Abstract
Background
The aim of this study was to detect the expression of epidermal growth factor-containing fibulin-like extracellular matrix protein 1 (EFEMP1) and estimate its diagnostic value in prostate cancer (PCa).
Material/Methods
EFEMP1 expression in serum and urine of patients with PCa, benign controls and healthy controls at mRNA and protein level were measured by quantitative reverse transcription polymerase chain reaction (qRT-PCR) and enzyme-linked immunosorbent assay (ELISA) analysis, respectively. The chi-square test was used to analyze the relationship between EFEMP1 expression and clinical factors of patients with PCa. A receiver operating characteristic (ROC) curve was established to evaluate the potential values of EFEMP1 for the diagnosis of PCa.
Results
The relative expression of EFEMP1 was significantly decreased in patients with PCa compared with that in the benign controls and healthy individuals, both at mRNA and protein levels (P<0.05). In the postoperative serum, the EFEMP1 expression was significantly higher than that in preoperative serum at 2 levels. Urine EFEMP1 expression was also down-regulated in patients with PCa compared to that in the other 2 control groups. The low expression of EFEMP1 was obviously affected by Gleason’s score, serum PSA, pathological stage, and lymph node metastasis. Moreover, there was a significant inverse correlation between EFEMP1 expression and PSA levels. The ROC curve revealed that EFEMP1 distinguished PCa patients from healthy controls, with a high AUC of 0.908, corresponding with high sensitivity and specificity, which was significantly higher than the PSA value.
Conclusions
Serum EFEMP1 is down-regulated and involved in the progression of PCa. It may serve as a useful diagnostic biomarker, with better diagnostic accuracy than PSA in PCa.
MeSH Keywords: Diagnosis, Epidermal Growth Factor, Prostatic Neoplasms
Background
Prostate cancer (PCa), originating from a gland in the male reproductive system near the bladder, is one of the most commonly diagnosed malignancies and the sixth leading cause of cancer-related death in men worldwide, especially in developed countries [1]. According to Gleason’s score method, PCa is stratified to stages I, II, III, and IV [2]. Early detection (diagnosed at stage I, II, or III) is crucial for the successful management of this disease, with an increase 5-year survival rate from 33% to 100%, whereas the 5-year survival rate in patients with stage IV cancer is low (27%) [3,4]. Although this disease progresses very slowly, patients usually miss appropriate treatment due to the absence of apparent symptoms at early stage. Currently, the prostate-specific antigen (PSA) assay and the digital rectal examination (DRE) are considered as the most useful screening methods for detection of PCa [5–7]. However, PSA remains controversial because it is not significantly specific for PCa and its elevated level frequently presents in benign pathologies, which have led to high rates of overdiagnosis [7–9]. Therefore, available biomarkers are needed for the early diagnosis of PCa.
Epidermal growth factor-containing fibulin-like extracellular matrix protein 1 (EFEMP1, also known as fibulin-3), is a member of the fibulin family of secreted glycoproteins, which contains a series of epidermal growth factor (EGF)-like modules and is distributed widely [10,11]. Initially, the EFEMP1 is identified as a senescence protein, which takes part in processes such as regulation of body weight and behavioral control [12–14]. In human cancers, the other fibulin family members have been reported to play crucial roles in tumorigenesis through modulating cell morphology, growth, adhesion, and motility [10,15]. The roles of EFEMP1 in tumorigenesis remain controversial due to its association with tumor suppressive functions and oncogenic activities [16], and there has been little research on the relationship between EFEMP1 and PCa.
In this study, we detected the expression of EFEMP1 in serum of PCa patients, benign controls, and healthy controls and analyzed its relationship with clinical factors of patients. We also evaluated the diagnostic value of EFEMP1 in PCa.
Material and Methods
Patients and specimens
The study was approved by the Institutional Review Board of the Affiliated Tumor Hospital of Xinjiang Medical University, and written informed consent was obtained from each participant. We enrolled 96 patients diagnosed with PCa: 60 patients with benign prostate hyperplasia (BPH) were recruited as benign controls and 26 healthy donors were recruited as healthy controls.
We obtained 5-ml blood samples from patients with PCa, benign controls, and healthy controls. Corresponding urine samples were collected from all participants in the study. All patients with PCa underwent radical prostatectomy, and 40 postoperative serum samples were acquired. All blood samples were immediately centrifuged at 3000 rpm for 10 min at 4°C and then the supernate was stored at −80°C until use. Clinicopathological characteristics of patients participating in the study are summarized in Table 1.
