Abstract
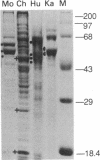
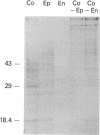
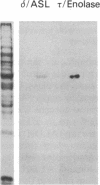
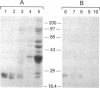
Studies of others have shown that class 3 aldehyde dehydrogenase is a major component of the epithelial cells of the mammalian cornea. Here we demonstrate by peptide sequencing that other major proteins of the corneal epithelium are also identical or related to enzymes in the human, mouse, kangaroo, chicken, and squid. Aldehyde dehydrogenase class 3 was found to be the major protein of human, mouse, and kangaroo corneal epithelial cells. Peptidyl prolyl cis-trans isomerase (cyclophilin) or a homologue thereof is strikingly abundant in the corneal epithelial cells of chicken, but not mammals, and appears to be absent from the cornea of squid. By contrast, enolase or its homologue is relatively abundant in both the mammalian and chicken corneal epithelial cells. In some instances, abundant enzymes are common to cornea and lens in the same species--for example, arginino-succinate lyase/delta 1-crystallin in the chicken and glutathione S-transferase-like protein in the squid; in other cases, the abundant proteins in the cornea have not been found as lens crystallins in any species--for example, aldehyde dehydrogenase class 3 and cyclophilin. These data suggest that enzymes and certain enzyme-crystallins have been recruited as major corneal proteins in a taxon-specific manner and may serve structural rather than, or as well as, enzymatic roles in corneal epithelial cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abedinia M., Pain T., Algar E. M., Holmes R. S. Bovine corneal aldehyde dehydrogenase: the major soluble corneal protein with a possible dual protective role for the eye. Exp Eye Res. 1990 Oct;51(4):419–426. doi: 10.1016/0014-4835(90)90154-m. [DOI] [PubMed] [Google Scholar]
- Aebersold R. H., Leavitt J., Saavedra R. A., Hood L. E., Kent S. B. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6970–6974. doi: 10.1073/pnas.84.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad H., Singh S. V., Medh R. D., Ansari G. A., Kurosky A., Awasthi Y. C. Differential expression of alpha, mu and pi classes of isozymes of glutathione S-transferase in bovine lens, cornea, and retina. Arch Biochem Biophys. 1988 Nov 1;266(2):416–426. doi: 10.1016/0003-9861(88)90273-1. [DOI] [PubMed] [Google Scholar]
- Alexander R. J., Silverman B., Henley W. L. Isolation and characterization of BCP 54, the major soluble protein of bovine cornea. Exp Eye Res. 1981 Feb;32(2):205–216. doi: 10.1016/0014-4835(81)90009-9. [DOI] [PubMed] [Google Scholar]
- Bours J., Van Doorenmaalen W. J. Th presence of lens antigens in the intra-ocular tissues of the chick eye. Exp Eye Res. 1972 May;13(3):236–247. doi: 10.1016/0014-4835(72)90105-4. [DOI] [PubMed] [Google Scholar]
- Chiou S. H. Physicochemical characterization of a crystallin from the squid lens and its comparison with vertebrate lens crystallins. J Biochem. 1984 Jan;95(1):75–82. doi: 10.1093/oxfordjournals.jbchem.a134605. [DOI] [PubMed] [Google Scholar]
- Clayton R. M., Campbell J. C., Truman D. E. A re-examination of the organ specificity of lens antigens. Exp Eye Res. 1968 Jan;7(1):11–29. doi: 10.1016/s0014-4835(68)80022-3. [DOI] [PubMed] [Google Scholar]
