Abstract
Polyethylene glycol (PEG) is a low-cost and advantageous embedding medium, which maintains the majority of cell contents unaltered during the embedding process. Some hard or complex plant materials are better embedded in PEG than in other usual embedding media. However, the histochemical tests for phenolics and lignins in PEG-embedded plant tissues commonly result in false negatives. We hypothesize that these false negatives should be prevented by the use of distinct fixatives, which should avoid the bonds between PEG and phenols. Novel protocols for phenolics and flavanols detection are efficiently tested, with fixation of the samples in ferrous sulfate and formalin or in caffeine and sodium benzoate, respectively. The differentiation of lignin types is possible in safranin-stained sections observed under fluorescence. The Maule’s test faultlessly distinguishes syringyl-rich from guaiacyl- and hydroxyphenyl-rich lignins in PEG-embedded material under light microscopy. Current hypothesis is corroborated, that is, the adequate fixation solves the false-negative results, and the new proposed protocols fill up some gaps on the detection of phenolics and lignins.
Keywords: epifluorescence, flavanols, lignins, plant anatomy, plant histochemistry, polyphenols, tannins
Introduction
Polyethylene glycol (PEG) has been attested as a good embedding medium for plant histochemistry, except for the detection of phenolic substances.1 PEG general efficiency relies on its solubility in water and also in the maintenance of the lipophilic cell contents.1,2 In addition, paraffin and methyl methacrylate-based resin (Historesin) are more expensive media than PEG, and their use demands dehydration of the material in a gradual series of ethanol, butanol, isoamyl acetate, butyl acetate, xylene, and so on.2,3 In contrast, PEG-embedding protocol involves a few steps in gradual PEG aqueous solutions, and is efficient for plant samples with cells and tissues of different sizes and degrees of hardness.1,2
The routines of plant anatomy laboratories commonly involve the use of paraffin, paraffin-like polymers (such as Paraplast), or methyl methacrylate (Historesin). PEG has been used for difficult-embedding materials such as wood fragments,4–8 but it is adequate for animal or plant samples, with distinct hardness and complex tissue arrangements. For instance, hard insect galls (with trichomes covering, and a mix of parenchymatic and lignified tissue layers), bean seeds, and leaves with several trichomes may be successfully embedded in PEG, sectioned in microtome (20–40 µm thickness), and submitted to double staining or histochemical tests.1
The histochemical detection of starch, reducing sugars, proteins, lipids, and terpenoids is perfectly possible both in plant materials embedded in PEG and in hand-sectioned materials. However, the detection of polyphenols, flavanols, and lignins has been unsuccessful with PEG-embedded material.1 Such technical failure probably occurs because PEG links to the hydroxyl groups of the phenolics through strong hydrogen bonds,9,10 precipitating the phenol molecules11,12 or simply covering the reactive sites.1
Phenolics are secondary metabolites produced mainly by land plants,13 usually deposited in vacuoles or cell walls.13,14 Polyphenols, tannins, and flavonoids are largely considered plant protectors against herbivores and pathogens.13,14 However, the correlation between defenses against herbivory and levels of secondary metabolites, such as phenolics, tannins, and flavonoids, has been doubted.15 In contrast, phenolics and flavanols have been proved to play important developmental, physiological, and structural roles,13 for instance, acting as inhibitors of indole-3-acetic acid (IAA) oxidases.16 IAA oxidases degrade the IAA, one of the most important plant hormones, associated with cell hypertrophy and plant growth. Further studies on the co-localization of phenolics, flavanols, auxins, and other hormones in plant tissues may indicate additional steps on how these compounds act in plant cell development.17,18 The phenolics act in the avoidance of excessive damage of reactive oxygen species (ROS) in cellular membranes and organelles.13,18–22 They are also important antioxidant compounds, capturing the ROS, protecting plant structures from ultraviolet (UV) radiation, and avoiding the programmed cell death.23,24 Such functions may be triggered in biotic stresses, water deficiency, and excessive light exposition.13,14,19,20,22,25 Some flavonoids, such as anthocyanins in the vacuoles, are important to give purple, pink, or blue color to some plant organs, acting as attractive traits for specialist pollinators and dispersers.13,23,24
The lignins are deposited in the cell walls, rigidifying and waterproofing these outer structures of plant cells.26,27 They are important in plant support, and are largely present in sclereids, fibers, and tracheary elements, and are key innovations for the conquest of the terrestrial environments by land plants.27,28 Lignins in vessel elements and tracheids avoid the collapse of these plant cells, which are submitted to the negative pressure of leaf transpiration, essential for water transport from soil toward leaves.29 The proportion of lignin residues may be distinct among taxonomic plant groups, as well as among cell types of the same species.27,30 However, the functional relationship of such distinctness remains unclear.
Lignin precursors capture ROS both for ordinary differentiation processes and under stressing conditions. Therefore, the accumulation of lignins in parenchymatic cells are direct responses to distinct types of stress.20,22,27 Also, the development of sclerenchyma layers in insect galls, and of sclereids and lignified fiber sheaths in xeromorphous leaves, involves stressful conditions.18,22 Moreover, agronomists are studying, selecting, and/or genetically enhancing cultivars with the lower possible amount of lignins.30 In addition, as less stressed plants produce less lignins and polyphenols, studies on the effects of distinct stresses in agricultural plants are demanded.19,20,31 Consequently, cheaper and faster ways of diagnosing such kinds of substances in plant structures may be useful for monitoring the distribution of phenolics and lignins in plants.
