Abstract
Introduction:
Ethambutol (EMB) is an anti-mycobacterial agent that is most commonly used in combination with other anti-tuberculosis (TB) drugs in the treatment of TB. Studies have shown that primary resistance rates of Mycobacterium tuberculosis to EMB vary widely, that is, from 1% to as high as 14%. In this study, we aimed to determine the exact prevalence of primary EMB resistance among pulmonary TB cases.
Methods:
Several databases, including Medline, Embase, and Iranian databases, were searched from March 2000 to January 2016 to identify studies addressing EMB-resistant TB in Iran. Comprehensive meta-analysis (V2.2, Biostat) software was used to analyze the data.
Results:
Of the 112 records identified from the databases, 10 studies fulfilled the eligibility criteria. The pooled prevalence of primary EMB-resistant TB was estimated at 4.2% [95% confidence interval (CI) 1.8–9.0]. No evidence of publication bias was observed among the included studies (p = 0.4 for Begg rank correlation analysis; p = 0.2 for Egger weighted regression analysis).
Conclusion:
Results of systematic review and meta-analyses indicated that effective strategies to minimize the acquired drug resistance, to improve the drug susceptibility testing (DST) capability, and to control the transmission of resistance should be attached importance for control of TB in Iran.
Keywords: ethambutol, Iran, meta-analysis, systematic review, tuberculosis
Introduction
Tuberculosis (TB) caused by Mycobacterium tuberculosis is a major global health problem. In 2014, there were an estimated 9.6 million new TB cases and 1.5 million deaths from the disease [World Health Organization (WHO), 2015]. Some important challenges for control of TB include the emergence of multidrug-resistant TB (MDR-TB), lack of rapid methods for detection of drug-resistant TB, and widespread irrational use of anti-TB drugs without known drug susceptibility patterns [Nasiri et al. 2014; Varahram et al. 2014a]. The currently recommended treatment for new cases of drug-susceptible TB includes an induction phase consisting of isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), and ethambutol (EMB) [WHO, 2015]. EMB, the key component in the first-line treatment for TB, is added as a protection against unrecognized resistance to one of the three core drugs [Horsburgh et al. 2015]. However, treatment of patients infected with M. tuberculosis strains showed simultaneous resistance to EMB and INH or EMB and RIF, and using the current chemotherapy methods has been associated with increased risk of treatment failure and further acquired resistance [WHO, 2011]. Thus, understanding the exact extent of mono- or poly-resistant TB is crucial for the programmatic management of TB cases. The incidence rate of TB in Iran, which is located between high TB-burden countries, was 22 cases per 100,000 people in the year 2014 [WHO, 2015]. Studies from Iran have also shown that primary resistance rates of M. tuberculosis to INH and RIF vary widely, that is, from 10% to 40% [Merza et al. 2011; Velayati et al. 2014; Nasiri et al. 2016]. However, little is known about the prevalence of EMB-resistant TB in Iran. In this study, we aimed to investigate the true prevalence of primary EMB resistance among new pulmonary TB cases, using a systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [Moher et al. 2009].
Method
Literature search and inclusion criteria
To identify relevant studies, we conducted a literature search in the bibliographic databases Medline (via PubMed), Web of Science, Embase, and Iranian databases from March 2000 to January 2016. Key words used in the search included ‘tuberculosis’, ‘primary drug-resistance’, ‘ethambutol’, and related terms. Only studies that used standard methods for drug susceptibility testing (DST) of M. tuberculosis, reported primary drug-resistant data, and enrolled patients who presented with symptoms of TB were included. Studies that did not report the number of new cases with active TB, the patterns of primary drug resistance, or that considered only specific groups were excluded. To minimize the potential bias caused by a sample size too small, studies with less than 50 subjects were excluded. Studies identified by the search strategy were reviewed for eligibility based on title and abstract by two reviewers. Two investigators assessed full manuscripts of the records kept based on title and abstract independently. The term ‘primary resistance’ refers to new TB cases that have never received anti-TB drugs. The term ‘mono-resistance’ refers to resistance to one anti-TB drug. ‘Any drug resistance’ defines resistance to any drug regardless of ‘mono-resistance’ or MDR-TB, whereas ‘poly-resistance’ refers to resistance to two or more drugs excluding MDR-TB.
Data extraction
Data were extracted using an extraction form by the first author and were verified by the second author. The following data were extracted: first author, publication year, study setting, enrollment time, number of patients investigated, drug resistance profile, human immunodeficiency virus (HIV) status, and distribution of age and sex in the study population.
