasites of Rhombomys opimus from GolestanProvince, Northeast Iran. Iran J Parasitol. 2013; 8( 1): 78– 84. [PMC free article] [PubMed] [Google Scholar]
- 22. Changbunjong T, Weluwanarak T, Chamsai T, Sedwisai P, Ngamloephochit S, et al. Occurrence of Ectoparasites on Rodents in Sukhothai Province, Northern Thailand. Southeast Asian J Trop Med Public Health. 2010; 41( 6): 1324– 1330. [PubMed] [Google Scholar]
- 23. Pakdel N, Naem S, Rezaei F, Chalehchaleh A. A survey on helminthic infection in mice (Mus musculus) and rats (Rattus norvegicus and Rattus rattus) in Kermanshah, Iran. Vet Res Forum. 2013; 4( 2): 105– 109. [PMC free article] [PubMed] [Google Scholar]
- 24. Nourollahi-Fard SR, Khalili M. PCR-Detection of Coxiella burnetii in Ticks Collected from Sheep and Goats in Southeast Iran. Iran J Arthropod Borne Dis. 2011; 5( 1): 1– 6. [PMC free article] [PubMed] [Google Scholar]
- 25. School of public Health and Institute of public Health research Geographical pathology of Iran: Publications of University of Tehran ; 1970 . .
- 26. Motevalli-Haghi F, Golami Sh, Sharif M, Moabedi I, Sobhani Z, et al. Study of rodents ectoparasites in urban areas of Mazandaran province. Iran J Mazandaran Uni Med Sci. 2002; 36( 12): 77– 78. [Google Scholar]
- 27. Kia EB, Moghddas-Sani H, Hassanpoor H, Vatandoost H, Zahabiun F, et al. Ectoparasites of Rodents Captured in Bandar Abbas, Southern Iran. Iran J Arthropod-Borne Dis. 2009; 3( 2): 44– 49. [PMC free article] [PubMed] [Google Scholar]