To develop novel protocols for detection of lignins and polyphenols in native and cultivated plants, we hypothesized that distinct fixative methodologies could avoid the bonds between PEG and phenolic molecules. In an effort to detect phenolics and lignins in PEG-embedded samples, we test several fixatives, stainings, and histochemical reagents in stems of two native plants of distinct families (Miconia albicans, Melastomataceae, and Schinus polygamus, Anacardiaceae), and of a largely studied agricultural plant (tobacco, Nicotiana tabacum). Current analyses provide protocols for the detection of phenolics and lignins in sections with standardized thickness, and the avoidance of false-negative results.
Materials and Methods
Stem fragments (0.5 cm) (n = 3) of M. albicans (Sw.) Steud. (Melastomataceae), S. polygamus (Cav.) Cabrera (Anacardiaceae), and N. tabacum L. (cultivar Havana [cv Hav.] 425) (Solanaceae) in secondary growth were sampled and submitted to distinct treatments (T1, T2, T3, T4, and T5, analyzed under light microscopy; and T6, analyzed under epifluorescence microscopy).
T1—Control—No Fixatives
The freshly collected fragments were hand-sectioned, submitted to histochemical reactions for polyphenols, proanthocyanidins (condensed tannins), lignins, and flavanols (Table 1).
Table 1.
Staining Procedures and Respective Detected Metabolites.
| Staining | Substance Detected | Steps | References |
|---|---|---|---|
| 3% ferric chloride | Soluble polyphenols (black precipitates) | 3% ferric chloride (5–15 min) | Perkin et al.32; Johansen3 |
| DMACA | Flavanols (black or dark blue precipitates) | (1) Fixation of sections (5 min; 0.5 g pure caffeine + 0.5 g sodium benzoate + 10 ml distilled water + 90 ml butyl alcohol) (2) Staining in DMACA solution (15–120 min; 1 g DMACA + 50 ml distilled water + 9.2 ml HCl + 50 ml ethyl alcohol) |
McMurrough and McDowell33; Feucht et al.34 |
| Vanillin/hydrochloric acid | Proanthocyanidins (condensed tannins; pink precipitates) | (1) 0.5 g (vanillin) + 100 ml (9% HCl) for 15–30 min (2) Mount in 10% HCla or glycerin acidified medium |
Gardner35 |
| Wiesner’s test | Lignin (pink cell walls) | (1) Solution A: 1 g phloroglucinol + 100 ml of 95% ethyl alcohol (2) Solution B: 18% HCl. (3) Submit the sections to Solution A + B (1:1) for 1–5 min, and mount slides with solution Ba |
Allen and Tollens36; Kisser and Lohwag37; Johansen3 |
| Maule’s test | Lignin (red, orange, or brown cell walls) | (1) 1% KMnO4 (5 min) (2) Wash in distilled water (3) 10% HCl (until the sections become dark brown: 5–10 min) (4) Wash in distilled water (2×) (5) Mount on slides with concentrated ammonium hydroxidea and immediately take photographs (6) Syringyl lignins (S-type) stain red; guaiacyl (G-type), and hydroxyphenyl (H-type) lignins stain brown |
Patten et al.30 |
| Sudan black B | Oils, suberin, wax, cutin (dark blue or black) | Saturated and filtered solution of Sudan black B in 70% ethanol | Jensen38 |
| Astra blue and basic fuchsin | Lignin (fluorescence) | (1) 0.5% basic fuchsin in alcohol (2 min) (2) 5% astra blue (15 sec) (3) 0.5% basic fuchsin in alcohol (20 sec) (4) Wash in distilled water (10 sec) (5) Mount in glycerin 50% |
Kraus et al.39; Souza et al.40 |
| Astra blue and safranin | Lignin (fluorescence) | (1) 1% astra blue and 1% safranin (20 sec) (2) Wash in distilled water (10 sec) (3) Mount with 50% glycerin |
Souza et al.40; Bukatsch41 |
Abbreviation: DMACA, p-dimethylamino-cinnamaldehyde.
Be careful: The corrosive mounting solution should not leak out and reach the microscope.
T2—Fixation in Karnovsky
The samples were fixed in Karnovsky’s solution42 (Table 2); embedded in PEG; sectioned; submitted to histochemical reactions for detection of polyphenols, lignins, flavanols, and proanthocyanidins (Table 1); and mounted in slides with distilled water. Blank sections (without staining) were also mounted and analyzed. Suberin and lignins were distinguished in stem sections submitted to Sudan black B (Table 1).
Table 2.
Fixation Procedures Previous to Embedding in PEG.
| Fixative | Composition | Objective | References |
|---|---|---|---|
| Karnovsky’s solution | 2.5% glutaraldehyde and 4.5% formaldehyde in phosphate buffer (0.1 M, pH 7.2) | Histological fixation | Karnovsky42 |
| Ferrous sulfate/formalin | 4 ml of 37% formaldehyde, 10 g of Iron(II) sulfate heptahydrate, and fill up to 100 ml with distilled water | Fixation with precipitation of soluble phenolics (black precipitates) | Johansen3 |
| Caffeine/sodium benzoate | 0.5 g pure caffeine, 0.5 g sodium benzoate, 10 ml distilled water, 90 ml butyl alcohol | Fixation of the polyphenols/flavanols | Feucht et al.34 |
Abbreviation: PEG, Polyethylene glycol.
T3—Fixation With Ferrous Sulfate/Formalin
Fresh fragments fixed in ferrous sulfate and formalin3 (Table 2) were embedded in PEG, sectioned, and mounted in distilled water for polyphenols detection.
T4—Fixation in Caffeine/Sodium Benzoate
Fresh fragments fixed in caffeine and sodium benzoate (Tables 1 and 2) for 72 hr were embedded in PEG, sectioned, and divided in three groups. Sections of group 1 were incubated in DMACA (p-dimethylaminocinnamaldehyde), group 2 in ferric chloride, and group 3 in vanillin/hydrochloridric acid (Table 1), for detection of flavanols, polyphenols, and condensed tannins, respectively.