Quality assessment of studies
Quality assessment of included studies was performed using checklist provided by the Joanna Briggs Institute [2014].
Statistical analysis
Meta-analyses were performed using Compre-hensive Meta-Analysis version 2.2 (Biostat). Heterogeneity in the reported effects was examined using a fixed and a random effects model. Heterogeneity between studies was measured using I2 statistics in conjunction with Q-statistics. The random effects model was used if either the p value was below 0.05 or I2 was above 60, as an I2 above 60 indicates that 60% of the total observed variation is due to true heterogeneity. Begg’s rank correlation and Egger weighted regression methods in combination with a funnel plot were used to assess publication bias (p < 0.05 was considered indicative of statistically significant publication bias; funnel plot asymmetry also suggests bias in meta-analysis).
Results
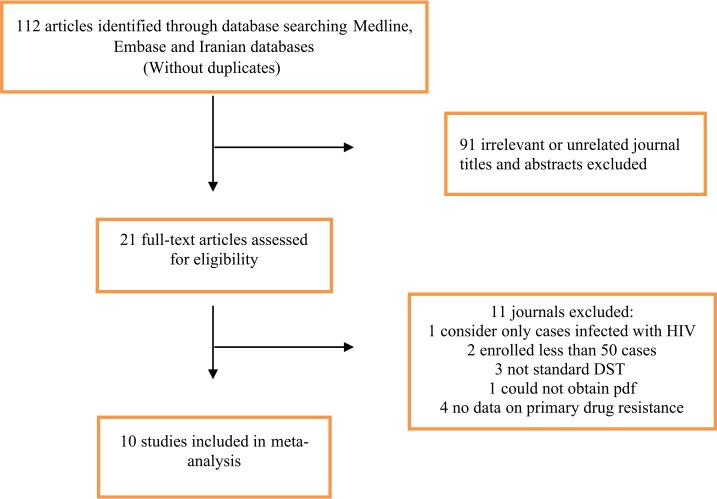
Of the 112 records identified from the Medline (Via PubMed), Web of Science, Embase, and the Iranian database searches, 21 were considered for evaluation of the full manuscript. Of the full-text articles reviewed, 10 studies fulfilled the eligibility criteria (Table 1). Figure 1 shows why records were excluded based on the assessment of title/abstract and full-text articles. The final dataset included data from different TB centers of Iran.
Table 1.
Characteristics of studies included in the meta-analysis.
| First author | Published time | Start and end date | City | Mean age | Males | Total No. of TB cases | No. of new cases | EMB resistance* |
|---|---|---|---|---|---|---|---|---|
| Mansoori [Mansoori et al. 2003] | 2003 | 1996–2000 | Tehran | 38 | 273 | 273 | 187 | 51 |
| Bahrmand [Bahrmand et al. 2000] | 2000 | 1998–1999 | Tehran | 48 | 290 | 563 | 563 | 17 |
| Heidarnejad [Heidarnejad and Nagili, 2001] | 2001 | 1999–2000 | Tabriz | 44 | 88 | 155 | 148 | 0 |
| Shamaei [Shamaei et al. 2009] | 2009 | 2000–2003 | Tehran | 45 | 317 | 548 | 363 | 11 |
| Namaei [Namaei et al. 2006] | 2006 | 2001–2002 | Mashhad | 56 | 55 | 105 | 105 | 2 |
| Mirsaeidi [Mirsaeidi et al. 2007] | 2007 | 2003–2004 | Tehran | 48 | 102 | 264 | 196 | 7 |
| Marjani [Marjani et al. 2012] | 2012 | 2003–2008 | Tehran | 52 | 251 | 554 | 508 | 17 |
| Nasiri [Nasiri et al. 2014] | 2014 | 2010–2012 | Tehran | 45 | – | 252 | 252 | 10 |
| Farazi [Farazi et al. 2013] | 2014 | 2011–2012 | Arak | 52 | 56 | 115 | 103 | 15 |
| Tavanaee Sani [Tavanaee Sani et al. 2015] | 2015 | 2012–2013 | Mashhad | 53 | 45 | 100 | 74 | 1 |
EMB: ethambutol.
Any EMB resistance.
Figure 1.
Flow diagram of search strategy.
