Abstract
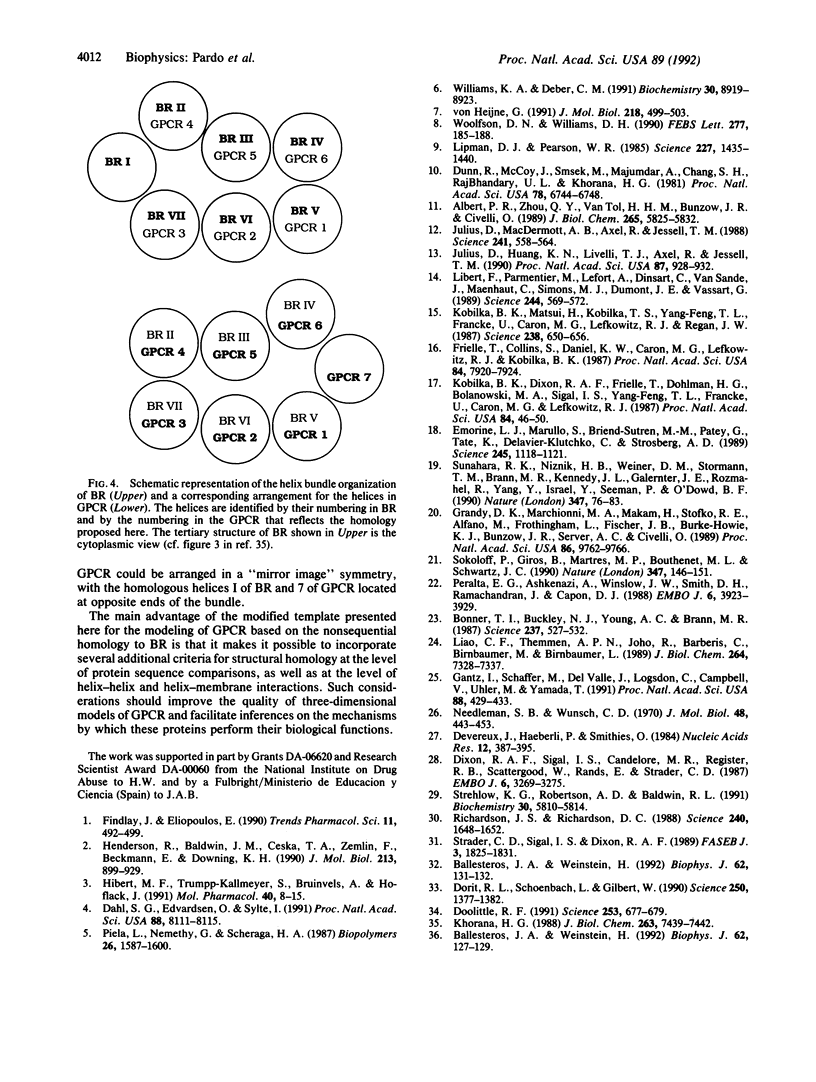
The molecular architecture of bacteriorhodopsin (BR) is commonly regarded as a structural template for the three-dimensional structure of membrane receptors that are functionally coupled to guanine nucleotide-binding regulatory proteins (GPCR). More recently, specific molecular models of such GPCR were constructed on the basis of the functional and structural relation of rhodopsin to BR as well as the sequence homology between rhodopsin and the GPCR. Such models of GPCR leave unresolved the difficulty caused by the apparent lack of any significant degree of sequence homology between the seven transmembrane helices (TMH) of BR and the portions in the sequence of the various GPCR that are considered to constitute their transmembrane domains. Evolutionary arguments offered in favor of the structural relation between BR and the opsins, and hence the GPCR, prompted our investigation of the possibility that the sequence homology, including any similarity in the distribution of kink-inducing proline residues among the helices, might have been obscured by the assumption that the TMH maintained their sequential order from BR in the evolution of the mammalian proteins. With a definition of the TMH in the neurotransmitter GPCR guided by hydropathicity predictions, and additional criteria used to define the span of each helix, optimal alignment of each pair of sequences was determined with no gaps allowed in the matching. The resulting alignment proposed here reveals considerable homology between the TMH in BR and those in GPCR, if the sequential order of the helices is ignored. These findings suggest the possibility that exon shuffling could have occurred in the proposed evolution of the GPCR gene from BR and point to a modification of the BR template to account for the correct packing of the helices in the tertiary structures of GPCR. These findings could guide the construction of three-dimensional models of the neurotransmitter GPCR on the basis of specific interhelical interactions observed in BR.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert P. R., Zhou Q. Y., Van Tol H. H., Bunzow J. R., Civelli O. Cloning, functional expression, and mRNA tissue distribution of the rat 5-hydroxytryptamine1A receptor gene. J Biol Chem. 1990 Apr 5;265(10):5825–5832. [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Dahl S. G., Edvardsen O., Sylte I. Molecular dynamics of dopamine at the D2 receptor. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8111–8115. doi: 10.1073/pnas.88.18.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Sigal I. S., Candelore M. R., Register R. B., Scattergood W., Rands E., Strader C. D. Structural features required for ligand binding to the beta-adrenergic receptor. EMBO J. 1987 Nov;6(11):3269–3275. doi: 10.1002/j.1460-2075.1987.tb02645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F. Counting and discounting the universe of exons. Science. 1991 Aug 9;253(5020):677–680. doi: 10.1126/science.1871603. [DOI] [PubMed] [Google Scholar]