T5—Fixation in Karnovsky and Caffeine/Sodium Benzoate
Fresh fragments fixed in Karnovsky’s solution (Table 2) were postfixed in caffeine and sodium benzoate (Tables 1 and 2) for 48 hr, embedded in PEG, sectioned, and incubated in DMACA, ferric chloride, and vanillin/hydrochloridric acid (Table 1), for detection of flavanols, polyphenols, and condensed tannins, respectively.
T6—Fixation in Karnovsky—Epifluorescence Microscopy
For distinguishing the types of lignins, sections of N. tabacum stems, fixed in Karnovsky and embedded in PEG, were mounted (1) without staining, (2) stained with basic fuchsin and astra blue, and (3) stained with safranin and astra blue (Table 1), and analyzed under light and epifluorescence microscopy (EKB-2F, Eikonal; São Paulo, Brazil). Violet (λ390–400), blue (λ460–470), and green light (λ520–530) filters were used for the detection of lignins. Also, sections submitted to Wiesner’s and Maule’s reagents were observed under light and epifluorescence microscopes.
Embedding in PEG
Following the steps described by Ferreira et al.,1 plant samples were transferred from the fixative solution, washed in distilled water (3× of 5 min), and placed in 25% PEG (w/v) in a 60C stove. We used PEG 6000, but it is also possible to use PEG polymers with molecular mass between 1500 and 6000. When the volume of the recipient reached half of the initial volume (24–48 hr), a solution of 90% PEG was added up to the initial volume. After 24 to 48 hr of evaporation, the fragments were embedded in blocks, using 90% to 100% PEG2 (modified by the suppression of Arabic gum). The suppression of Arabic gum resulted in friable blocks, and extra care was necessary during sectioning in microtome. The blocks were kept in the freezer (−16C) before sectioning, to maintain them hard and cold. The hardened PEG blocks were bonded in wood blocks with pure PEG or 90% PEG, to be stuck in the microtome for sectioning.
PEG Blocks Sectioning
During sectioning in microtome (20–40 µm), the blocks usually crumble/break. To prevent such breaking, the block surface was moisturized with a wet paintbrush before every single section. The humid sections were carefully collected with a wet paintbrush or a stylet, and immediately immersed in warm water in a Petri dish (35C–50C).
The histochemical analyses or histological staining were performed (T2–T6) with the sections directly collected from the Petri dishes, similarly to fresh handmade sections (T1). After the histochemical tests or histological staining, the sections were mounted between slides and coverslips with water (50% glycerin or jelly glycerin may be used; see references2,3,40; or other plant histology manuals).
Results
T1 (Controls)
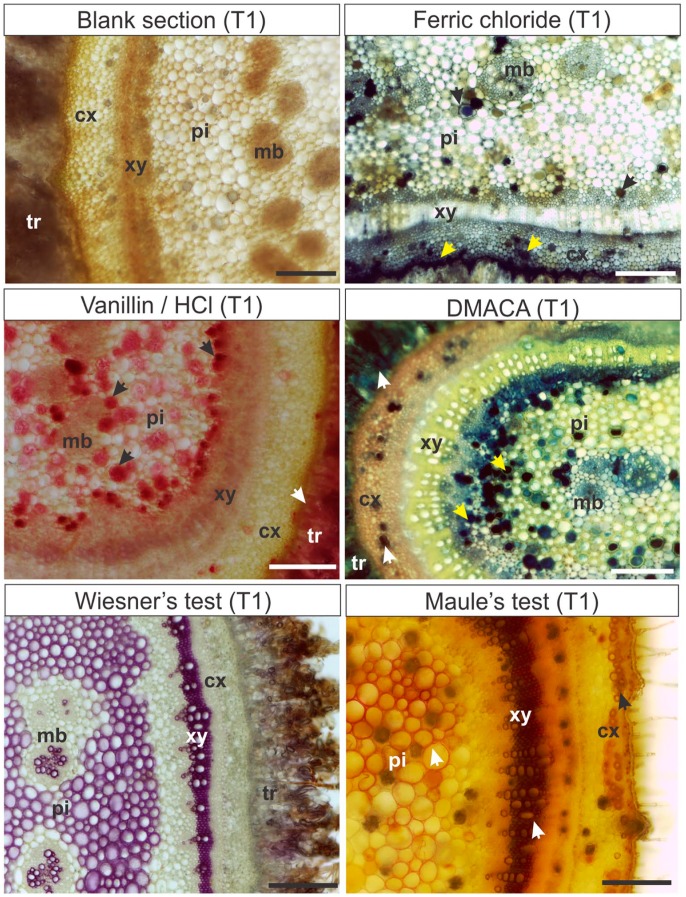
The T1 treatment was used as control for the other treatments (Table 3). Histochemical tests in fresh material detected soluble polyphenols, flavanols, and proanthocyanidins in trichomes, and cortical and pith parenchyma of M. albicans stems (Fig. 1). In these stems, lignins were detected by Wiesner’s test in arachnoid trichomes, cortical sclereids, secondary xylem, pith parenchyma, and xylem of the medullary bundles (Fig. 1). The Maule’s test applied to the fresh material stained lignins in orange in the xylem, and in brownish-orange in pith parenchyma and cortical sclereids (Fig. 1).
Table 3.