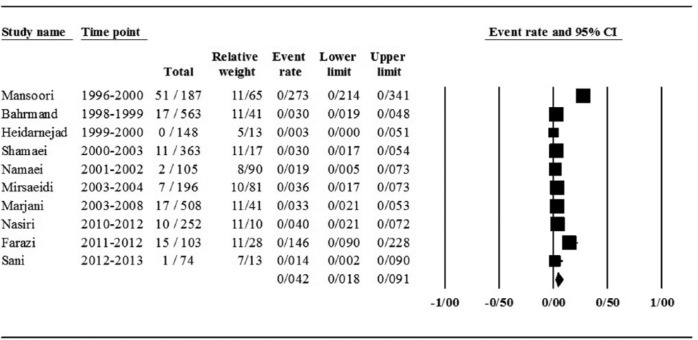
Of the 10 studies, the pooled prevalence of primary EMB-resistant TB was estimated at 4.2% [95% confidence interval (CI) 1.8–9.0]. The results were found to be frequently heterogeneous (I2 = 90.0; p = 0.00 test for heterogeneity). Details of the meta-analysis of different forms of EMB resistance, model used for heterogeneity, and the p values are provided in Table 2. Forest plot for meta-analysis of EMB-resistant TB is shown in Figure 2.
Table 2.
Meta-analysis of prevalence of primary EMB resistance in Iran.
| No. of study | Pooled estimate of EMB resistance (95% CI) | n/N* | Heterogeneity test |
Publication bias (p value) |
|||
|---|---|---|---|---|---|---|---|
| I2** | p value | Begg’s method | Egger’s test | ||||
| Resistance to EMB: | |||||||
| Any-resistance | 10 | 4.2% (1.8–9.0) | 131/2499 | 90.0 | 0.0 | 0.4 | 0.06 |
| Poly-resistance | 7 | 1.5% (0.4–6.0) | 41/1376 | 82.0 | 0.0 | 0.5 | 0.00 |
| Mono-resistance | 9 | 1.0% (0.4–3.0) | 21/1991 | 70.0 | 0.0 | 0.7 | 0.00 |
EMB: ethambutol; TB: tuberculosis.
n, number of events (EMB resistance); N, total number of new TB cases.
Random effects model were used.
Figure 2.
Forest plot of the meta-analysis on any EMB-resistant cases.
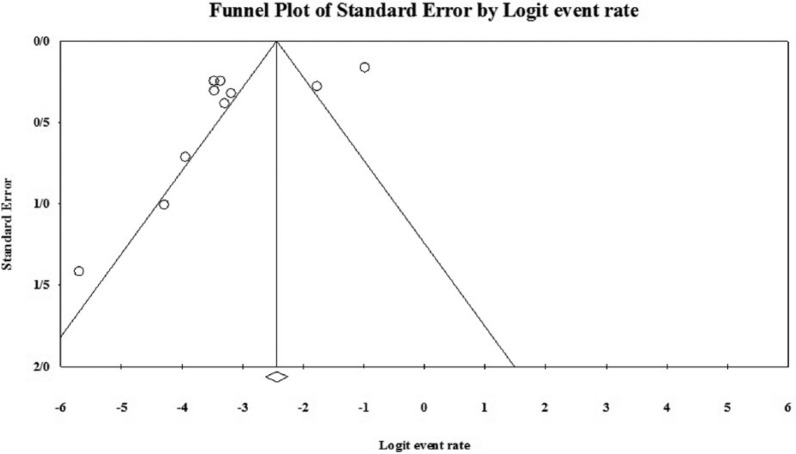
As shown in Table 2 and Figure 3, no evidence of publication bias was observed (p = 0.4 for Begg rank correlation analysis; p = 0.2 for Egger weighted regression analysis).
Figure 3.
Funnel plot of the meta-analysis on any EMB-resistant cases.
Discussion
EMB is one of the key components of the first-line anti-TB drugs and is added to the current treatment regimen for TB as a protection against unrecognized resistance to other core drugs (INH, RIF, and PZA) [Horsburgh et al. 2015]. Increasing resistance to EMB is associated with increased risk of unsuccessful TB treatment outcome and acquiring resistance to other anti-TB drugs [WHO, 2011]. Therefore, understanding the current situation of primary EMB-resistant TB is necessary for the programmatic management of TB cases in Iran. Studies have shown that primary resistance rates of M. tuberculosis to EMB vary widely, that is, from 1% to as high as 14% [Namaei et al. 2006; Farazi et al. 2013; Seif et al. 2015]. We used meta-analysis to summarize these findings, reporting on 10 studies that included 2499 patients. The pooled prevalence of primary EMB-resistant TB in Iran was found to be 4.2% (95% CI 1.8–9.0).
