Abstract
Background
There is some evidence to support the use of acupuncture as an alternative therapy for asthma. However, the mechanisms underlying its effects are not fully understood. We have reported previously that acupuncture has beneficial effects on asthma without changing the concentration of serum cortisol, although endogenous glucocorticoid (GC) plays an important role in regulating immune responses.
Objective
In this study, bilateral adrenalectomy (removal of both adrenal glands) was performed in rats before asthma model induction to investigate whether acupuncture influences asthma in a GC-dependent manner.
Methods
Adrenal-intact and adrenalectomised rats were injected with ovalbumin to induce asthma and then left untreated or treated with manual acupuncture (MA) at GV14, bilateral BL12 and bilateral BL13, or manual restraint without MA. Healthy and sham-adrenalectomised control groups were also included. Pulmonary resistance (RL), serum concentrations of corticosterone, and eosinophil counts were measured at the end of the experimental course. Sera from adrenal-intact and adrenalectomised asthmatic rats treated with acupuncture were injected into untreated adrenal-intact and adrenalectomised asthmatic rats to investigate further the potential role of GC in the effect of acupuncture.
Results
Acupuncture significantly decreased RL and eosinophil count in both adrenal-intact and adrenalectomised asthmatic rats. Moreover, administration of sera derived from acupuncture-treated adrenal-intact and adrenalectomised asthmatic rats attenuated the increase in RL and eosinophil count in both asthmatic models.
Conclusions
Results of this study suggest that endogenous GC is not a key contributor to the effects of acupuncture on asthma, and that acupuncture may have potentially therapeutic effects on asthma in a GC-independent manner.
Keywords: ACUPUNCTURE
Introduction
Asthma is a common chronic disease with a multifactorial aetiology and only partially understood mechanisms.1 2 The global prevalence, morbidity, mortality, and economic burden of asthma have increased over the past few decades.3 Most of the currently available treatments, such as anti-inflammatory drugs and muscle relaxants, do not prevent the natural course of asthma.4 Therefore many asthma sufferers turn to alternative or complementary therapies to improve management of their condition.5 6
Historically, acupuncture has been used to treat asthma for thousands of years in China. The WHO listed asthma as an indication for acupuncture in 1979,7 and the National Institutes of Health have since recommended acupuncture as an adjunctive treatment in comprehensive management programmes for asthma.8 9 Previous studies have shown that acupuncture may be effective as a treatment for asthma by alleviating symptoms and improving quality of life in affected patients.10 11
Asthma is the result of inflammation of the airways that subsequently leads to bronchoconstriction and airway hyperresponsiveness.12 Several studies have reported that acupuncture treatment has an immunomodulatory effect (via inflammatory cells and cytokines) in asthma.13 14 Our previous research has indicated that acupuncture downregulates the expression of proinflammatory proteins and upregulates the expression of anti-inflammatory proteins.15 Glucocorticoids (GCs) are potent anti-inflammatory steroid hormones that are mainly produced endogenously in the adrenal cortex and have been shown to provide protection against the pathological process of airway inflammation that occurs in asthma.16 Inhaled exogenous GCs are one of the major drug classes used in asthma treatment and have been shown to regulate leucocyte trafficking and inhibit the synthesis and release of inflammatory cytokines.17 However, the role of GC in the efficacy of acupuncture in asthma is still unclear.
Our previous clinical study indicated that acupuncture reduced the degree and frequency of asthma exacerbations and regulated mucosal and cellular immunity in asthmatic patients without changing the concentration of serum cortisol;10 this implies that GC activity may not in fact be a key contributor to the effect of acupuncture on asthma with respect to alleviating the symptoms and modulating the immune system. The aim of this study was to investigate whether acupuncture influences asthma in a GC-dependent manner in a rat model by assessing the relative impact of bilateral adrenalectomy (removal of both adrenal glands), which would be unethical in humans. We also aimed to evaluate the effect of serum derived from acupuncture-treated adrenal-intact and adrenalectomised asthmatic rats to investigate further the role of GC in the potentially therapeutic effect of acupuncture.
Methods
Experimental animals and study design
One hundred and eighty specific pathogen-free (SPF) male Sprague-Dawley rats (4 weeks old, weighing 100–120 g) were purchased from the Shanghai Laboratory Animal Centre (Shanghai, China) and housed in a pathogen-free animal facility maintained at a controlled temperature of 20–22°C and air humidity of 45–55% with a 12 h/12 h light/dark schedule. Animals were allowed to adapt for at least 1 week before investigations began and were provided with food and water ad libitum. All animal experiments were reviewed and approved by the Committee on the Ethics of Animal Experiments of Shanghai University of Traditional Chinese Medicine (reference no. 08001) and performed according to the National Institutes of Health ‘Guide for the Care and Use of Laboratory Animals’ as well as the guidelines of the Animal Welfare Act.