Summary of Results After Each Treatment (T1, T2, T3, T4, T5, and T6).
| Staining | T1 | T2 | T3 | T4 | T5 | T6 |
|---|---|---|---|---|---|---|
| Blank sections | NA | NA | + (*) | NA | NA | + |
| Ferric chloride | + | − | NA | + (*) | + (*) | NA |
| Vanillin/HCl | + | − | NA | − | − | NA |
| DMACA | + | − | NA | + (*) | + (*) | NA |
| Wiesner’s test | + | ± | NA | NA | NA | + |
| Maule’s test | + | + (*) | NA | NA | NA | NA |
| Safranin/astra blue | + | + | NA | NA | NA | + (*) |
| Basic fuchsin/astra blue | + | + | NA | NA | NA | + |
| Sudan black B | + | + | NA | NA | NA | NA |
Results: (+) positive; (−) false negative; (±) sometimes false negatives; (*) best treatments for detection of each phenolic derivative; (NA) not applicable. Abbreviation: DMACA, p-dimethylamino-cinnamaldehyde.
Figure 1.
Histochemical analyses with hand-sectioned fresh material (T1) in Miconia albicans stems. T1: Hand-sectioned fresh material submitted to the histochemical reactions. Arrows: Sites of positive results. Scale bars: 200 µm. Abbreviations: cx, cortex; pi, pith; xy, xylem; mb, medullary bundle; tr, trichome; DMACA, p-dimethylamino-cinnamaldehyde.
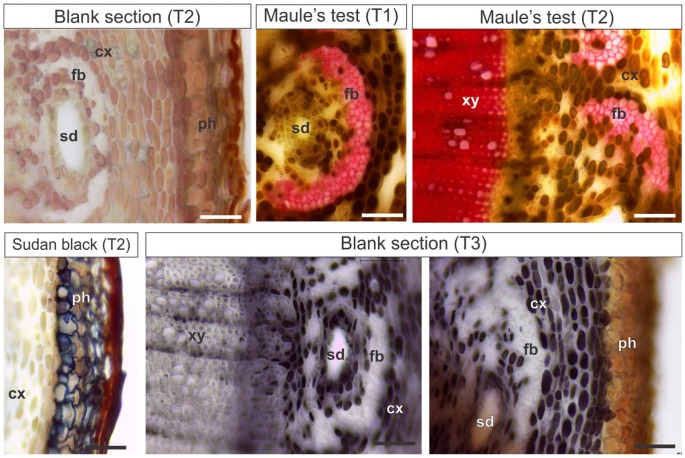
In S. polygamus, the perivascular fibers, secondary xylem, and pith parenchyma stained red with Maule’s test (Fig. 2) and pink with Wiesner’s test. Sudan black B detected the presence of suberin in cell walls of the phellem (suber; Fig. 2). Phenolics were detected in gray by ferric chloride in cortical and pith parenchyma cells.
Figure 2.
Histochemical analyses in Schinus polygamus stems (T1, T2, and T3). T1: Hand-sectioned fresh material submitted to the histochemical reactions. T2: Material fixed in Karnovsky’s solution, embedded in PEG, sectioned, and submitted to the histochemical reactions. T3: Material fixed in ferrous sulfate and formalin, embedded in PEG, and sectioned. Scale bars: 50 µm. Abbreviations: PEG, polyethylene glycol; cx, cortex; fb, perivascular fibers; sd, secretory duct; ph, phellem (suber); xy, xylem.
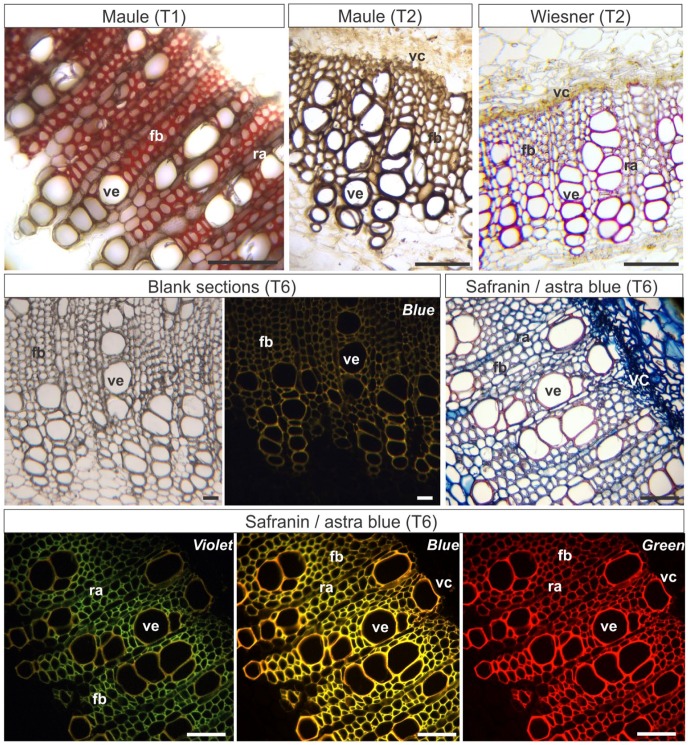
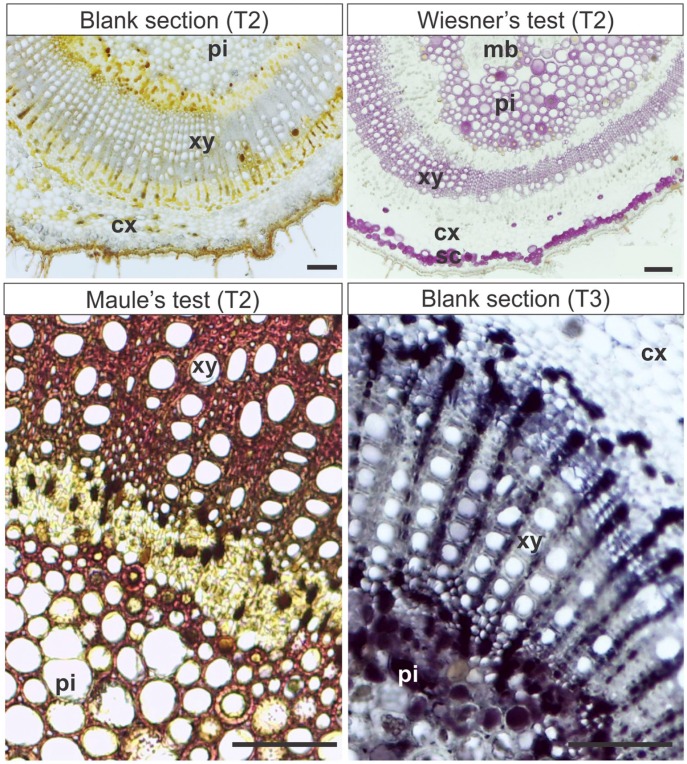
In N. tabacum stems, soluble polyphenols were not detected, and lignins were detected in radial parenchyma, fibers, and vessel elements of the secondary xylem with Wiesner’s reaction. The test of Maule in fresh material stained lignins in brown in the vessel elements and radial parenchyma cells of the secondary xylem, and in reddish-orange in secondary xylem fibers (Fig. 3).
