Abstract
Background:
Aurones are naturally occurring compounds that belong to flavenoids family and have antiplasmodial effects. This study investigated some new aurones derivatives against chloroquine sensitive Plasmodium falciparum. Here we report the synthesis, in vitro antiplasmodial activity and cytotoxic evaluation of 11 compound from derivatives of (Z)-2- benzylidene-4, 6-dimethoxybenzofuran-3(2H)-one.
Methods:
The cytotoxic evaluations of active compounds were performed with MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5 diphenyltetrazolium bromide) assay on human breast cancer cell lines; MCF7 and T47D.
Results:
From 11 compounds M3, M6 and M7 compounds showed good anti-plasmodial effect against chloroquine-sensitive 3D strain of P. falciparum with IC50 (50% inhibitory concentration) values of 7.82, 7.27 and 2.3 μM respectively. No noticeable toxicity was observed with these compounds when tested against tested cell lines.
Conclusion:
The replacement of the 4 and 5 positions at ring B of aurone derivatives, with propoxy and bromide (Br) respectively was revealed highly advantageous for their antiplasmodial effect.
Keywords: Plasmodium falciparum, Aurones, Cytotoxicity
Introduction
Malaria is an infectious disease that caused by Plasmodium species (1). Plasmodium falciparum, P. vivax, P. malarie, P. ovale, and P. knowlesi have been known as causes of human malaria (2–3). The most severe and deadly form of disease is caused by P. falciparum subspecies that causes the death of 1% of patients (2). According to WHO Malaria Report 2012, there was an approximately 219 million cases of malaria all around the world and 660,000 people died from this disease (4).
Nowadays after AIDS and tuberculosis, malaria is the most important human diseases. Unfortunately, despite using different methods for eradication or control of malaria, this disease is endemic in many tropical and subtropical countries (about 104 countries worldwide). Developing and spreading multidrug-resistant strains of P. falciparum, developing resistance in malaria vectors to the usual effective insecticides and unsuccessfulness in developing an effective and inexpensive vaccine are the most important reasons of failure in malaria eradication or control programs (4).
Today artemisinin-based combination therapy is the last defense against the malaria but there are some reports about the resistance against atremisinin and its derivatives (5). Furthermore, these drugs are too expensive for widespread use (6). Therefore, we need effective and inexpensive drugs to replace chloroquine, which was the main stay of chemotherapy of malaria for more than 50 years.
Aurones (2-benzylidenbenzofuran-3(2H)-ones), are secondary metabolite belonging to the flavonoids family and have an important role for the pigmentation of flowers in which they are found (7). They are structural isomers of flavones and there are reports about their inhibitory effects on erythrocytic stages of P. falciparum strains in vitro (8–10). Other effects of aurones are antileshmanial (11–12), trypanocidal (13), antiviral, and antifungal effects (7).
The aim of this study was to investigate aurones analogs in order to find new drug candidates against malaria.
Materials and Methods
Chemicals
Reagents and materials obtained from Merck (Darmstadt, Germany) and Sigma Aldrich (Steinheim, Germany), and DMSO was purchased from Fluka (Steinheim, Germany) and RPMI 1640 medium from Gibco-Invitrogen (Paisley, Scotland, UK).
Tested compounds
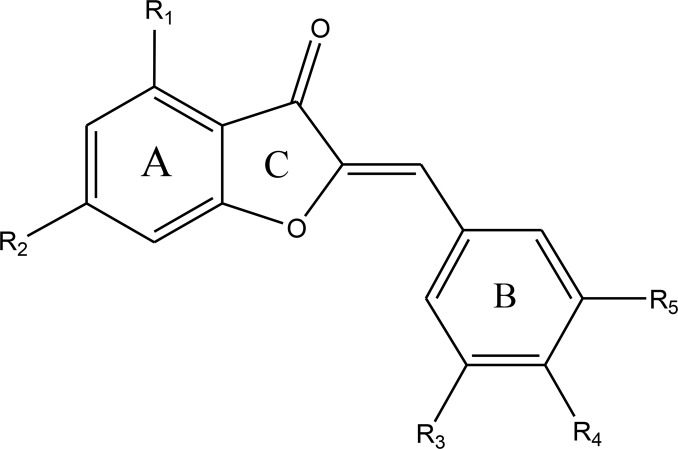
Synthesis of these compounds has been described previously (10, 14–15). The basic structure of aurones derivatives is indicated in Fig. 1. The structures of synthesized aurones have been shown in Table 1.