- Dorit R. L., Schoenbach L., Gilbert W. How big is the universe of exons? Science. 1990 Dec 7;250(4986):1377–1382. doi: 10.1126/science.2255907. [DOI] [PubMed] [Google Scholar]
- Dunn R., McCoy J., Simsek M., Majumdar A., Chang S. H., Rajbhandary U. L., Khorana H. G. The bacteriorhodopsin gene. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6744–6748. doi: 10.1073/pnas.78.11.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emorine L. J., Marullo S., Briend-Sutren M. M., Patey G., Tate K., Delavier-Klutchko C., Strosberg A. D. Molecular characterization of the human beta 3-adrenergic receptor. Science. 1989 Sep 8;245(4922):1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- Findlay J., Eliopoulos E. Three-dimensional modelling of G protein-linked receptors. Trends Pharmacol Sci. 1990 Dec;11(12):492–499. doi: 10.1016/0165-6147(90)90050-i. [DOI] [PubMed] [Google Scholar]
- Frielle T., Collins S., Daniel K. W., Caron M. G., Lefkowitz R. J., Kobilka B. K. Cloning of the cDNA for the human beta 1-adrenergic receptor. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7920–7924. doi: 10.1073/pnas.84.22.7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz I., Schäffer M., DelValle J., Logsdon C., Campbell V., Uhler M., Yamada T. Molecular cloning of a gene encoding the histamine H2 receptor. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):429–433. doi: 10.1073/pnas.88.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandy D. K., Marchionni M. A., Makam H., Stofko R. E., Alfano M., Frothingham L., Fischer J. B., Burke-Howie K. J., Bunzow J. R., Server A. C. Cloning of the cDNA and gene for a human D2 dopamine receptor. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9762–9766. doi: 10.1073/pnas.86.24.9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Hibert M. F., Trumpp-Kallmeyer S., Bruinvels A., Hoflack J. Three-dimensional models of neurotransmitter G-binding protein-coupled receptors. Mol Pharmacol. 1991 Jul;40(1):8–15. [PubMed] [Google Scholar]
- Julius D., Huang K. N., Livelli T. J., Axel R., Jessell T. M. The 5HT2 receptor defines a family of structurally distinct but functionally conserved serotonin receptors. Proc Natl Acad Sci U S A. 1990 Feb;87(3):928–932. doi: 10.1073/pnas.87.3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D., MacDermott A. B., Axel R., Jessell T. M. Molecular characterization of a functional cDNA encoding the serotonin 1c receptor. Science. 1988 Jul 29;241(4865):558–564. doi: 10.1126/science.3399891. [DOI] [PubMed] [Google Scholar]
- Khorana H. G. Bacteriorhodopsin, a membrane protein that uses light to translocate protons. J Biol Chem. 1988 Jun 5;263(16):7439–7442. [PubMed] [Google Scholar]
- Kobilka B. K., Dixon R. A., Frielle T., Dohlman H. G., Bolanowski M. A., Sigal I. S., Yang-Feng T. L., Francke U., Caron M. G., Lefkowitz R. J. cDNA for the human beta 2-adrenergic receptor: a protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet-derived growth factor. Proc Natl Acad Sci U S A. 1987 Jan;84(1):46–50. doi: 10.1073/pnas.84.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilka B. K., Matsui H., Kobilka T. S., Yang-Feng T. L., Francke U., Caron M. G., Lefkowitz R. J., Regan J. W. Cloning, sequencing, and expression of the gene coding for the human platelet alpha 2-adrenergic receptor. Science. 1987 Oct 30;238(4827):650–656. doi: 10.1126/science.2823383. [DOI] [PubMed] [Google Scholar]