Table 1.
Relationship between EFEMP1 expression and clinical factors of patients with PCa.
| Clinicopathological characteristics | Cases (n=96) | EFEMP1 expression | P | |
|---|---|---|---|---|
| High (n=45) | Low (n=51) | |||
| Age | 0.101 | |||
| <70 | 48 | 26 | 22 | |
| ≥70 | 48 | 19 | 29 | |
| Gleason’s score | 0.002 | |||
| 4–6 | 32 | 22 | 10 | |
| 7 | 40 | 17 | 23 | |
| 8–10 | 24 | 6 | 18 | |
| Serum PSA | 0.000 | |||
| <4 ng/ml | 29 | 6 | 23 | |
| 4–10 ng/ml | 37 | 13 | 24 | |
| >10 ng/ml | 30 | 26 | 4 | |
| Pathological stage | 0.001 | |||
| T1 | 47 | 30 | 17 | |
| T2/T3 | 49 | 15 | 34 | |
| Lymph node metastasis | 0.000 | |||
| Negative | 49 | 32 | 17 | |
| Positive | 47 | 13 | 34 | |
| Angiolymphatic invasion | 0.108 | |||
| Negative | 50 | 20 | 30 | |
| Positive | 46 | 25 | 21 | |
RNA extraction and qRT-PCR
Total RNA was extracted from the serum samples using TRIzol reagent following the manufacturer’s instructions, and the purity of RNA was determined using the Nanodrop ND-2000 device. The first chain of cDNAs was synthesized by reverse transcription using a First-Strand cDNA Synthesis kit. RT-PCR reaction was conducted using the SuperScript III Platinum SYBR Green One-Step qRT-PCR kit (Invitrogen) according to the recommended protocol. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was taken as an internal control. The sequences of primers for EFEMP1 were as follows: forward-5′-GAGGGGAGCAGTGCGTAGACA-3′ and reverse-5′-TCGGCACATGGCATTTGAGAC-3′. The relative mRNA expression of EFEMP1 was calculated using the 2−ΔΔCt method. Each sample was analyzed in triplicate.
Enzyme-linked immunosorbent assay (ELISA) analysis
The serum sample was diluted with EIA buffer (1% BSA, 0.05% Tween 20 in phosphate buffer) and incubated for 2 h at 37°C. Then horseradish peroxidase-conjugated antibodies were added and incubated for 30 min at 4°C after being washed with EIA buffer 4 times. After another 4 washes, 100 μl of tetramethyl benzidine solution was added and incubated for 30 min at room temperature. Finally, 100 μl of 1 N sulfuric acid was added to stop the reaction, and the relative expression of EFEMP1 was measured by use of an ELISA reader at 450 nm.
PSA measurement
The serum level of PSA was assessed by PSA ELISA kit (MyBiosource, MBS494521) according to the manufacturer’s instructions.
Statistical analysis
SPSS 18.0 and GraphPad Prism 5.0 software were used for the statistical analysis and the design of figures. All data are presented as mean ± standard deviation (SD). The differences between 2 groups were analyzed by the t test. The correlations between 2 groups were estimated by Spearman correlation test and the relationship between EFEMP1 expression and clinical factors was compared by chi-square test. An ROC curve was built for evaluating the diagnostic value of EFEMP1 in patients with PCa. Differences were considered to be statistically significant when P value was less than 0.05.