- Cooper D. L., Baptist E. W., Enghild J. J., Isola N. R., Klintworth G. K. Bovine corneal protein 54K (BCP54) is a homologue of the tumor-associated (class 3) rat aldehyde dehydrogenase (RATALD). Gene. 1991 Feb 15;98(2):201–207. doi: 10.1016/0378-1119(91)90174-a. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn T. J., Koleske A. J., Lindahl R., Pitot H. C. Phenobarbital-inducible aldehyde dehydrogenase in the rat. cDNA sequence and regulation of the mRNA by phenobarbital in responsive rats. J Biol Chem. 1989 Aug 5;264(22):13057–13065. [PubMed] [Google Scholar]
- Evces S., Lindahl R. Characterization of rat cornea aldehyde dehydrogenase. Arch Biochem Biophys. 1989 Nov 1;274(2):518–524. doi: 10.1016/0003-9861(89)90465-7. [DOI] [PubMed] [Google Scholar]
- Farrés J., Guan K. L., Weiner H. Primary structures of rat and bovine liver mitochondrial aldehyde dehydrogenases deduced from cDNA sequences. Eur J Biochem. 1989 Mar 1;180(1):67–74. doi: 10.1111/j.1432-1033.1989.tb14616.x. [DOI] [PubMed] [Google Scholar]
- Harding M. W., Handschumacher R. E. Cyclophilin, a primary molecular target for cyclosporine. Structural and functional implications. Transplantation. 1988 Aug;46(2 Suppl):29S–35S. doi: 10.1097/00007890-198808001-00006. [DOI] [PubMed] [Google Scholar]
- Harding M. W., Handschumacher R. E., Speicher D. W. Isolation and amino acid sequence of cyclophilin. J Biol Chem. 1986 Jun 25;261(18):8547–8555. [PubMed] [Google Scholar]
- Hempel J., Harper K., Lindahl R. Inducible (class 3) aldehyde dehydrogenase from rat hepatocellular carcinoma and 2,3,7,8-tetrachlorodibenzo-p-dioxin-treated liver: distant relationship to the class 1 and 2 enzymes from mammalian liver cytosol/mitochondria. Biochemistry. 1989 Feb 7;28(3):1160–1167. doi: 10.1021/bi00429a034. [DOI] [PubMed] [Google Scholar]
- Hohman R. J., Hultsch T. Cyclosporin A: new insights for cell biologists and biochemists. New Biol. 1990 Aug;2(8):663–672. [PubMed] [Google Scholar]
- Holmes R. S., van Oorschot R. A., Vandeberg J. L. Genetics of alcohol dehydrogenase and aldehyde dehydrogenase from Monodelphis domestica cornea: further evidence for identity of corneal aldehyde dehydrogenase with a major soluble protein. Genet Res. 1990 Oct-Dec;56(2-3):259–265. doi: 10.1017/s0016672300035369. [DOI] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. E., Jr, Brennan M. D., Hempel J., Lindahl R. Cloning and complete nucleotide sequence of a full-length cDNA encoding a catalytically functional tumor-associated aldehyde dehydrogenase. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1782–1786. doi: 10.1073/pnas.85.6.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R., Fröhli E., Steiger R. H., Schäfer R., Aoyama A. Alpha B-crystallin is a small heat shock protein. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3652–3656. doi: 10.1073/pnas.88.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh H., Araki I., Yasuda K., Matsubasa T., Mori M. Expression of the chicken 'delta 2-crystallin' gene in mouse cells: evidence for encoding of argininosuccinate lyase. Gene. 1991 Mar 15;99(2):267–271. doi: 10.1016/0378-1119(91)90137-z. [DOI] [PubMed] [Google Scholar]
- Lane W. S., Galat A., Harding M. W., Schreiber S. L. Complete amino acid sequence of the FK506 and rapamycin binding protein, FKBP, isolated from calf thymus. J Protein Chem. 1991 Apr;10(2):151–160. doi: 10.1007/BF01024778. [DOI] [PubMed] [Google Scholar]