- Liao C. F., Themmen A. P., Joho R., Barberis C., Birnbaumer M., Birnbaumer L. Molecular cloning and expression of a fifth muscarinic acetylcholine receptor. J Biol Chem. 1989 May 5;264(13):7328–7337. [PubMed] [Google Scholar]
- Libert F., Parmentier M., Lefort A., Dinsart C., Van Sande J., Maenhaut C., Simons M. J., Dumont J. E., Vassart G. Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science. 1989 May 5;244(4904):569–572. doi: 10.1126/science.2541503. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Peralta E. G., Ashkenazi A., Winslow J. W., Smith D. H., Ramachandran J., Capon D. J. Distinct primary structures, ligand-binding properties and tissue-specific expression of four human muscarinic acetylcholine receptors. EMBO J. 1987 Dec 20;6(13):3923–3929. doi: 10.1002/j.1460-2075.1987.tb02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piela L., Némethy G., Scheraga H. A. Proline-induced constraints in alpha-helices. Biopolymers. 1987 Sep;26(9):1587–1600. doi: 10.1002/bip.360260910. [DOI] [PubMed] [Google Scholar]
- Richardson J. S., Richardson D. C. Amino acid preferences for specific locations at the ends of alpha helices. Science. 1988 Jun 17;240(4859):1648–1652. doi: 10.1126/science.3381086. [DOI] [PubMed] [Google Scholar]
- Sokoloff P., Giros B., Martres M. P., Bouthenet M. L., Schwartz J. C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990 Sep 13;347(6289):146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Dixon R. A. Structural basis of beta-adrenergic receptor function. FASEB J. 1989 May;3(7):1825–1832. doi: 10.1096/fasebj.3.7.2541037. [DOI] [PubMed] [Google Scholar]
- Strehlow K. G., Robertson A. D., Baldwin R. L. Proline for alanine substitutions in the C-peptide helix of ribonuclease A. Biochemistry. 1991 Jun 11;30(23):5810–5814. doi: 10.1021/bi00237a026. [DOI] [PubMed] [Google Scholar]
- Sunahara R. K., Niznik H. B., Weiner D. M., Stormann T. M., Brann M. R., Kennedy J. L., Gelernter J. E., Rozmahel R., Yang Y. L., Israel Y. Human dopamine D1 receptor encoded by an intronless gene on chromosome 5. Nature. 1990 Sep 6;347(6288):80–83. doi: 10.1038/347080a0. [DOI] [PubMed] [Google Scholar]
- Williams K. A., Deber C. M. Proline residues in transmembrane helices: structural or dynamic role? Biochemistry. 1991 Sep 17;30(37):8919–8923. doi: 10.1021/bi00101a001. [DOI] [PubMed] [Google Scholar]
- Woolfson D. N., Williams D. H. The influence of proline residues on alpha-helical structure. FEBS Lett. 1990 Dec 17;277(1-2):185–188. doi: 10.1016/0014-5793(90)80839-b. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Proline kinks in transmembrane alpha-helices. J Mol Biol. 1991 Apr 5;218(3):499–503. doi: 10.1016/0022-2836(91)90695-3. [DOI] [PubMed] [Google Scholar]