Figure 3.
Histochemical and epifluorescence tests in stems in secondary growth of Nicotiana tabacum. T1: Hand-sectioned fresh material submitted to the histochemical reactions. T2: Material fixed in Karnovsky’s solution, embedded in PEG, sectioned, and submitted to the histochemical reactions. T6: Material fixed in Karnovsky’s solution, embedded in PEG, sectioned, and stained. Violet, Blue, Green: Epifluorescence microscopy photographs were taken under violet, blue, or green light (indicated in each image). Scale bars: 50 µm. Abbreviations: PEG, Polyethylene glycol; fb, xylem fibers; ra, radial parenchyma; ve, vessel element; vc, vascular cambium.
T2
The blank sections fixed in Karnovsky’s solution and embedded in PEG showed some brown colored vacuoles (Fig. 4). The tests with ferric chloride, vanillin, and DMACA in samples fixed with Karnovsky’s solution did not stain the polyphenols after embedding in PEG (Table 3).
Figure 4.
Histochemical analyses in Miconia albicans stems embedded in PEG (T2 and T3). T2: Material fixed in Karnovsky’s solution, embedded in PEG, sectioned, and submitted to the histochemical reactions. T3: Material fixed in ferrous sulfate and formalin, embedded in PEG, and sectioned. Scale bars: 100 µm. Abbreviations: PEG, Polyethylene glycol; pi, pith; xy, xylem; cx, cortex; mb, medullary bundle; sc, cortical sclereids.
Wiesner’s test weakly stained lignins in M. albicans and N. tabacum (Figs. 3 and 4), but did not stain any tissues in S. polygamus. Maule’s test detected lignins in red in perivascular fibers, secondary xylem, and pith parenchyma of S. polygamus (Fig. 2). In N. tabacum stems, Maule’s test stained the cell walls of the vessel elements in dark brown, and radial parenchyma and secondary xylem fibers in brownish-orange (Fig. 3). The pith lignified parenchyma and the secondary xylem of M. albicans stained red, cortical sclereids stained light brown (Fig. 4). Sudan Black B detected suberin in cell walls of phellem (suber; Fig. 2).
T3
The polyphenols were detected (Table 3) in S. polygamus (Fig. 2) and M. albicans (Fig. 4), in sections previously fixed in ferrous sulfate in formalin and embedded in PEG. As in controls, N. tabacum had no detectable phenolic inclusions.
T4
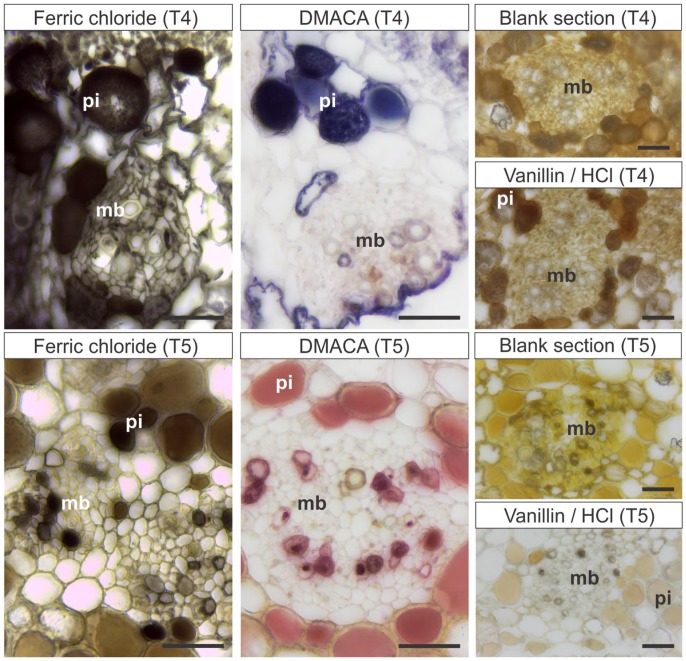
The polyphenols (ferric chloride test) and flavanols (DMACA test) were detected (Table 3), but the fixation with caffeine and sodium benzoate had not adequately preserved the tissues (Fig. 5). Vanillin/hydrochloridric acid did not detect condensed tannins (Table 3).
Figure 5.
Histochemical analyses for detection of polyphenols and flavanols in stems of Miconia albicans fixed or postfixed in caffeine and sodium benzoate, previous to embedding in PEG (T4, T5). T4: Material fixed in caffeine and sodium benzoate, embedded in PEG, sectioned, and submitted to histochemical reactions. T5: Material fixed in Karnovsky’s solution, postfixed in caffeine and sodium benzoate, embedded in PEG, sectioned, and submitted to histochemical reactions. The vanillin/hydrochloride acid tests show false-negative results in T4 and T5. Scale bars: 50 µm. Abbreviations: PEG, Polyethylene glycol; DMACA, p-dimethylamino-cinnamaldehyde; pi, pith; mb, medullary bundle.