To determine the sample size requirement, an a priori power calculation for a two-tailed test was performed based on our pre-selected primary outcome measure of pulmonary resistance (RL). Based on a pilot study, we estimated that 10 animals per group would be required to detect a reduction of 0.003 kPa/mL/s in adrenal-intact rats, assuming a standard deviation (SD) of 0.00295, with an α level of 0.05 and 80% power. For adrenalectomised rats, we estimated that 19 animals per group would be required to detect a reduction of 0.0019 kPa/mL/s, assuming an SD of 0.0028, with an α level of 0.05 and 80% power. Considering the small possibility of death following adrenalectomy, we chose to allocate 20 rats into the adrenalectomy groups.
The 180 rats were divided into 12 groups using a random number table. The following six groups remained adrenal-intact: a normal control group (Control, n=10) left untreated, an asthmatic group induced by intraperitoneal injection of ovalbumin (OVA, n=10) and left untreated; an asthmatic group treated with manual acupuncture (OVA+MA, n=10); a manually restrained asthmatic group (OVA+MR, n=10); an adrenal-intact asthmatic group injected with serum from the adrenal-intact OVA+MA group (OVA+SI, n=10); and an adrenal-intact asthmatic group injected with serum from acupuncture-treated adrenalectomised asthmatic rats (OVA+SI-ADX, n=10). The remaining six groups all underwent adrenalectomy: an adrenalectomised asthmatic group (ADX+OVA, n=20) left untreated; a sham adrenalectomised asthmatic group (SADX+OVA, n=20) left untreated; an adrenalectomised asthmatic group treated with MA (ADX+OVA+MA, n=20); a manually restrained adrenalectomised asthmatic group (ADX+OVA+MR, n=20); an adrenalectomised asthmatic group injected with serum from the adrenal-intact OVA+MA group (ADX+OVA+SI, n=20); and an adrenalectomised asthmatic group injected with serum from the adrenalectomised ADX+OVA+MA group (ADX+OVA+SI-ADX, n=20).
RL (used for the power calculation) and corticosterone values were the primary outcome measures, and eosinophil count was measured as a secondary outcome. The investigators responsible for RL measurement, corticosterone analysis, and eosinophil counting were blinded to the experimental group allocation when assessing animals or serum samples, respectively.
Adrenalectomy
The protocol for adrenalectomy in rats was modified from that of Farris and Griffith.18 Briefly, 2 days before sensitisation, rats were anaesthetised with pentobarbital sodium (50 mg/kg bodyweight) and disinfected with 0.1% benzalkonium bromide tincture and 75% alcohol. A single dorsal midline incision was made at the level of the kidneys. After lateral retraction of the skin, the muscle was exposed by cutting through the subcutaneous fascia. A longitudinal incision was made along the lateral border of the dorsal muscle mass, from the level of the centre of the kidney toward the apex of the angle between the lowest rib and the dorsal muscle mass. The adrenal gland was removed from the upper pole of the kidney. The second adrenal was then removed in the same manner (figure 1A). The dorsal muscle and subcutaneous tissues were closed with catgut suture, whereas the skin was closed with 4-0 silk suture (Jinhuan Medical, Shanghai Pudong Jinhuan Medical Products Co Ltd, Shanghai, China). All adrenalectomised rats were warmed by incandescent lamps to keep their temperature at approximately 28°C until they awoke, and were provided with water containing 5% glucose and 1% sodium chloride. Sham surgeries were identical to adrenalectomy except that the adrenals were not removed. Rats received postoperative pain relief for 3 days after surgery in the form of 12 h subcutaneous buprenorphine (0.05 mg/kg).
Figure 1.
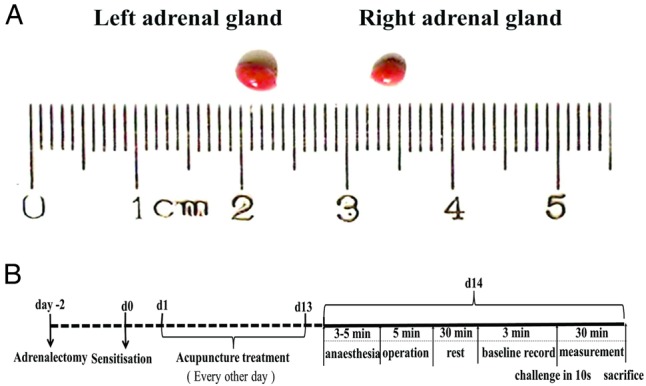
(A) Photograph of the exteriorised rat adrenal glands. The right adrenal gland is pyramidal in shape. The left adrenal gland is semilunar in shape, larger in size and positioned lower than the right adrenal gland. (B) A schematic representation of the experimental design including adrenalectomy, establishment of the rat model of asthma, and measurement of physiological parameters.
