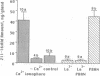
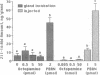
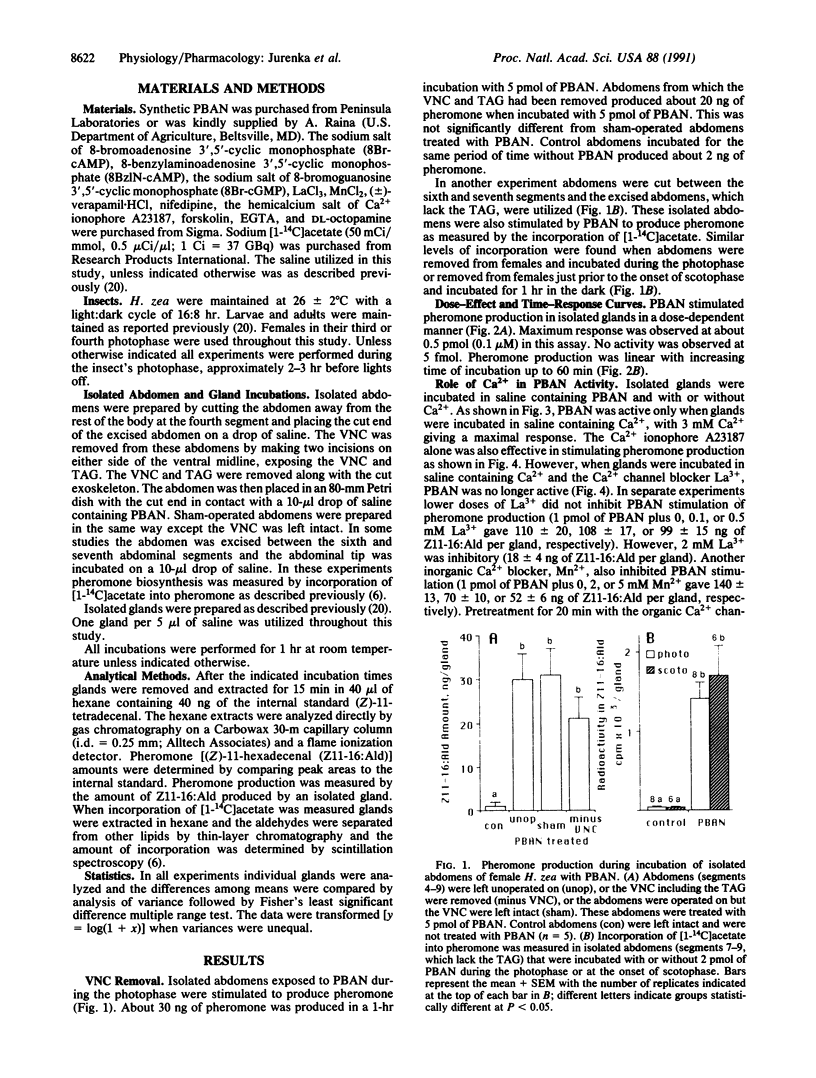
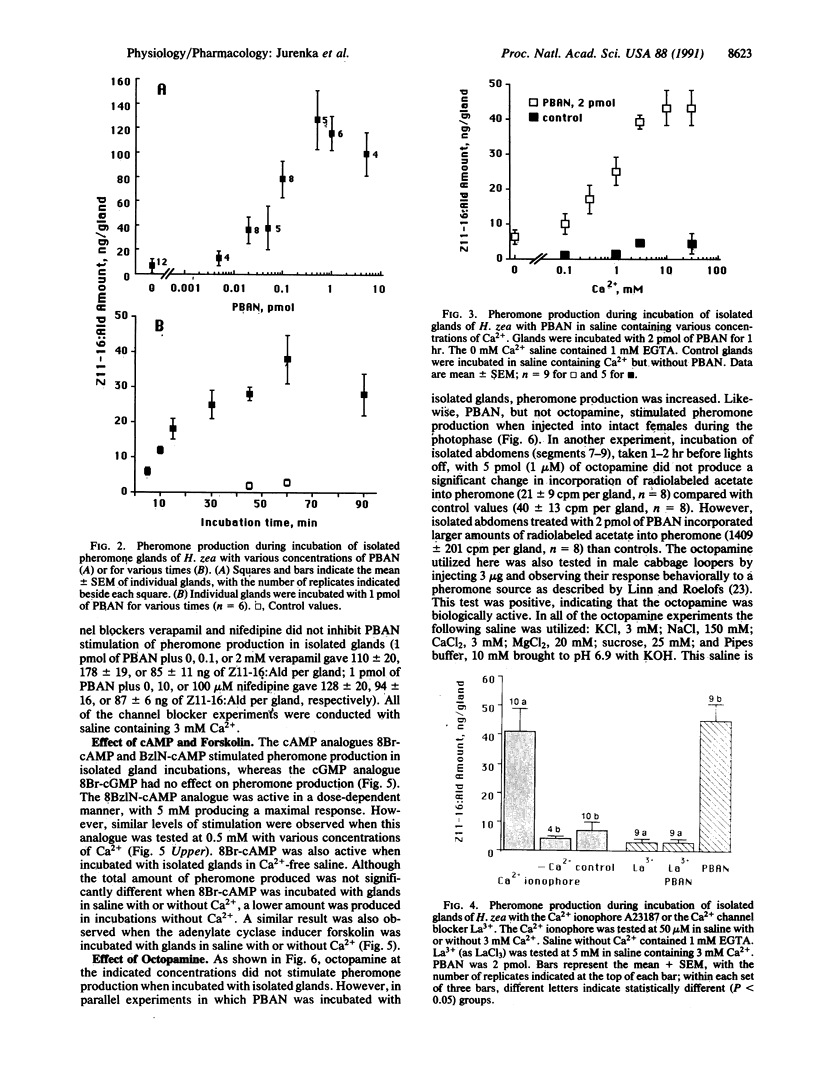
Abstract
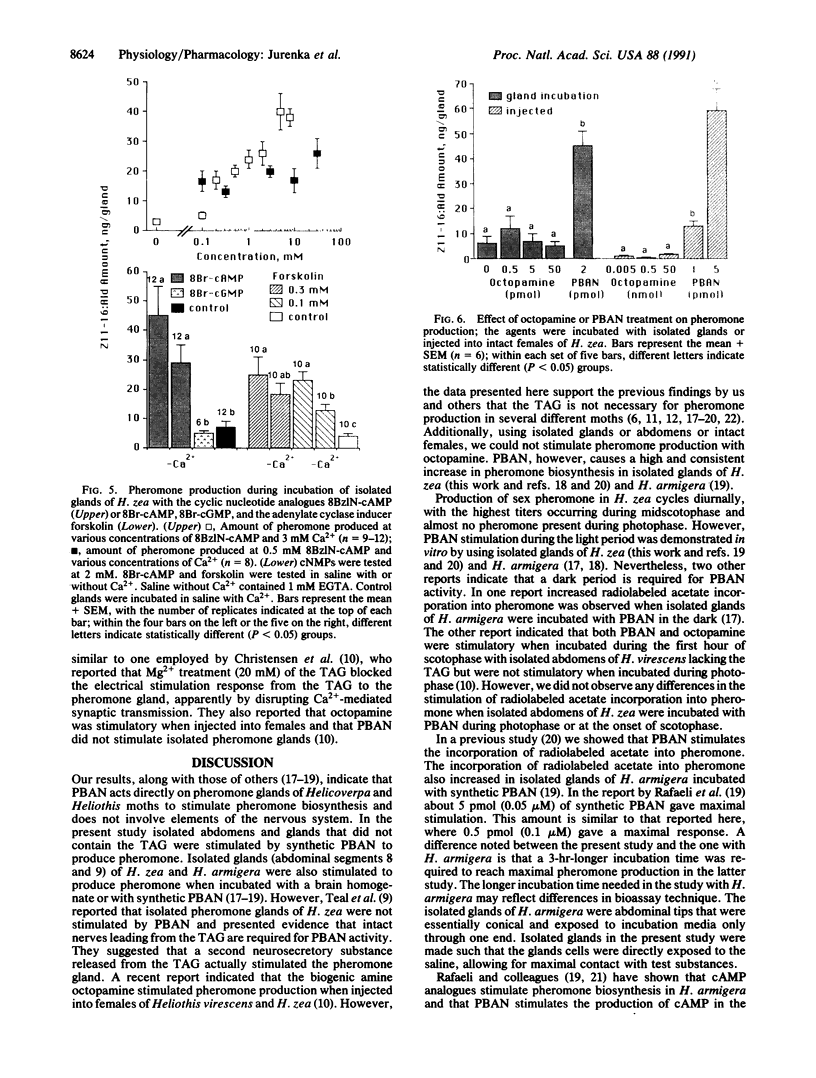
Isolated abdomen and pheromone gland bioassays were utilized to determine the physiological action of the pheromone-biosynthesis-activating neuropeptide (PBAN) in the corn earworm moth Helicoverpa (= Heliothis) zea. An isolated pheromone gland bioassay showed that synthetic PBAN was active at 0.02 pmol, with maximal activity occurring at 0.5 pmol and 60 min of incubation. Second-messenger studies demonstrated that extracellular Ca2+ is necessary for PBAN activity on isolated pheromone glands. The Ca2+ ionophore A23187 stimulated pheromone biosynthesis alone, whereas the Ca2+ channel blockers La3+ and Mn2+ inhibited PBAN activity. However, the organic Ca2+ channel blockers verapamil and nifedipine did not inhibit PBAN activity. Both forskolin and two cAMP analogues stimulated pheromone biosynthesis in the absence of extracellular Ca2+, indicating that Ca2+ may activate an adenylate cyclase. The biogenic amine octopamine did not elicit pheromone production in isolated gland or abdomen bioassays or when injected into intact female moths. Removal of the ventral nerve chord, including the terminal abdominal ganglia in isolated abdomens, did not affect PBAN stimulation of pheromone production. Similar levels of stimulation were found when isolated abdomens were treated with PBAN in scotophase or photophase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Calcium oscillations. J Biol Chem. 1990 Jun 15;265(17):9583–9586. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Christensen T. A., Itagaki H., Teal P. E., Jasensky R. D., Tumlinson J. H., Hildebrand J. G. Innervation and neural regulation of the sex pheromone gland in female Heliothis moths. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4971–4975. doi: 10.1073/pnas.88.11.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusson M., McNeil J. N. Involvement of juvenile hormone in the regulation of pheromone release activities in a moth. Science. 1989 Jan 13;243(4888):210–212. doi: 10.1126/science.243.4888.210. [DOI] [PubMed] [Google Scholar]
- Eliot L. S., Dudai Y., Kandel E. R., Abrams T. W. Ca2+/calmodulin sensitivity may be common to all forms of neural adenylate cyclase. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9564–9568. doi: 10.1073/pnas.86.23.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert L. I., Combest W. L., Smith W. A., Meller V. H., Rountree D. B. Neuropeptides, second messengers and insect molting. Bioessays. 1988 May;8(5):153–157. doi: 10.1002/bies.950080506. [DOI] [PubMed] [Google Scholar]
- Kitamura A., Nagasawa H., Kataoka H., Ando T., Suzuki A. Amino acid sequence of pheromone biosynthesis activating neuropeptide-II (PBAN-II) of the silkmoth, Bombyx mori. Agric Biol Chem. 1990 Sep;54(9):2495–2497. [PubMed] [Google Scholar]
- Kitamura A., Nagasawa H., Kataoka H., Inoue T., Matsumoto S., Ando T., Suzuki A. Amino acid sequence of pheromone-biosynthesis-activating neuropeptide (PBAN) of the silkworm, Bombyx mori. Biochem Biophys Res Commun. 1989 Aug 30;163(1):520–526. doi: 10.1016/0006-291x(89)92168-2. [DOI] [PubMed] [Google Scholar]
- Meller V. H., Combest W. L., Smith W. A., Gilbert L. I. A calmodulin-sensitive adenylate cyclase in the prothoracic glands of the tobacco hornworm, Manduca sexta. Mol Cell Endocrinol. 1988 Sep;59(1-2):67–76. doi: 10.1016/0303-7207(88)90196-7. [DOI] [PubMed] [Google Scholar]