T5
The polyphenols were detected with the ferric chloride test (Table 3), and the cytological content was preserved as well (Fig. 5). Flavanols stained magenta with DMACA reaction (Fig. 5), but condensed tannins were not detected with vanillin/hydrochloridric acid (Table 3).
T6
The lignified and non-lignified cell walls were well distinguishable in the sections of N. tabacum stems analyzed under epifluorescence microscopy (Table 3). Lignins in blank sections were weakly marked under epifluorescence (Fig. 3). Lignins stained pink with safranin and astra blue, or basic fuchsin and astra blue under light microscopy (Fig. 3). The autofluorescence of totally and partially lignified cell walls was observed both in blank and stained sections (Fig. 3). Best results were obtained with safranin/astra blue staining, with distinctions among lignified cell types evidenced with violet light/violet filter (Fig. 3). Cell walls of fibers were green, of radial parenchyma were yellowish-green, and of vessel elements were yellow under violet light. All the used light wavelengths provided good contrast in sections stained with astra blue/safranin (Fig. 3).
Discussion
Samples embedded in PEG, previously fixed in Karnovsky’s solution (T2), had produced false-negative results for the detection of polyphenols, tannins, flavanols, and lignins (Table 3), reinforcing the observations of Ferreira et al.1 Herein, we proposed other successful protocols for the detection of these secondary compounds in PEG-embedded samples.
Total Phenolics
Total polyphenols may be detected by fixation in ferrous sulfate and formalin (T3) previous to PEG embedding. The fixation with ferrous sulfate and formalin previous to the PEG-embedding procedures precipitates the polyphenols and consequently avoids their binding to PEG. Moreover, the fixation with caffeine and sodium benzoate before the incubation of the sections in DMACA coagulates the flavanols,34 avoiding their leaching during the staining. Therefore, T4 and T5 may be used for the detection of phenolics. The overall presence of polyphenols in the native plants, such as M. albicans and S. polygamus, confirms the previous knowledge on the chemical profile of the Melastomataceae and Anacardiaceae,1,21,24 and may be related to molecular defenses against ROS and UV radiation damages on these plants, when growing under excessive irradiation.13,14,25 On the contrary, the absence of polyphenols in cultivated stems of N. tabacum cv. Hav. indicates that the plants were grown under low stress, which is an ideal condition for agricultural plants.19,20,31 Histochemical evaluation of agricultural plants should help agronomists to select the plant cultivars of interest.
Flavanols
Flavanols are commonly leached during the PEG-embedding process (T2). The incubation in caffeine and sodium benzoate (before the embedding in PEG; T4 and T5) allows the maintenance of the flavanols, even after fixation of the samples in Karnovsky’s solution. Such result indicates that the washing of the phenolic compounds during the PEG-embedding process1 was avoided due to the postfixation process.
The reactions with DMACA are positive after T4 and T5, and, therefore, either the fixation or postfixation with caffeine and sodium benzoate preserves the flavanols. The vacuolar content stained in magenta (T5) was also considered positive for flavanols, for the reaction of the DMACA starts dark-green between 1 and 2 hr of reaction, and thereafter may turn to magenta.33 Current results confirm that at least part of the phenolics observed in M. albicans and S. polygamus are flavanols, and, therefore, they also participate in plant development, probably avoiding excessive damage of oxidative stress in these native plants.23,24
Lignins
The comparison of Wiesner’s and Maule’s test indicates distinct types of lignin in the stems of S. polygamus, N. tabacum, and M. albicans. The lignins are always detected by Maule’s test, in red or brown, depending on the lignin type.30 The lignin chains rich in syringyl propane (S-type, sinapyl alcohol) stain red, whereas guaiacyl (G-type, coniferyl alcohol) and hydroxyphenyl (H-type, coumaryl alcohol) lignins stain brown. The orange or brownish-orange reactions with Maule’s test indicate intermediary levels of sinapyl and coniferyl/coumaryl alcohols in M. albicans stems. The variation in color with Maule’s test is also observed in N. tabacum stems, whose cell walls of the fibers reacted in red (syringyl lignin) in T1, and brownish-orange (mixed lignin) in T2. The mixed patterns in M. albicans and N. tabacum may indicate the compartmentalization of functions among distinct lignified cell types. Nevertheless, the differences on lignin composition within a single tissue or organ are not well understood, and future studies should elucidate its implication in plant physiology.
The differentiation of lignin types under blue and violet lights was also observed under epifluorescence, and could be distinguished on the basis of fluorochromasia in safranin-stained sections,43 which confirmed Maule’s test results under light microscopy. Under epifluorescence with violet light, the vessel elements with hydroxyphenyl or guaiacyl lignin (detected by Maule’s test) stained yellow, and the fibers with syringyl lignin (also detected by Maule’s test) stained green. Another use of safranin in epifluorescence has been supposedly the improving of discerning lignified and non-lignified tissues,44,45 or even distinct lignification levels of cell walls.43,44,46
Whereas lignin, cutin, and suberin are autofluorescent cell wall substances,47 we indicate Sudan black B as an additional counter-test to distinguish the suberization or lignification of cell walls.38,47 This staining technique detects the presence of lipids both in fresh samples or samples embedded in PEG.1 If suberin—a waxy substance48—is deposited in the cell wall, it will react with Sudan black B. Cutin and waxes, present in epidermal surfaces, will also react with this dye. This counter-test may be used as complementary to epifluorescence tests for lignins,47 avoiding misinterpretations. The protocols herein proposed, with distinct fixation procedures previous to embedding in PEG, solve the false negatives for the histochemical detection of polyphenols, flavanols, and lignins. For polyphenols detection, previous fixation in ferrous sulfate/formalin (T3) is indicated. Flavanols may be conserved by previous fixation with caffeine and sodium benzoate (T4) or with fixation with Karnovsky’s solution, followed by postfixation caffeine/sodium benzoate (T5). Finally, lignin types may be successfully distinguished with Maule’s test for light microscopy observation (T2), or by staining with safranin for observation under epifluorescence (T6) with blue or violet light.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: BGF and RMSI designed the experiment, performed analyses, and wrote the manuscript. LAS, LMG, RF, SCA, and WCA performed analyses and wrote the manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), Brazil; CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), Brazil; and FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais), Brazil.