Sensitisation and ovalbumin challenge
Rats were sensitised and challenged according to a previously described protocol.19 Briefly, on day 0, all rats assigned to asthmatic model groups (all except Control) were intraperitoneally injected with 1 mg ovalbumin (grade V, Sigma, Taufkirchen, Germany) that had been precipitated with 10 mg of aluminium hydroxide gel in 1 mL of saline for sensitisation. On day 14, following completion of acupuncture (or control) treatment, the sensitised rats were challenged with ovalbumin at a dose of 15 mg/kg (figure 1B).
Acupuncture treatment
Three acupuncture points commonly used in the treatment of pulmonary disease were selected and needled at the same time in the groups allocated to receive MA treatment (OVA+MA and ADX+OVA+MA). These acupuncture points were GV14 (Dazhui), located between the C7 and T1 vertebrae, bilateral BL12 (Fengmen), located laterally between the T2 and T3 vertebrae, and bilateral BL13 (Feishu), located laterally between the T3 and T4 vertebrae.10 MA was performed by a well-trained acupuncturist once every other day for 2 weeks starting on day 3 (seven times in total). Rats were restrained but conscious. Disposable stainless steel needles (0.30 mm×13 mm, Hwato, Suzhou Medical Appliance Factory, Suzhou, China) were inserted to a depth of 5 mm and twisted evenly through 360° at a frequency of 60 times/min for 20 s. The needles were manipulated every 5 min and were then withdrawn after 20 min. Rats in the manual restraint groups (OVA+MR and ADX+OVA+MR) were handled in the same manner as animals in the MA groups but were not needled.20
Measurement of pulmonary resistance
The protocol for RL measurement was modified from that of Glaab et al.21 22 Briefly, rats were anaesthetised with pentobarbital sodium (50 mg/kg bodyweight) and placed in a supine position on a wooden plate that was warmed by an incandescent lamp. A T-shaped incision was made in the upper part of the trachea and a T-shaped cannula, which was directly attached to a pneumotachograph (Godart 17212, Bilthoven, Holland), was gently inserted into the trachea. The tidal volume (VT) and flow rate (V) were measured by connection of the pneumotachograph to a differential pressure transducer. The intrapleural pressure (IPP) was replaced by oesophageal pressure, which was measured using a water-filled cannula coupled with a blood pressure transducer (YZ0.1-I, Shanghai Medical Electronic Instrument Factory, Shanghai, China) that was placed into the oesophagus and processed by a biological signal processing system. RL was calculated using Respiratory Flow Measurement Software (Shanghai Medical College, Fudan University, Shanghai, China) with the integration of the VT, V and IPP values. After the above steps were completed, the rats were allowed to rest for 30 min. The values during a 3 min period were then recorded as a baseline. Thereafter sensitised rats (all except the Control group) were challenged with a 15 mg/kg ovalbumin injection into the external jugular vein over 10 s. The Control group received an equivalent volume of saline. In all experimental groups, respiratory parameters were recorded for 30 min following administration of ovalbumin or saline by operators blinded to experimental group allocation.
Euthanasia and blood sampling
Following RL measurement, all rats were euthanased by exsanguination under terminal anaesthesia with pentobarbital sodium and blood was collected by cardiac puncture. Samples were anticoagulated with EDTA pending eosinophil counting or allowed to clot on ice pending separation by centrifugation. Approximately 3 mL of the resultant serum per rat was diluted with normal saline at a ratio of 1:20 and either stored at −80°C pending corticosterone quantification or, in the case of the OVX+MA and ADX+OVA+MA groups, pooled ready for injection into untreated adrenal-intact and adrenalectomised asthmatic rats (see below).
Measurement of serum corticosterone
Corticosterone values (ng/mL) were determined in rat serum using commercially available radioimmunoassay (RIA) kits (catalogue no: TKRC1, Coat-A-Count, DPC, Los Angeles, California, USA) by investigators blinded to experimental group allocation. This procedure is based on a solid-phase RIA in which 125I-labelled corticosterone competes with the corticosterone present in the rat sample for antibody sites over a fixed period of time. All serum samples were assayed in duplicate, and mean values were calculated.
Eosinophil count
Eosinophil numbers were determined in EDTA-treated whole blood samples following application of staining solution (eosin: acetone=9:1) in a Neubauer counting chamber. After staining at room temperature for 10 min, eosinophils were counted under an optical microscope (Olympus, Japan) by at least two independent observers who were blinded to experimental group allocation.