Fig. 1:
Basic structures of aurones derivatives
Table 1:
the structure of synthesized aurones derivatives
| Compound | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|
| M1 | OMe | OMe | OMe | OMe | Br |
| M2 | OMe | OMe | OMe | OMe | OMe |
| M3 | OMe | OMe | - | OMe | OMe |
| M4 | OMe | OMe | OMe | OH | Cl |
| M5 | OMe | OMe | OMe | OH | Br |
| M6 | OMe | OMe | OMe | OEt | Br |
| M7 | OMe | OMe | OMe | OPr | Br |
| M8 | - | - | OMe | OMe | Cl |
| M9 | - | - | OMe | OMe | Br |
| M10 | - | - | OMe | OH | Cl |
| M11 | - | - | OMe | OH | Br |
Parasite Culture
The P. falciparum strain used in this study was 3D7 chloroquine (CQ) sensitive. In vitro culture of P. falciparum was carried out according to the method described by Trager and Jensen with some modifications (16–18). Parasites were maintained in continuous culture on human erythrocytes (blood group O+), provided by the Blood Transfusion Organization (Zanjan, Iran), in RPMI 1640 medium with 5% of human AB+ serum, 0.3 g/100 mL Albumax I, 25 mM HEPES, 19 mM sodium carbonate and 30 μg/mL gentamicin sulfate at pH 7.2. The growth medium was replaced daily, and cultures were gassed with a mixture of 91% N2, 6% CO2, and 3% O2. Synchronization to the ring stage was achieved by sorbitol method (19).
In vitro antimalarial tests
Compounds were prepared in DMSO at concentration of 10 mg/mL and serially diluted with culture medium to reach 1mg/mL before use. Twenty μL of 2-fold dilution series (50 – 0.3906 μg/mL) of compounds prepared in assay medium and added to each well of 96-well plates in triplicate. One hundred eighty μL of synchronous P. falciparum culture (1% parasitemia and 2% hematocrit) added to each well reaching a final volume of 200 μL per well. Plates were incubated at 37° C for 24 h. Chloroquine was used as positive control and parasitized erythrocytes without drug were used as negative control. After 24 h incubation, Giemsa stained thin smears were made and parasitemia was confirmed by the numeration of 1000 erythrocytes (20). Data were imported in SPSS 16.0 software and IC50 values were calculated by means of Finney’s Probit analysis (21).
In vitro cytotoxicity assay
The toxic effects of active compounds against P. falciparum were assessed on human breast cancer cell lines (MCF7 and T47D) by using MTT (3–[4, 5-dimethylthiazol-2-yl] -2, 5 diphenyltetrazolium bromide) assay (22–23) and results were compared with untreated control. The cells were cultured in RPMI 1640 medium containing 10% FBS (Fetal Bovine Serum) and incubated at 37° C with 5% CO2 and 96% humidity. After several subcultures, cells were distributed in 96-well plates at 1,000 cells in 100 μL of culture medium and incubated for 24 h at same condition to allow attachment of cells to the bottom of wells. Then culture medium removed and 100 μL of the same medium containing the drugs at various concentrations (100, 30, 10, 3, 1, 0.1 μM) added to each well in triplicate. Plates further incubated for 5 days in same condition. The last column of plate containing 1000 cells in100 μL of culture medium was regarded as control. After 5 days incubation the drug-containing medium discharged. For evaluation of cell survival, 25 μL of MTT solution (4 mg/mL in PBS) added to each well and plates incubated for 3 h (in same condition). Then 100 μL of DMSO added to each well and plates were gently shaken to dissolve the formed formazan crystals. The absorbance of each well measured at 540 nm using an ELISA plate reader (Infinite M200, Tecan). The GI% (Growth Inhibition percent) was calculated using the formula; %Growth Inhibition = 100 − (ODtest − ODcontrol) × 100, where ODtest is the mean absorbance of treated cells and ODcontrol is the mean absorbance of a negative control. The cell survival of control assumed 100% and IC50values generated from dose-response curves for each cell line.