Literature Cited
- 1. Ferreira BG, Teixeira CT, Isaias RMS. Efficiency of the polyethylene-glycol (PEG) embedding medium for plant histochemistry. J Histochem Cytochem. 2014;62:577–83. [DOI] [PubMed] [Google Scholar]
- 2. Kraus JE, Arduin M. [Manual básico de métodos em morfologia vegetal]. Seropédica, Brazil: Editora da Universidade Federal Rural do Rio de Janeiro; 1997. Portuguese. [Google Scholar]
- 3. Johansen DA. Plant microtechnique. New York: McGraw-Hill Book; 1940. [Google Scholar]
- 4. Rupp P. [Polyglykol als Einbettungsmedium zum Schneiden botanischer Präparate]. Mikrokosmos. 1964;53:123–8. German. [Google Scholar]
- 5. Theiler R. [Polyäthylenglykol als Hilfsmittel beim Gefrierschneiden]. Mikrokosmos. 1973;62:59–62. German. [Google Scholar]
- 6. Araújo GUC, Costa CG. [Anatomia do caule de Serjania corrugata Radlk. (Sapindaceae)]. Acta Bot Bras. 2007;21:489–97. Portuguese. [Google Scholar]
- 7. Barbosa ACF, Pace MR, Witovisk L, Angyalossy V. A new method to obtain good anatomical slides of heterogeneous plant parts. IAWA J. 2010;31:373–83. [Google Scholar]
- 8. Tamaio N. [Caracterização anatômica das madeiras de lianas de Sapindaceae utilizadas comercialmente em São Paulo – SP]. Cerne. 2011;17:533–40. Portuguese. [Google Scholar]
- 9. Patel NK, Foss NE. Interaction of some pharmaceuticals with macromolecules. I. Effect of temperature on the binding of parabens and phenols by polysorbate 80 and polyethylene glycol 4000. J Pharm Sci. 1964;53:94–7. [DOI] [PubMed] [Google Scholar]
- 10. Silanikove N, Perevolotsky A, Provenza FD. Use of tannin-binding chemicals to assay for tannins and their negative postingestive effects in ruminants. Anim Feed Sci Technol. 2001;91:69–81. [Google Scholar]
- 11. Gehrig HH, Winter K, Cushman J, Borland A, Taybi T. An improved RNA isolation method for succulent plant species rich in polyphenols and polysaccharides. Plant Mol Biol Rep. 2000;18:369–76. [Google Scholar]
- 12. Henning T. Polyethylene glycols (PEGs) and the pharmaceutical industry. Fine, Specialty & Performance Chemicals. Pharmachem. 2002;1:57–9. [Google Scholar]
- 13. Lattanzio V, Lattanzio VMT, Cardinali A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In: Imperato F, editor. Phytochemistry: Advances in research. Trivandrum, Kerala: Research Signpost; 2006. p. 23–67. [Google Scholar]
- 14. Hättenschwiler S, Vitousek PM. The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol Evol. 2000;15:238–43. [DOI] [PubMed] [Google Scholar]
- 15. Carmona D, Lajeunesse MJ, Johnson MTJ. Plant traits that predict resistance to herbivores. Funct Ecol. 2010;25:305–432. [Google Scholar]
- 16. Hori K. Insect secretions and their effect on plant growth, with special reference to hemipterans. In: Shorthouse JD, Rohfritsch O, editors. Biology of insect-induced galls. Oxford: Oxford University Press; 1992. p. 157–70. [Google Scholar]
- 17. Bedetti CS, Modolo LV, Isaias RMS. The role of phenolics in the control of auxin in galls of Piptadenia gonoacantha (Mart.) MacBr (Fabaceae: Mimosoideae). Biochem System Ecol. 2014;55:53–9. [Google Scholar]
- 18. Oliveira DC, Isaias RMS, Fernandes GW, Ferreira BG, Carneiro RGS, Fuzaro L. Manipulation of host plant cells and tissues by gall-inducing insects and adaptive strategies used by different feeding guilds. J Insect Physiol. 2016;84:103–13. [DOI] [PubMed] [Google Scholar]
- 19. Sahai OP, Shuler ML. Environmental parameters influencing phenolics production by batch cultures of Nicotiana tabacum. Biotechnol Bioeng. 1984;26:111–20. [DOI] [PubMed] [Google Scholar]
- 20. Lagrimini LM. Wound-induced deposition of polyphenols in transgenic plants overexpressing peroxidase. Plant Physiol. 1991;96:577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dias GG, Ferreira BG, Moreira GRP, Isaias RMS. Developmental pathway from leaves to galls induced by a sap-feeding insect on Schinus polygamus (Cav.) Cabrera (Anacardiaceae). An Acad Bras Cienc. 2013;85:187–200. [DOI] [PubMed] [Google Scholar]
- 22. Isaias RMS, Oliveira DC, Moreira ASFP, Soares GLG, Carneiro RGS. The imbalance of redox homeostasis in arthropod-induced plant galls: mechanisms of stress generation and dissipation. Biochim Biophys Acta. 2015;1850:1509–17. [DOI] [PubMed] [Google Scholar]
- 23. Hatier JHB, Gould KS. Anthocyanin: function in vegetative organs. In: Gould K, Davies K, Winefield C, editors. Anthocyanins: Biosynthesis, functions, and applications. New York: Springer Science and Business Media; 2009. p. 1–12. [Google Scholar]