Effect of serum from acupuncture-treated rats
To further investigate the role of GC in the effect of acupuncture, sera extracted from the OVA+MA group (SI) and ADX+OVA+MA group (SI-ADX) were collected and intravenously injected into adrenal-intact asthmatic rats (OVA+SI and OVA+SI-ADX groups) and adrenalectomised asthmatic rats (ADX+OVA+SI and ADX+OVA+SI-ADX groups) at a dose of 2.5 mL/kg 10 min before ovalbumin challenge, RL measurement and blood sampling for eosinophil counts (as described).
Statistical analysis
Data are presented as mean±SEM. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by post-hoc test of least significant difference (LSD) or Games-Howell tests (depending on the data and on the hypothesis tested). A level of p<0.05 was considered to indicate formal statistical significance and p=0.05–0.1 was considered to represent a statistical tendency towards a significant difference.
Results
Three rats randomised to the sham adrenalectomised asthmatic (SADX+OVA) group died after surgery, which reduced the size of this group to n=17. There were no other fatalities or exclusions from the study.
Effect of acupuncture on adrenal-intact asthmatic rats
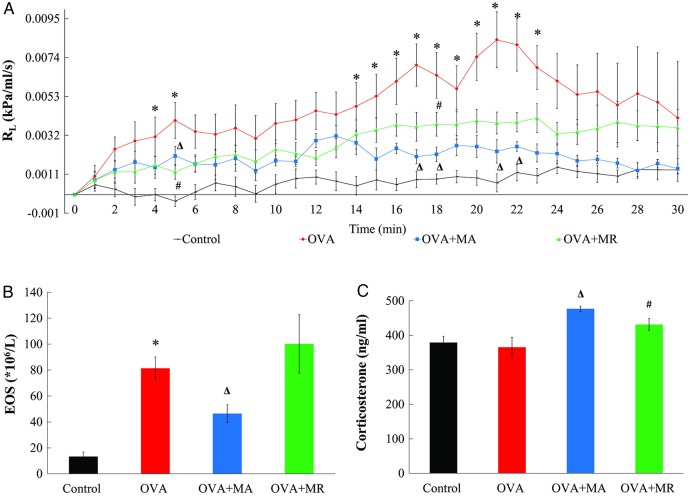
The ovalbumin challenge in the sensitised rats in the OVA group induced an increase in RL relative to the Control group, with statistically significant differences noted at 4–5 min and 14–23 min after challenge (p=0.001–0.036; figure 2A). After acupuncture was performed on the ovalbumin-sensitised rats, RL was significantly decreased at 5 min, 17–18 min, and 21–22 min (p=0.006–0.04), whereas manual restraint of ovalbumin-sensitised rats reduced RL at 5 and 18 min (p=0.002 and 0.028, respectively). As shown in figure 2B, ovalbumin challenge significantly increased the eosinophil count (OVA vs Control groups: 81.4±8.7 vs 13.2±3.6×106/L, p<0.001). The eosinophil count in the OVA+MA group (46.4±6.9×106/L) was 43% lower than the OVA group (p=0.027). By contrast, the eosinophil count in the OVA+MR group (100.2±22.6×106/L) did not differ from the OVA group (p=0.323). Although serum corticosterone concentrations were not significantly different between Control and OVA groups (figure 2C), acupuncture increased serum corticosterone values to 1.3 times that of the OVA group (476.6±7.8 vs 365.3±28.3 ng/mL, p<0.001). Serum corticosterone concentrations were also elevated in the OVA+MR group (431.1±17.8 ng/mL, p=0.025 compared with that in the OVA group) but the increase was approximately 41% less than that observed in the OVA+MA group.
Figure 2.
Measurements of: (A) pulmonary resistance (RL) over time; (B) eosinophil count (EOS); and (C) serum corticosterone concentrations, in 10 healthy rats (Control group) and 30 adrenal-intact rats sensitised to ovalbumin and treated with manual acupuncture (OVA+MA group, n=10), subjected to manual restraint without needling (OVA+MR group, n=10) or left untreated (OVA group, n=10) before ovalbumin challenge to model asthma. Data are mean±SEM. ∗p<0.05 OVA versus Control groups. Δp<0.05 OVA+MA versus OVA groups. #p<0.05, OVA+MR versus OVA group.