Results
In vitro antiplasmodial activity
All the 11 compounds screened for in vitro antiplasmodial activity against the CQ-sensitive (3D7) P. falciparum strain (Table 2). Of 11 compounds tested, 3 compounds had better antiplasmodial activity. Compound M7 showed significant antiplasmodial activity with IC50 value of 2.3 μM. Compounds M3 and M6 also showed good antiplasmodial activity with IC50 values of 7.82 and 7.27 μM respectively. The rest of compounds did not show noticeable antiplasmodial activity.
Table 2:
Antiplasmodial activity and toxicity assessment of tested compounds
| Compound | P. falciparum IC50 (μM) | MCF7 IC50 (μM) | T47D IC50 (μM) | SI for MCF7 | SI for T47D |
|---|---|---|---|---|---|
| M1 | 22.29 | 36.34 | 28.85 | 1.63 | 1.29 |
| M2 | ≥50 | - | - | - | - |
| M3 | 7.82 | 20.80 | 3.15 | 2.65 | 0.4 |
| M4 | ≥50 | - | - | - | - |
| M5 | 28.64 | 34.75 | 19.39 | 1.21 | 1.21 |
| M6 | 7.27 | 29.90 | 9.35 | 4.11 | 1.28 |
| M7 | 2.3 | 17.19 | 11.65 | 7.47 | 5.06 |
| M8 | 22.04 | 20.60 | 9.07 | 0.93 | 0.41 |
| M9 | 32.32 | 34.99 | 29.47 | 1.08 | 0.91 |
| M10 | 36.38 | 20.27 | 11.72 | 0.55 | 0.55 |
| M11 | ≥50 | - | - | - | - |
| CQ | 0.775 | - | - | - | - |
In vitro cytotoxicity assay
Cytotxicity of compounds with IC50 value less than 50 μM assessed on MCF7 and T47D cell lines. Results of toxicity activity of the tested compounds and selectivity index (SI) are shown in Table 2. The SI is defined as the ratio of the toxicity to the antiplasmodial activity and the higher selectivity should offer the potential of safer therapy.
The M7 compound had high selectivity for P. falciparum than studied cell lines in comparison with other compounds (P = 0.008).
Discussion
Several studies have been done concerning the aurones as chemotherapeutic antimalarial agents (8–10). Aurones [2-benzylidenebenzofuran-3(2H)-ones] are natural compounds belong to the flavonoids family. The natural entity of aurones, their inhibitory activity on Leishmania and malaria parasites (8, 24–25), has prompted us to investigate aurones analogs in order to gather more structural elements required for the antiplasmodial activity.
Three out of 11 compounds (M3, M6 and M7) showed good antiplasmodial activity with IC50 values of 7.82, 7.27 and 2.3 μM against 3D7 starin of P. falciparum respectively. The M7 compound had better selectivity index than other two active compound (with SI value of 7.47 and 5.06 for MCF7 and T47D cell lines respectivly). This let us believe that activity obtained with this compound is not due to general toxicity against P. falciparum but can be explained by specific anti-plasmodial activity andshould offer potential for safer therapy.
In one study a library comprising 44 different substituted aurones derivatives was synthesized and screened against the chloroquine resistant P. falciparum W2 strain and demonstrated that some compounds showed anti-plasmodial activity in micromolar range (10). “A series of naturally occurring aurones was synthesized and evaluated for their ability to inhibit erythrocytic stages of P. falciparum strains in vitro” (8). The most active compound was 4,6,4′-triacetyl-3′,5′-dimethoxy-2-aurone with IC50 values of 0.007 μM and 0.18 μM for the P. falciparum strains K1 and NF54, respectively.