- 24. Dias GG, Moreira GRP, Ferreira BG, Isaias RMS. Why do the galls induced by Calophya duvauae Scott on Schinus polygamus (Cav.) Cabrera (Anacardiaceae) change colors? Biochem System Ecol. 2013;48:111–22. [Google Scholar]
- 25. Grassman J, Hippeli S, Elstner EF. Plant’s defence and its benefits for animals and medicine: role of phenolics and terpenoids in avoiding oxygen stress. Plant Physiol Biochem. 2002;40:471–8. [Google Scholar]
- 26. Sarkanen KV, Ludwig CH. Lignins: occurrence, formation, structure, and reactions. New York: Wiley-Interscience; 1971. [Google Scholar]
- 27. Boerjan W, Ralph J, Baucher M. Lignin biosynthesis. Annu Rev Plant Biol. 2003;54:519–46. [DOI] [PubMed] [Google Scholar]
- 28. Jones L, Ennos AR, Turner SR. Cloning and characterization of irregular xylem4 (irx4): a severely lignin-deficient mutant of Arabidopsis. Plant J. 2001;26:205–16. [DOI] [PubMed] [Google Scholar]
- 29. Turner SR, Somerville CR. Collapsed xylem phenotype of Arabidopsis identifies mutants deficient in cellulose deposition in the secondary cell wall. Plant Cell. 1997;9:689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patten AM, Jourdes M, Brown EE, Laborie MP, Davin LB, Lewis NG. Reaction tissue formation and stem tensile modulus properties in wild-type and p-coumarate-3-hydroxylase downregulated lines of alfalfa, Medicago sativa (Fabaceae). Am J Bot. 2007;94:912–25. [DOI] [PubMed] [Google Scholar]
- 31. Van Cutsem E, Simonart G, Degand H, Faber AM, Morsomme P, Boutry M. Gel-based and gel-free proteomic analysis of Nicotiana tabacum trichomes identifies proteins involved in secondary metabolism and in the (a)biotic stress response. Proteomics. 2011;11:440–54. [DOI] [PubMed] [Google Scholar]
- 32. Perkin AG. The yellow colouring principles of various tannin matters. J Chem Soc London. 1897;71:1131–8. [Google Scholar]
- 33. McMurrough I, McDowell J. Chromatographic separation and automated analysis of flavanols. Anal Biochem. 1978;91:92–100. [DOI] [PubMed] [Google Scholar]
- 34. Feucht W, Schmid PPS, Christ E. Distribution of flavanols in meristematic and mature tissues of Prunnus avium shoots. J Plant Physiol. 1986;125:1–8. [Google Scholar]
- 35. Gardner RO. Vanillin-hydrochloric acid as a histochemical test for tannin. Stain Technol. 1975;50:315–7. [DOI] [PubMed] [Google Scholar]
- 36. Allen EW, Tollens B. [Ueber Holzzucker (Xylose) und Holzgummi (Xylan)]. Justus Liebigs Ann Chem. 1890;260:289–306. German. [Google Scholar]
- 37. Kisser J, Lohwag K. [Kritische Betrachtungen Über Die Von G. Friesen Empfohlene Holzreaktion]. Mikrochemie. 1938; 24:179–91. [Google Scholar]
- 38. Jensen WA. Botanical histochemistry. San Francisco: W.H. Freeman; 1962. [Google Scholar]
- 39. Kraus JE, Sousa HC, Rezende MH, Castro NM, Vecchi C, Luque R. Astra blue and basic fuchsin double staining of plant materials. Biotech Histochem. 1998;73:235–43. [DOI] [PubMed] [Google Scholar]
- 40. Souza LA, Rosa SM, Moscheta IS, Mourão KSM, Rodella RA, Rocha DC, Lolis MIGA. [Morfologia e anatomia vegetal: técnicas e práticas]. Ponta Grossa, Brazil: Editora Universidade Estadual de Ponta Grossa; 2005. Portuguese. [Google Scholar]
- 41. Bukatsch F. [Bemerkungen zur Doppelfärbung Astrablau-Safranin]. Mikrokosmos. 1972;61:255. [Google Scholar]
- 42. Karnovsky MJ. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol. 1965;27:137–8. [Google Scholar]
- 43. De Micco V, Aronne G. Combined histochemistry and autofluorescence for identifying lignin distribution in cell walls. Biotech Histochem. 2007;82:209–16. [DOI] [PubMed] [Google Scholar]
- 44. Vazquez-Cooz I, Meyer RW. A differential staining method to identify lignified and unlignified tissues. Biotech Histochem. 2002;77:277–82. [DOI] [PubMed] [Google Scholar]
- 45. Lux A, Morita S, Abe J, Ito K. An improved method for clearing and staining free-hand sections and whole-mount samples. Ann Bot. 2005;96:989–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bond J, Donaldson L, Hill S, Hitchcock K. Safranine fluorescent staining of wood cell walls. Biotech Histochem. 2008;83:161–71. [DOI] [PubMed] [Google Scholar]
- 47. Biggs AR. Intracellular suberin: occurrence and detection in tree bark. IAWA J. 1984;5:243–8. [Google Scholar]
- 48. Espelie KE, Sadek NZ, Kolattukudy PE. Composition of suberin-associated waxes from the subterranean storage organs of seven plants: parsnip, carrot, rutabaga, turnip, red beet, sweet potato and potato. Planta. 1980;148:468–76. [DOI] [PubMed] [Google Scholar]