Effect of acupuncture on adrenalectomised asthmatic rats
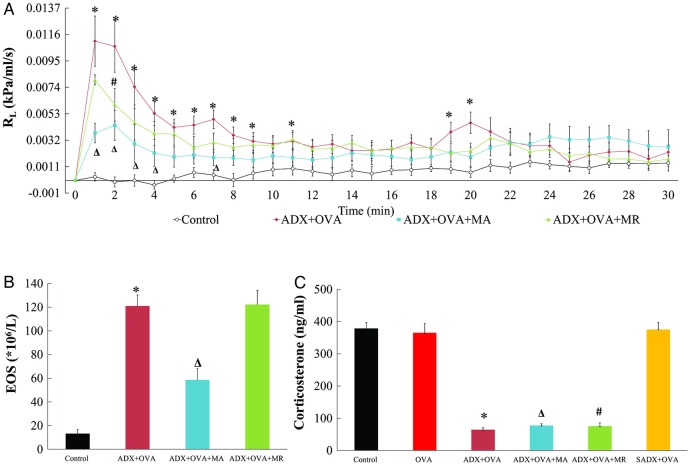
The ovalbumin challenge in the ADX+OVA group induced a significant increase in RL compared with the Control group, with statistically significant differences noted at 1–9 min, 11 min, 19 and 20 min after challenge (p<0.001–0.043; figure 3A). After acupuncture, RL in the ADX+OVA+MA group was significantly lower than that in the ADX+OVA group at 1–4 min and 7 min (p=0.001–0.003). In the second minute only following ovalbumin challenge, manual restraint also decreased RL in the ADX+OVA+MR group (p=0.044 compared with the ADX+OVA group).
Figure 3.
Measurements of: (A) pulmonary resistance (RL) over time; (B) eosinophil count (EOS); and (C) serum corticosterone concentrations, in 10 healthy rats (Control group), 17 rats sensitised to ovalbumin after sham adrenalectomy (SADX+OVA group), and 60 adrenalectomised rats sensitised to ovalbumin and treated with manual acupuncture (ADX+OVA+MA group, n=20), subjected to manual restraint without needling (ADX+OVA+MR group, n=20) or left untreated (ADX+OVA group, n=20) before ovalbumin challenge to model asthma. Data are mean±SEM. ∗p<0.05 ADX+OVA versus Control groups. Δp<0.05 ADX+OVA+MA versus ADX+OVA groups. #p<0.05, ADX+OVA+MR versus ADX+OVA groups.
As observed in adrenal-intact animals, the eosinophil count was significantly raised after ovalbumin challenge in adrenalectomised (ADX+OVA) rats (figure 3B). The eosinophil count in the ADX+OVA+MA group was decreased after acupuncture, falling to 49% of that of the ADX+OVA group (58.7±9.5 vs 121±9.3×106/L, p=0.001). However, restraint did not change the eosinophil count in the ADX+OVA+MR group (122.2±12.2×106/L) compared with the ADX+OVA group (p=1.000).
As anticipated, adrenalectomy induced a significant decrease in the serum corticosterone concentration (p<0.001; figure 3C), which was not recovered by MA. Accordingly, serum corticosterone values did not differ between the ADX+OVA, ADX+OVA+MA, and ADX+OVA+MR groups (64.5±6.2, 77.6±5.3, and 75.1±10.4 ng/mL, respectively) and were each approximately 20% of the value observed in the Control group. Sham adrenalectomy did not change the corticosterone concentration when compared to the adrenal-intact rats (SADX+OVA vs Control group: 375.6±22.2 vs 378.8±18.1 ng/mL, p=1.000), indicating a successful adrenalectomy model.
Effect of serum derived from acupuncture-treated asthmatic rats
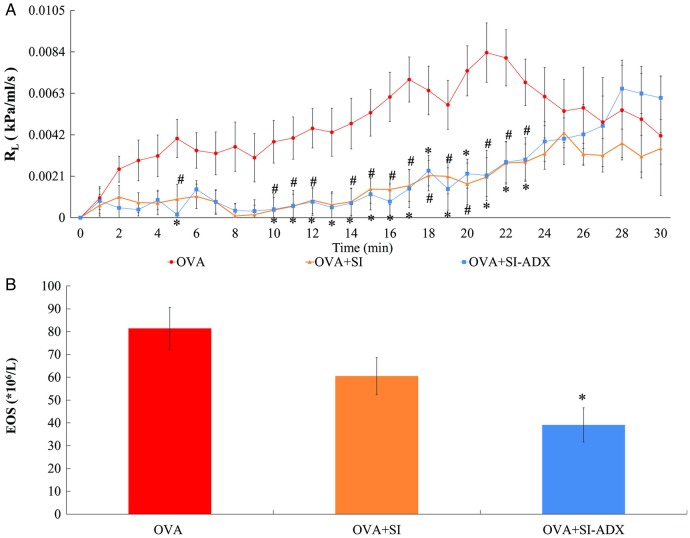
As shown in figure 4, administration of serum derived from acupuncture-treated asthmatic rats had a similar effect on adrenal-intact asthmatic rats as acupuncture per se (figure 2). Sera from the two different types of acupuncture-treated asthmatic rats (adrenal-intact and adrenalectomised) inhibited the increase in RL, reaching statistical significance at the 5, 10–12, and 14–23 min time points in the OVA+SI group (p=0.001–0.029) and the 5 min and 10–23 min time points in the OVA+SI-ADX group (p=0.001–0.049) compared with the OVA group (figure 4A). The eosinophil count in the OVA+SI-ADX group was significantly decreased to 48% that of the OVA group (39.1±6.1 vs 81.4±8.7×106/L, p<0.001; figure 4B) and there was also a tendency towards a lower eosinophil count in the OVA+SI group (60.5±6.9×106/L; p=0.062).