- Messiha F. S., Price J. Properties and regional distribution of ocular aldehyde dehydrogenase in the rat. Neurobehav Toxicol Teratol. 1983 Mar-Apr;5(2):251–254. [PubMed] [Google Scholar]
- Piatigorsky J., O'Brien W. E., Norman B. L., Kalumuck K., Wistow G. J., Borras T., Nickerson J. M., Wawrousek E. F. Gene sharing by delta-crystallin and argininosuccinate lyase. Proc Natl Acad Sci U S A. 1988 May;85(10):3479–3483. doi: 10.1073/pnas.85.10.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatigorsky J., Wistow G. J. Enzyme/crystallins: gene sharing as an evolutionary strategy. Cell. 1989 Apr 21;57(2):197–199. doi: 10.1016/0092-8674(89)90956-2. [DOI] [PubMed] [Google Scholar]
- Siezen R. J., Shaw D. C. Physicochemical characterization of lens proteins of the squid Nototodarus gouldi and comparison with vertebrate crystallins. Biochim Biophys Acta. 1982 Jun 4;704(2):304–320. doi: 10.1016/0167-4838(82)90160-1. [DOI] [PubMed] [Google Scholar]
- Stapel S. O., de Jong W. W. Lamprey 48-kDa lens protein represents a novel class of crystallins. FEBS Lett. 1983 Oct 17;162(2):305–309. doi: 10.1016/0014-5793(83)80777-7. [DOI] [PubMed] [Google Scholar]
- Thomas G., Zelenka P. S., Cuthbertson R. A., Norman B. L., Piatigorsky J. Differential expression of the two delta-crystallin/argininosuccinate lyase genes in lens, heart, and brain of chicken embryos. New Biol. 1990 Oct;2(10):903–914. [PubMed] [Google Scholar]
- Tomarev S. I., Zinovieva R. D., Piatigorsky J. Crystallins of the octopus lens. Recruitment from detoxification enzymes. J Biol Chem. 1991 Dec 15;266(35):24226–24231. [PubMed] [Google Scholar]
- Tomarev S. I., Zinovieva R. D. Squid major lens polypeptides are homologous to glutathione S-transferases subunits. Nature. 1988 Nov 3;336(6194):86–88. doi: 10.1038/336086a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistow G. J., Piatigorsky J. Gene conversion and splice-site slippage in the argininosuccinate lyases/delta-crystallins of the duck lens: members of an enzyme superfamily. Gene. 1990 Dec 15;96(2):263–270. doi: 10.1016/0378-1119(90)90262-p. [DOI] [PubMed] [Google Scholar]
- Wistow G. J., Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- Wistow G., Anderson A., Piatigorsky J. Evidence for neutral and selective processes in the recruitment of enzyme-crystallins in avian lenses. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6277–6280. doi: 10.1073/pnas.87.16.6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistow G., Kim H. Lens protein expression in mammals: taxon-specificity and the recruitment of crystallins. J Mol Evol. 1991 Mar;32(3):262–269. doi: 10.1007/BF02342749. [DOI] [PubMed] [Google Scholar]
- Wistow G., Piatigorsky J. Recruitment of enzymes as lens structural proteins. Science. 1987 Jun 19;236(4808):1554–1556. doi: 10.1126/science.3589669. [DOI] [PubMed] [Google Scholar]
- Yin S. J., Vagelopoulos N., Wang S. L., Jörnvall H. Structural features of stomach aldehyde dehydrogenase distinguish dimeric aldehyde dehydrogenase as a 'variable' enzyme. 'Variable' and 'constant' enzymes within the alcohol and aldehyde dehydrogenase families. FEBS Lett. 1991 May 20;283(1):85–88. doi: 10.1016/0014-5793(91)80559-l. [DOI] [PubMed] [Google Scholar]
- de Jong W. W., Hendriks W., Mulders J. W., Bloemendal H. Evolution of eye lens crystallins: the stress connection. Trends Biochem Sci. 1989 Sep;14(9):365–368. doi: 10.1016/0968-0004(89)90009-1. [DOI] [PubMed] [Google Scholar]