- Murray R. K., Kotlikoff M. I. Receptor-activated calcium influx in human airway smooth muscle cells. J Physiol. 1991 Apr;435:123–144. doi: 10.1113/jphysiol.1991.sp018501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafaeli A., Soroker V. Cyclic AMP mediation of the hormonal stimulation of 14C-acetate incorporation by Heliothis armigera pheromone glands in vitro. Mol Cell Endocrinol. 1989 Aug;65(1-2):43–48. doi: 10.1016/0303-7207(89)90163-9. [DOI] [PubMed] [Google Scholar]
- Raina A. K., Klun J. A. Brain factor control of sex pheromone production in the female corn earworm moth. Science. 1984 Aug 3;225(4661):531–533. doi: 10.1126/science.225.4661.531. [DOI] [PubMed] [Google Scholar]
- Smith W. A., Gilbert L. I., Bollenbacher W. E. Calcium-cyclic AMP interactions in prothoracicotropic hormone stimulation of ecdysone synthesis. Mol Cell Endocrinol. 1985 Jan;39(1):71–78. doi: 10.1016/0303-7207(85)90093-0. [DOI] [PubMed] [Google Scholar]
- Smith W. A., Gilbert L. I. Early events in peptide-stimulated ecdysteroid secretion by the prothoracic glands of Manduca sexta. J Exp Zool. 1989 Dec;252(3):264–270. doi: 10.1002/jez.1402520309. [DOI] [PubMed] [Google Scholar]
- Tang J. D., Charlton R. E., Jurenka R. A., Wolf W. A., Phelan P. L., Sreng L., Roelofs W. L. Regulation of pheromone biosynthesis by a brain hormone in two moth species. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1806–1810. doi: 10.1073/pnas.86.6.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teal P. E., Tumlinson J. H., Oberlander H. Neural regulation of sex pheromone biosynthesis in Heliothis moths. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2488–2492. doi: 10.1073/pnas.86.7.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]