In many low- and middle-income settings, due to inadequate laboratory capabilities for DST, most of the MDR-TB cases are treated based on the standardized regimens that are recommended by WHO and the Union [Caminero and Van Deun, 2013; WHO, 2014]. These regimens use drug resistance survey data from a representative patient population but do not use DST results from the individual patient’s isolate [Caminero and Van Deun, 2013; Daley and and Caminero, 2013]. However, there are conflicting opinions about drug-resistant cases other than MDR-TB, largely because of the paucity of strong evidence. Definitive randomized or controlled studies have not been performed to determine the best treatment regimens for various patterns of drug resistance [WHO, 2011]. Treatment of these cases with first-line drugs alone will likely result in failure. Thus, when a decision has been made to modify standard chemotherapy, the most effective regimen should be chosen to maximize the likelihood of cure. In this regard, different regimens have been suggested by WHO for common patterns of mono- and poly-resistant TB [WHO, 2014]. For example, for EMB- and INH-resistant TB, the WHO Guide recommends a regimen with 9–12 months of RIF, PZA, and levofloxacin [WHO, 2014]. Some experts also recommend using a second-line injectable agent for treatment [Rich, 2013; Varaine and Rich, 2013]. It should be taken into consideration that, if there is a high probability that the effectiveness of any of the first-line drugs has been compromised, the patient should be treated with an MDR-TB regimen [WHO, 2014]. Therefore, using rapid DST methods to evaluate the status of resistance to anti-TB drugs during therapy is crucial for treatment of patients with mono- and poly-resistant TB.
The heterogeneity of estimates between studies and limitations associated with potential publication bias is considered a limitation of this study.
In conclusion, this review provides an overview of prevalence of primary EMB-resistant TB in Iran. Our results suggest that effective strategies to improve the DST capabilities, to minimize the acquired drug resistance, and to control the transmission of resistance should be attached importance for control of TB in Iran.
Acknowledgments
We also would like to thank from the “Clinical Research Development Center of Baqiyatallah hospital” for their kindly cooperation.
Footnotes
Funding: This study was supported by Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Mohammad Javad Nasiri, Department of Microbiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Abbas Ali Imani Fooladi, Applied Microbiology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran.
Hossein Dabiri, Department of Microbiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Ali Pormohammad, Department of Microbiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Alireza Salimi Chirani, Department of Microbiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Masoud Dadashi, Department of Microbiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Hamidreza Houri, Department of Microbiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Mohsen Heidary, Department of Microbiology, School of Medicine, Iran University of Medical Science, Tehran, Iran.
Mohammad Mehdi Feizabadi, Department of Microbiology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
References
- Bahrmand A., Velayati A.A., Bakayev V. (2000) Treatment monitoring and prevalence of drug resistance in tuberculosis patients in Tehran. Int J Tuberc Lung Dis 4: 544–549. [PubMed] [Google Scholar]
- Caminero J., Van Deun A. (2013) Guidelines for Clinical and Operational Management of Drug-Resistant Tuberculosis. Paris: International Union against Tuberculosis and Lung Disease. [Google Scholar]
- Daley C., Caminero J. (2013) Management of multidrug resistant tuberculosis. Semin Respir Crit Care Med 34: 44–59. [DOI] [PubMed] [Google Scholar]
- Farazi A., Sofian M., Zarrinfar N., Katebi F., Hoseini S., Keshavarz R. (2013) Drug resistance pattern and associated risk factors of tuberculosis patients in Iran’s central province. Caspian J Intern Med 4: 785–789. [PMC free article] [PubMed] [Google Scholar]
- Heidarnejad H., Nagili B. (2001) Primary resistance of mycobacterium tuberculosis to isoniazid, streptomycin, rifampin, and ethambutol in pulmonary tuberculosis. Arch Irn Med 4: 1–4. [Google Scholar]