Figure 4.
Measurements of: (A) pulmonary resistance (RL) over time; and (B) eosinophil count (EOS), in 30 adrenal-intact rats sensitised to ovalbumin and left untreated (OVA group, n=10) or given a 2.5 mL/kg jugular venous injection of serum derived from acupuncture-treated adrenal-intact asthmatic rats (OVA+SI group, n=10) or acupuncture-treated adrenalectomised asthmatic rats (OVA+SI-ADX group, n=10) before ovalbumin challenge to model asthma. Data are mean±SEM. #p<0.05 OVA+SI versus OVA groups. ∗p<0.05 OVA+SI-ADX versus OVA groups.
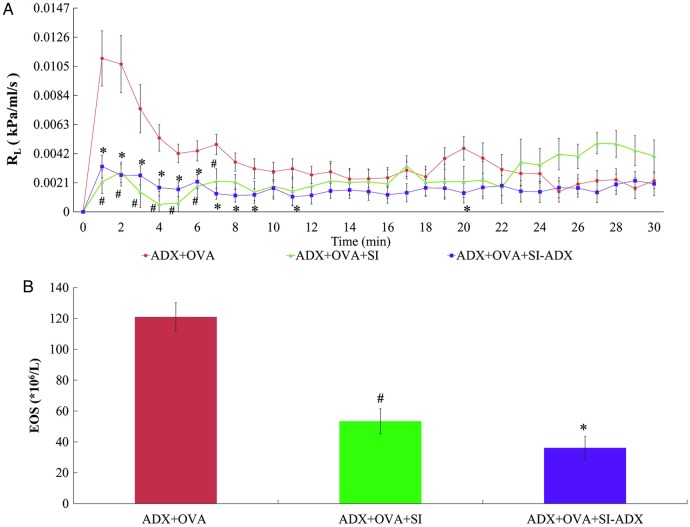
As demonstrated in figure 5, serum derived from acupuncture-treated asthmatic rats also reduced RL in adrenalectomised asthmatic rats. As per adrenal-intact rats, sera from both types of acupuncture-treated asthmatic rats inhibited the increase in RL, with significant differences at the 1–7 min time points in the ADX+OVA+SI group and the 1–9, 11, and 20 min time points in the ADX+OVA+SI-ADX group, respectively, compared with the ADX+OVA group (p=0.001–0.045; figure 5A). Similarly, eosinophil counts were also lower in the groups receiving serum injection (figure 5B). The eosinophil counts in the ADX+OVA+SI and ADX+OVA+SI-ADX groups were decreased to 44.1% and 29.8%, respectively, of the eosinophil count in the ADX+OVA group (53.4±8.1 and 36.1±7.5 vs 121±9.3×106/L, p<0.001 each).
Figure 5.
Measurements of: (A) pulmonary resistance (RL) over time; and (B) eosinophil count (EOS), in 60 adrenalectomised rats sensitised to ovalbumin and left untreated (ADX+OVA group, n=20) or given a 2.5 mL/kg jugular venous injection of serum derived from acupuncture-treated adrenal asthmatic rats (ADX+OVA+SI group, n=20) or acupuncture-treated adrenalectomised asthmatic rats (ADX+OVA+SI-ADX group, n=20) before ovalbumin challenge to model asthma. Data are mean±SEM. #p<0.05 ADX+OVA+SI versus ADX+OVA groups. ∗p<0.05 ADX+OVA+SI-ADX versus ADX+OVA groups.
Discussion
Although GC has an inhibitory effect on inflammatory processes and is widely used in the treatment of asthma, the role of GC in the mechanism of action underlying the putative efficacy of acupuncture remains poorly understood. Our previous study showed that acupuncture was effective in patients with asthma but did not change the concentration of GC in the serum. In the current study, acupuncture treatment effectively reduced the RL and eosinophil count with a concomitant increase in the concentration of corticosterone in adrenal-intact asthmatic rats. Acupuncture treatment also had a significant and positive effect on the RL and eosinophil count in the adrenalectomised asthmatic model, which is characterised by very low values of corticosterone. In rats, corticosterone is the predominant endogenous GC,23 so these results suggest that endogenous GC is not a key contributor to the efficacy of acupuncture in asthma.