According to previous investigations, the structure activity relationship confirmed that methoxylation at 4 and 6 carbons were beneficial for antiplasmodial activity of aurones derivatives (9). In our study, these substitutions were confirmed (compound M1 versus M9 and M5 versus M11). This substitution pattern involved in binding of aurones derivatives to protozoal target protein ATP-binding cassette (ABC) transporters like P-glycoprotein (26–27). At the ring B, presence of a hydrophobic group was highly beneficial for antiplasmodial effect as illustrated by comparing derivatives M6 versus M5 and M9 versus M11. The elongation of chain from methoxy to ethoxy and then to propoxy leads to an increase in anti-plasmodial effect (compound M1 versus M6 and M7). The presence of a hydrophobic halogen atom at the ring B at 5 positions was quite beneficial as illustrated by comparing derivatives M1 and M2. In conclusion, throughout the present study, we report the preliminary results regarding the structural requirements for the antiplasmodial activity of aurones. We have found that the replacement of the 4 and 5 positions at ring B with propoxyand bromide (Br) respectively was revealed to be highly advantageous for the activity. We also optimized (at least in part) the nature of substituents to be present at the A and B-rings.
Conclusion
The present results bring essential elements, which will be used, for the synthesis of more active aurones against malaria parasite. Further studies need to confirm the in vivo antiplasmodial properties of active aurones derivatives against rodent malaria.
Acknowledgments
We thank the Research Deputy of Zanjan University of Medical Sciences (Pharm. D. thesis) and Iran National Science Foundation (INSF) for financial support of this project. The authors declare that there is no conflict of interest.
References
- 1. Harrison TR, Adams RD, Bennett IL, Jr, ResnIk WH, Thorn GW, Wintrobe M. Principles of internal medicine. Academic Medicine; 2012. p. 1246. [Google Scholar]
- 2. de Oliveira RB, de Souza-Fagundes EM, Soares RP, Andrade AA, Krettli AU, Zani CL. Synthesis and antimalarial activity of semicarbazone and thiosemicarbazone derivatives. Eur J Med Chem. 2008; 43( 9): 1983– 8. [DOI] [PubMed] [Google Scholar]
- 3. Singh B, Daneshvar C. Human infections and detection of Plasmodium knowlesi. Clin Microbiol Rev. 2013; 26( 2): 165– 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO World Malaria Report 2012. World Health Organization; 2012; Available from: http://www.who.int/malaria/publications/world_malaria_report_2012/en/. [Google Scholar]
- 5. Alker AP, Lim P, Sem R, Shah NK, Yi P, Bouth DM, et al. Pfmdr1 and in vivo resistance to artesunate-mefloquine in falciparum malaria on the Cambodian-Thai border. Am J Trop Med Hyg. 2007; 76( 4): 641– 7. [PubMed] [Google Scholar]
- 6. Mutabingwa TK. Artemisinin-based combination therapies (ACTs): best hope for malaria treatment but inaccessible to the needy! Acta Trop. 2005; 95( 3): 305– 15. [DOI] [PubMed] [Google Scholar]
- 7. Boumendjel A. Aurones: a subclass of flavones with promising biological potential. Curr Med Chem. 2003; 10( 23): 2621– 30. [DOI] [PubMed] [Google Scholar]
- 8. Kayser O, Kiderlen AF, Brun R. In vitro activity of aurones against Plasmodium falciparum strains K1 and NF54. Planta Med. 2001; 67( 8): 718– 21. [DOI] [PubMed] [Google Scholar]
- 9. Souard F, Okombi S, Beney C, Chevalley S, Valentin A, Boumendjel A. 1-Azaaurones derived from the naturally occuring auounes as potential antimalarial drugs. Bioorg Med Chem. 2010; 18( 15): 5724– 31. [DOI] [PubMed] [Google Scholar]