- Horsburgh C., Jr., Barry C., 3rd, Lange C. (2015) Treatment of tuberculosis. N Engl J Med 373: 2149–2160. [DOI] [PubMed] [Google Scholar]
- Mansoori S., Mirabolhasani Z., Farnia P., Velayati A. (2003) The pattern of drug resistance among newly diagnosed and old cases of pulmonary tuberculosis in NRITLD. Arch Irn Med 6: 255–260. [Google Scholar]
- Marjani M., Baghaei P., Tabarsi P., Shamaei M., Mansouri D., Masjedi M., et al. (2012) Drug resistance pattern and outcome of treatment in recurrent episodes of tuberculosis. East Mediterr Health J 18: 957–961. [DOI] [PubMed] [Google Scholar]
- Merza M., Farnia P., Tabarsi P., Khazampour M., Masjedi M., Velayati A. (2011) Anti-tuberculosis drug resistance and associated risk factors in a tertiary level TB center in Iran: a retrospective analysis. J Infect Dev Ctries 5: 511–519. [DOI] [PubMed] [Google Scholar]
- Mirsaeidi M., Tabarsi P., Farnia P., Ebrahimi G., Morris M., Masjedi M., et al. (2007) Trends of drug resistant mycobacterium tuberculosis in a tertiary tuberculosis center in Iran. Saudi Med J 28: 544–550. [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151: 264–269. [DOI] [PubMed] [Google Scholar]
- Namaei M., Sadeghian A., Naderinasab M., Ziaee M. (2006) Prevalence of primary drug resistant mycobacterium tuberculosis in Mashhad, Iran. Indian J Med Res 124: 77–80. [PubMed] [Google Scholar]
- Nasiri M., Chirani A., Amin M., Halabian R., Imani Fooladi A. (2016) Isoniazid-resistant tuberculosis in Iran: a systematic review. Tuberculosis 98: 104–109. [DOI] [PubMed] [Google Scholar]
- Nasiri M., Dabiri H., Darban-Sarokhalil D., Rezadehbashi M., Zamani S. (2014a) Prevalence of drug-resistant tuberculosis in Iran: systematic review and meta-analysis. Am J Infect Control 42: 1212–1218. [DOI] [PubMed] [Google Scholar]
- Nasiri M., Rezaei F., Zamani S., Darban-Sarokhalil D., Imani Fooladi A., Shojaei H., et al. (2014b) Drug resistance pattern of mycobacterium tuberculosis isolates from patients of five provinces of Iran. Asian Pac J Trop Med 7: 193–196. [DOI] [PubMed] [Google Scholar]
- Rich M. (2013) The PIH Guide to the Medical Management of Multidrug-Resistant Tuberculosis. Boston, MA: Partners in Health. [Google Scholar]
- Seif S., Malekshahian D., Shamsi M. (2015) Prevalence of primary ethambutol resistance in new smear-positive pulmonary TB cases. Int J Mycobacteriol 4: 171–172. [Google Scholar]
- Shamaei M., Marjani M., Chitsaz E., Kazempour M., Esmaeili M., Farnia P., et al. (2009) First-line anti-tuberculosis drug resistance patterns and trends at the national TB referral center in Iran – eight years of surveillance. Int J Infect Dis 13: e236–e240. [DOI] [PubMed] [Google Scholar]
- Tavanaee Sani A., Shakiba A., Salehi M., Bahrami Taghanaki H., Ayati Fard S., Ghazvini K. (2015) Epidemiological characterization of drug resistance among mycobacterium tuberculosis isolated from patients in northeast of Iran during 2012–2013. Biomed Res Int 2015: 747085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Joanna Briggs Institute. (2014) Joanna Briggs Institute Reviewers’ Manual. Adelaide, SA, Australia: The Joanna Briggs Institute. [Google Scholar]
- Varahram M., Nasiri M., Farnia P., Mozafari M., Velayati A. (2014) A retrospective analysis of isoniazid-monoresistant tuberculosis: among Iranian pulmonary tuberculosis patients. Open Microbiol J 8: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varaine F., Rich M. (2013) Tuberculosis, Practical Guide for Clinicians, Nurses, Laboratory Technicians and Medical Auxiliaries. Boston, MA: Partner in Health. [Google Scholar]
- Velayati A., Farnia P., Mozafari M., Sheikholeslami M., Karahrudi M., Tabarsi P., et al. (2014) High prevelance of rifampin-monoresistant tuberculosis: a Retrospective Analysis among Iranian pulmonary tuberculosis patients. Am J Trop Med Hyg 90: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO). (2011) Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis – 2011 Update. Geneva: WHO. [PubMed] [Google Scholar]
- World Health Organization (WHO). (2014) Companion Handbook to the WHO Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis. Geneva: WHO. [PubMed] [Google Scholar]
- World Health Organization (WHO). (2015) Global Tuberculosis Report 2015. Geneva: WHO. [Google Scholar]