It has been reported that serum sampled from acupuncture-treated individuals can provide comprehensive pharmacobiological information on acupuncture efficacy.24 Accordingly, we collected GC-deficient and GC-sufficient sera from the acupuncture-treated ADX+OVA+MA group and OVA+MA groups, respectively, and evaluated their effects on the asthmatic rats. Administration of the GC-deficient and GC-sufficient sera from acupuncture-treated asthmatic rats had a significant inhibitory effect on the increase in RL and eosinophil count that was otherwise observed in both adrenal-intact and adrenalectomised asthmatic rats, which provides further evidence that acupuncture has potentially therapeutic effects on asthma via a mechanism that is independent of GC activity.
Our results showed that adrenalectomy in rats induced a ∼80% decrease in the concentration of serum corticosterone compared with sham adrenalectomy. After ovalbumin challenge, the elevation of RL in sensitised adrenalectomised rats occurred earlier in time and was larger in magnitude than that observed in adrenal-intact rats. The earlier changes in RL may have been due to the lack of endogenous GC in the adrenalectomised rats, as GC can provide effective protection against the pathological process triggered by the ovalbumin challenge. Adrenalectomy has also been shown to induce a significant decrease in pulmonary surfactant,25 26 and this may lead to decreased pulmonary compliance and a larger increase in RL in adrenalectomised rats.
Restraint alone induces a stress response, which is accompanied by an increase in serum corticosterone. The manual restraint group was included here as a control to attempt to differentiate the specific effects of acupuncture from the restraint-induced physiological effects of stress. The effect of acupuncture was greater than that of restraint alone. Acupuncture not only decreased RL but also attenuated the increase in eosinophil count that is characteristic of this rat model of asthma.
Although the positive effect of acupuncture on asthma may not be related to GC, acupuncture stimulation does appear to produce changes in the concentration and/or activity of biologically active components in the serum, judging from the results of our experiments involving injection of sera from acupuncture-treated asthmatic rats into adrenal-intact and adrenalectomised asthmatic rats. These currently unidentified biologically active molecules and their underlying signalling transduction mechanisms potentially form the basis of acupuncture's efficacy in asthma. Further studies using proteomic techniques are needed to analyse the sera following acupuncture treatment, and identify candidate proteins in order to elucidate their biological functions and potential anti-asthma properties and mechanisms.
In summary, this study is the first to evaluate the role of GC in the efficacy of acupuncture in an animal model of asthma. Our results suggest that endogenous GC is not a key contributor to the effects of acupuncture, and that acupuncture has positive effects on asthma in a manner that is independent of the presence of an adrenal gland.
Footnotes
Contributors: YYQ supervised the study design and revised the manuscript. W-WQ and XYD contributed equally to this work, carried out all the experimental work, analysed the data and drafted the manuscript. CLP, YLM, WY, and LYY conceived the study, participated in the design and helped to draft the manuscript. All authors read and approved the final version.
Funding: This work was funded by the National Natural Science Foundation of China (No. 81202753, 81173332, 81173341, 81473760, 81574058), Shanghai Key Research Program of Shanghai Municipal Commission of Health and Family Planning (ZYSNXD-CC-ZDYJ039, ZY3-CCCX-3-3005).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Erle DJ, Sheppard D. The cell biology of asthma. J Cell Biol 2014;205:621–31. 10.1083/jcb.201401050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 2012;18:716–25. 10.1038/nm.2678 [DOI] [PubMed] [Google Scholar]
- 3.Pawankar R, Canonica G, Holgate S, et al. World Allergy Organization white book on allergy. Update 2013. http://www.worldallergy.org/definingthespecialty/white_book.php (accessed 26 Nov 2015).