Results
Down-regulated expression of serum EFEMP1 was observed in patients with PCa
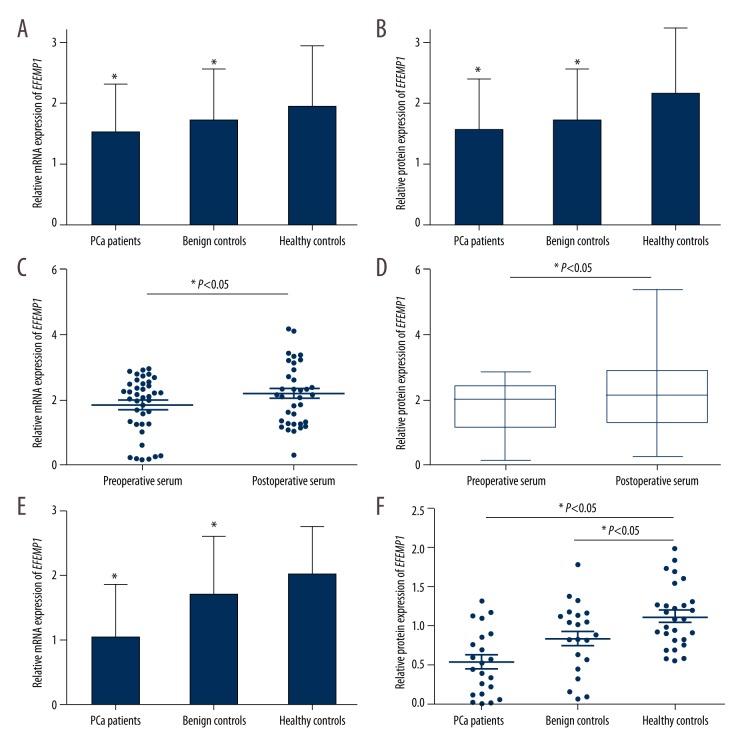
To investigate the relative EFEMP1 expression in serum of 96 PCa patients, 60 BPH patients, and 26 normal controls, we conducted the qRT-PCR and Western blot analysis, respectively. As shown in Figure 1A, the relative mRNA expression of serum EFEMP1 was significantly decreased in PCa patients compared with benign controls and healthy controls (1.528±0.797 vs. 1.715±0.854 vs. 1.955±0.986, P<0.05). The relative protein expression of EFEMP1 was also demonstrated to be lower in patients with PCa compared to that in benign controls and healthy controls (0.779±0.406 vs. 0.858±0.427 vs. 1.075±0.542, P<0.05; Figure 1B). We compared the relative expression of EFEMP1 in preoperative serum and postoperative serum, showing that postoperative serum EFEMP1 expression was significantly increased compared to that in preoperative serum (P<0.05, Figure 1C, 1D). Furthermore, as in the serum analysis, the EFEMP1 expression in PCa urine was significantly decreased compared to that in benign controls and healthy controls at both levels (P<0.001; Figure 1E, 1F).
Figure 1.
Relative EFEMP1 expression in patients with PCa, benign controls, and healthy controls. The relative expression of serum EFEMP1 in PCa patients was significantly decreased compared to that in benign controls and healthy controls, both at mRNA (A) and protein (B) levels (P<0.05). EFEMP1 expression was increased in postoperative serum compared to that in preoperative serum, both at mRNA (C) and protein (D) levels (P<0.05). Relative EFEMP1 expression in PCa urine was also lower than that in the urine of benign controls and healthy controls at mRNA (E) and protein (F) levels (P<0.05).
The relationship between EFEMP1 expression and clinical factors of patients with PCa
To determine whether serum EFEMP1 is involved in the development of PCa, we analyzed the association between its expression and clinical factors of patients. The outcomes demonstrated that the low serum EFEMP1 expression was significantly related to Gleason’s score (P=0.002), serum PSA (P=0.000), pathological stage (P=0.001), and lymph node metastasis (P=0.000), but we found no correlation with age or angiolymphatic invasion (Table 1).
The diagnostic significance of EFEMP1 in PCa
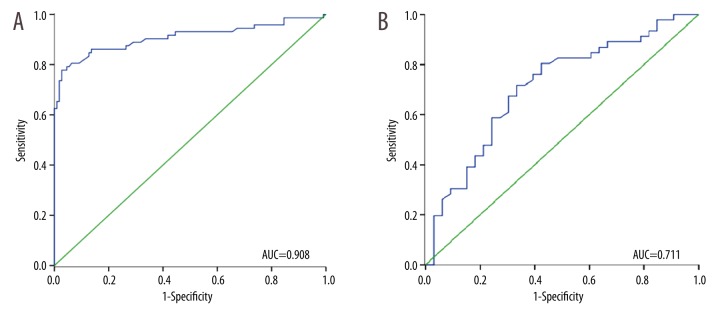
To further explore the potential clinical utility of EFEMP1 in PCa, the ROC curve was established, and we estimated the diagnostic value of PSA based on its expression. The correlation analysis indicated that the level of EFEMP1 in serum was significantly inversely correlated with PSA (r=−0.376, P=0.014; Figure 2). According to the ROC curve, EFEMP1 had a high diagnostic value, with an AUC of 0.908, a sensitivity of 77.8%, and a specificity of 97.3% (Figure 3A). Its value was higher than that of the PSA which had an AUC of 0.711, sensitivity of 71.7%, and specificity of 66.7% (Figure 3B). Importantly, multivariable logistic regression analysis showed that serum EFEMP1 expression could be a potential reliable diagnostic biomarker for PCa (OR=8.47 95% CI: 2.26–11.22, P=0.004; Table 2).
