Abstract
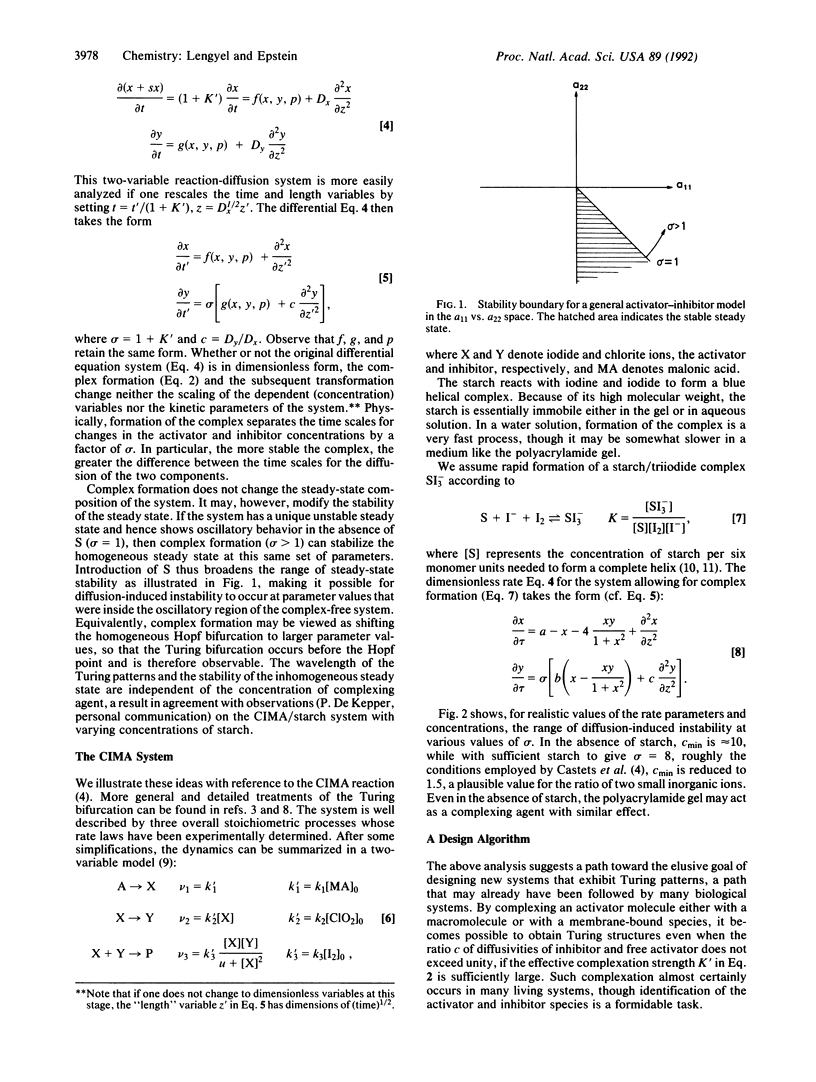
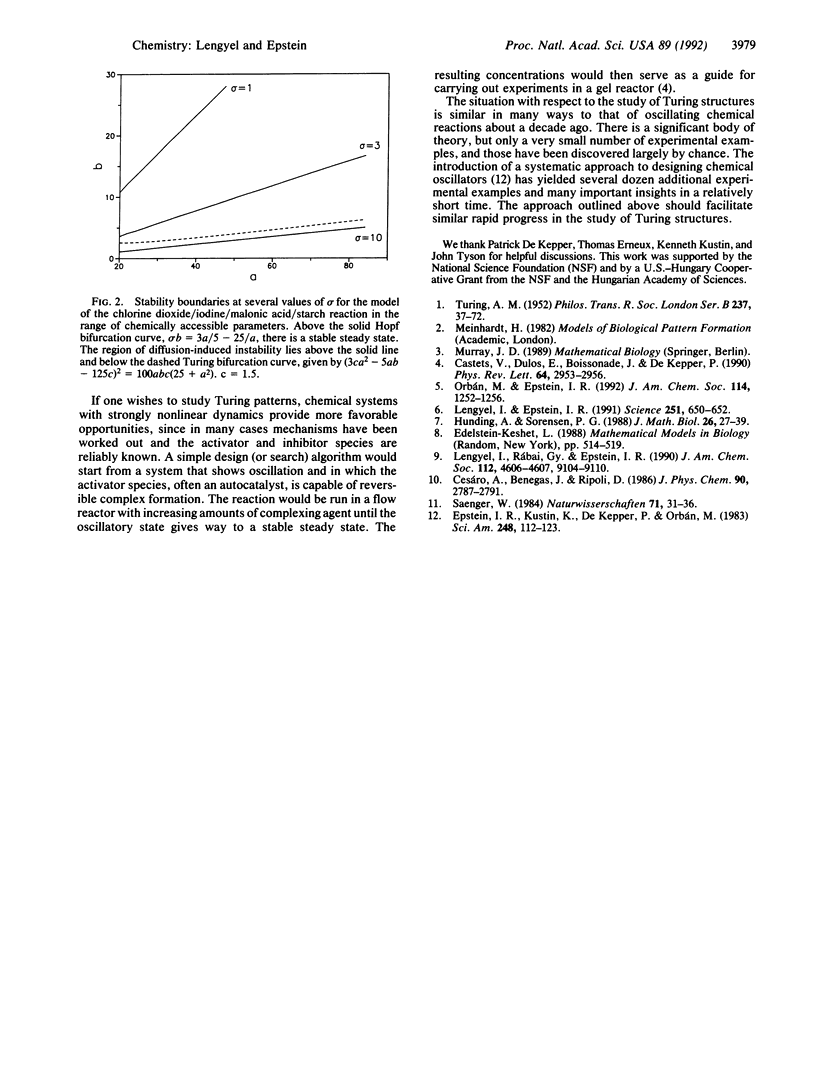
A systematic approach is suggested to design chemical systems capable of displaying stationary, symmetry-breaking reaction diffusion patterns (Turing structures). The technique utilizes the fact that reversible complexation of an activator species to form an unreactive, immobile complex reduces the effective diffusion constant of the activator, thereby facilitating the development of Turing patterns. The chlorine dioxide/iodine/malonic acid reaction is examined as an example, and it is suggested that a similar phenomenon may occur in some biological pattern formation processes.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castets V, V, Dulos E, Boissonade J, De Kepper P Experimental evidence of a sustained standing Turing-type nonequilibrium chemical pattern. Phys Rev Lett. 1990 Jun 11;64(24):2953–2956. doi: 10.1103/PhysRevLett.64.2953. [DOI] [PubMed] [Google Scholar]
- Hunding A., Sørensen P. G. Size adaptation of Turing prepatterns. J Math Biol. 1988;26(1):27–39. doi: 10.1007/BF00280170. [DOI] [PubMed] [Google Scholar]
- Lengyel I., Epstein I. R. Modeling of turing structures in the chlorite--iodide--malonic Acid--starch reaction system. Science. 1991 Feb 8;251(4994):650–652. doi: 10.1126/science.251.4994.650. [DOI] [PubMed] [Google Scholar]