- 4.Barnes PJ. New therapies for asthma: is there any progress? Trends Pharmacol Sci 2010;31:335–43. 10.1016/j.tips.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 5.George M, Topaz M, Rand C, et al. Inhaled corticosteroid beliefs, complementary and alternative medicine, and uncontrolled asthma in urban minority adults. J Allergy Clin Immunol 2014;134:1252–9. 10.1016/j.jaci.2014.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marino LA, Shen J. Characteristics of complementary and alternative medicine use among adults with current asthma, 2006. J Asthma 2010;47:521–5. 10.3109/02770900903576320 [DOI] [PubMed] [Google Scholar]
- 7.[No authors listed] Use of acupuncture in modern health care. WHO Chron 1980;34:294–301. [PubMed] [Google Scholar]
- 8.[No authors listed] Acupuncture. NIH Consens Statement 1997;15:1–34. [PubMed] [Google Scholar]
- 9.[No authors listed] NIH Consensus Conference. Acupuncture. JAMA 1998;280:1518–24. [PubMed] [Google Scholar]
- 10.Yang YQ, Chen HP, Wang Y, et al. Considerations for use of acupuncture as supplemental therapy for patients with allergic asthma. Clin Rev Allergy Immunol 2013;44:254–61. 10.1007/s12016-012-8321-3 [DOI] [PubMed] [Google Scholar]
- 11.Choi JY, Jung HJ, Kim JI, et al. A randomized pilot study of acupuncture as an adjunct therapy in adult asthmatic patients. J Asthma 2010;47:774–80. 10.3109/02770903.2010.485665 [DOI] [PubMed] [Google Scholar]
- 12.Manni ML, Trudeau JB, Scheller EV, et al. The complex relationship between inflammation and lung function in severe asthma. Mucosal Immunol 2014;7:1186–98. 10.1038/mi.2014.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carneiro ER, Xavier RA, De Castro MA, et al. Electroacupuncture promotes a decrease in inflammatory response associated with Th1/Th2 cytokines, nitric oxide and leukotriene B4 modulation in experimental asthma. Cytokine 2010;50:335–40. 10.1016/j.cyto.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 14.Wei Y, Dong M, Zhang H, et al. Acupuncture attenuated inflammation and inhibited Th17 and treg activity in experimental asthma. Evid Based Complement Alternat Med 2015;2015:340126 10.1155/2015/340126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu YD, Cui JM, Wang Y, et al. Proteomic analysis reveals the deregulation of inflammation-related proteins in acupuncture-treated rats with asthma onset. Evid Based Complement Alternat Med 2012;2012:850512 10.1155/2012/850512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang SQ, Shen ZY, Hu GR, et al. . Effects of endogenous glucocorticoids on allergic inflammation and T(H)1 /T(H)2 balance in airway allergic disease. Ann Allergy Asthma Immunol 2009;103:525–34. 10.1016/S1081-1206(10)60270-0 [DOI] [PubMed] [Google Scholar]
- 17.Urbano FL. Review of the NAEPP 2007 Expert Panel Report (EPR-3) on Asthma Diagnosis and Treatment Guidelines. J Manag Care Pharm 2008;14:41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farris EJ, Griffith JQ Jr. The rat in laboratory investigation. 2nd edn Philadelphia: JB Lippincott Company, 1949:444. [Google Scholar]
- 19.Yin LM, Jiang GH, Wang Y, et al. Serial analysis of gene expression in a rat lung model of asthma. Respirology 2008;13:972–82. 10.1111/j.1440-1843.2008.01398.x [DOI] [PubMed] [Google Scholar]
- 20.Yin LM, Jiang GH, Wang Y, et al. Use of serial analysis of gene expression to reveal the specific regulation of gene expression profile in asthmatic rats treated by acupuncture. J Biomed Sci 2009;16:46 10.1186/1423-0127-16-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glaab T, Mitzner W, Braun A, et al. Repetitive measurements of pulmonary mechanics to inhaled cholinergic challenge in spontaneously breathing mice. J Appl Physiol 2004;97:1104–11. 10.1152/japplphysiol.01182.2003 [DOI] [PubMed] [Google Scholar]
- 22.Glaab T, Ziegert M, Baelder R, et al. Invasive versus noninvasive measurement of allergic and cholinergic airway responsiveness in mice. Respir Res 2005;6:139 10.1186/1465-9921-6-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koren L, Whiteside D, Fahlman S, et al. Cortisol and corticosterone independence in cortisol-dominant wildlife. Gen Comp Endocrinol 2012;177:113–9. 10.1016/j.ygcen.2012.02.020 [DOI] [PubMed] [Google Scholar]
- 24.Yang YQ, Chen HP, Wang Y, et al. Material base of acupuncture effects. J Acupunct Tuina Sci 2006;4:65–7. [Google Scholar]
- 25.Fisher JH, McCormack F, Park SS, et al. In vivo regulation of surfactant proteins by glucocorticoids. Am J Respir Cell Mol Biol 1991;5:63–70. 10.1165/ajrcmb/5.1.63 [DOI] [PubMed] [Google Scholar]
- 26.Enhorning G, Duffy LC, Welliver RC. Pulmonary surfactant maintains patency of conducting airways in the rat. Am J Respir Crit Care Med 1995;151:554–6. 10.1164/ajrccm.151.2.7842219 [DOI] [PubMed] [Google Scholar]