- 10. Carrasco MP, Newton AS, Goncalves L, Gois A, Machado M, Gut J, et al. Probing the aurone scaffold against Plasmodium falciparum: design, synthesis and antimalarial activity. Eur J Med Chem. 2014; 80: 523– 34. [DOI] [PubMed] [Google Scholar]
- 11. Kayser O, Kiderlen A, Folkens U, Kolodziej H. In vitro leishmanicidal activity of aurones. Planta Med. 1999; 65( 4): 316– 9. [DOI] [PubMed] [Google Scholar]
- 12. Kayser O, Kiderlen A, Croft S. Natural products as antiparasitic drugs. Parasitol Res. 2003; 90( 2): S55– 62. [DOI] [PubMed] [Google Scholar]
- 13. Ameta K, Rathore NS, Kumar B, Verastegui M, Gilman RH, Verma B. Synthesis and trypanocidal evaluation of some novel 2-(substituted benzylidene)-5, 7-dibromo-6-hydroxy-1-benzofuran-3 (2H)-ones. Int J Org Chem. 2012; 2: 295– 301. [Google Scholar]
- 14. Zhang M, Xu XH, Cui Y, Xie LG, Kong CH. Synthesis and herbicidal potential of substituted aurones. Pest Manag Sci. 2012; 68( 11): 1512– 22. [DOI] [PubMed] [Google Scholar]
- 15. Lawrence NJ, Rennison D, McGown AT, Hadfield JA. The total synthesis of an aurone isolated from Uvaria hamiltonii: aurones and flavones as anticancer agents. Bioorg Med Chem Lett. 2003; 13( 21): 3759– 63. [DOI] [PubMed] [Google Scholar]
- 16. Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976; 193( 4254): 673– 5. [DOI] [PubMed] [Google Scholar]
- 17. Ramazani A, Sardari S, Zakeri S, Vaziri B. In vitro antiplasmodial and phytochemical study of five Artemisia species from Iran and in vivo activity of two species. Parasitol Res. 2010; 107( 3): 593– 9 [DOI] [PubMed] [Google Scholar]
- 18. Ramazani A, Zakeri S, Sardari S, Khodakarim N, Djadidt ND. In vitro and in vivo anti-malarial activity of Boerhavia elegans and Solanum surattense. Malar J. 2010; 9: 124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979; 65( 3): 418– 20. [PubMed] [Google Scholar]
- 20. Sangian H, Faramarzi H, Yazdinezhad A, Mousavi SJ, Zamani Z, Noubarani M, et al. Antiplasmodial activity of ethanolic extracts of some selected medicinal plants from the northwest of Iran. Parasitol Res. 2013; 112( 11): 3697– 701. [DOI] [PubMed] [Google Scholar]
- 21. Finney DJ. The adjustment for a natural response rate in probit analysis. Ann Appl Biol. 1949; 36( 2): 187– 95. [DOI] [PubMed] [Google Scholar]
- 22. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65( 1–2): 55– 63. [DOI] [PubMed] [Google Scholar]
- 23. Rajabi S, Ramazani A, Hamidi M, Naji T. Artemia salina as a model organism in toxicity assessment of nanoparticles. Daru. 2015; 23: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kayser O, Kiderlen AF. Leishmanicidal activity of aurones. Tokai J Exp Clin Med. 1998; 23( 6): 423– 6. [PubMed] [Google Scholar]
- 25. Sairafianpour M, Kayser O, Christensen J, Asfa M, Witt M, Staerk D, et al. Leishmanicidal and antiplasmodial activity of constituents of Smirnowia Iranica. J Nat Prod. 2002; 65( 12): 1754– 8. [DOI] [PubMed] [Google Scholar]
- 26. Di Pietro A, Conseil G, Perez-Victoria JM, Dayan G, Baubichon-Cortay H, Trompier D, et al. Modulation by flavonoids of cell multidrug resistance mediated by P-glycoprotein and related ABC transporters. Cell Mol Life Sci. 2002; 59( 2): 307– 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sim HM, Lee CY, Ee PL, Go ML. Dimethoxyaurones: Potent inhibitors of ABCG2 (breast cancer resistance protein). Eur J Pharm Sci. 2008; 35( 4): 293– 306. [DOI] [PubMed] [Google Scholar]