Figure 2.
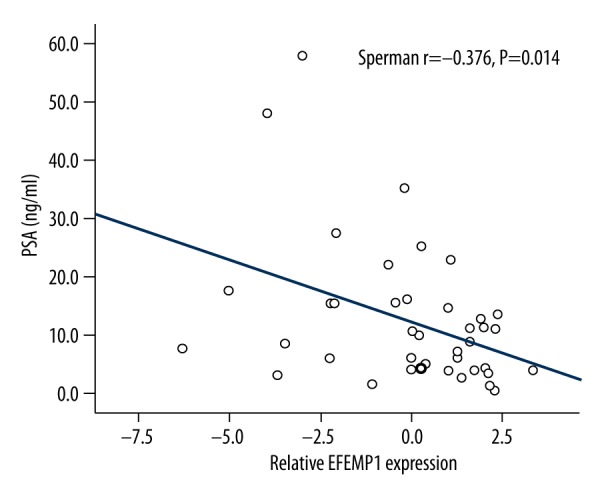
Correlation between EFEMP1 expression and PSA levels. A significant inverse correlation between EFEMP1 and PSA levels was observed (r=−0.376, P=0.014).
Figure 3.
Receiver operating characteristic (ROC) curves were established for estimating the diagnostic value of EFEMP1 and PSA. (A) The AUC for EFEMP1 was 0.908, with a sensitivity of 77.8% and a specificity of 97.3%. (B) The AUC for PSA was 0.711, with a sensitivity of 71.7% and a specificity of 66.7%.
Table 2.
Multivariable logistic regression analysis for the diagnostic value of EFEMP1 and clinical factors in the early detection of PCa.
| Parameters | OR (95% CI) | P value |
|---|---|---|
| Age | 1.2 (0.89–1.22) | 0.087 |
| PSA (ng/ml) | 2.63 (0.96–3.12) | 0.025 |
| EFEMP1 | 8.47 (2.26–11.22) | 0.004 |
Discussion
An increasing number of reports have revealed that appropriate diagnostic biomarkers are crucial and essential to ensure a correct treatment strategy and reduce mortality from human cancers [17–19]. However, modalities used for the biomarkers of PCa still suffer from various limitations. Hence, the identification of specific, predictive, and diagnostic markers for PCa still remains urgent.
Past research has shown that EFEMP1 acts as a tumor suppressor in several human cancers, such as lung cancer, hepatocellular carcinoma, breast cancer, nasopharyngeal carcinomas, colorectal cancer, and non-small cell lung carcinoma [20–25]. In contrast, a potential cancer oncogenic function of EFEMP1 was found in the study of glioma, pancreatic adenocarcinoma, and cervical cancer [26–28], which indicate that the relationship between EFEMP1 and tumorigenesis is unclear and controversial. As in PCa, EFEMP1 expression was confirmed to be significantly higher in tissue samples of patients with BPH than in those with PCa, and it was identified as a candidate methylation marker for PCa [29,30]. However, whether the dysregulation of EFEMP1 expression could distinguish patients with PCa from non-cancerous individuals remains unknown.
In the present study, we discovered decreased expression of EFEMP1 in serum and urine of PCa patients. Moreover, radical prostatectomy could significantly elevate serum EFEMP1 expression in PCa patients compared to its expression in postoperative serum and preoperative serum. These findings are consistent with the previous study and suggest EFEMP1 might act as a tumor suppressor in PCa. As in the abnormal expression of EFEMP1, we thought it might be correlated with the development of PCa, so we analyzed its correlation with clinical factors of patients. We found that low EFEMP1 expression was influenced by many clinical factors, such as Gleason’s score, serum PSA, pathological stage, and lymph node metastasis, which confirmed our inference.
Currently, the PSA screening test with is most commonly used for the detection of PCa, and has led to a 20% reduction in PCa-associated deaths [31]. However, it has limitations and no available biomarkers have been identified to have better of diagnostic accuracy than the PSA test. PSA testing is controversial due to its increased expression in parallel with increased incidence of the BPH [32,33], and it is not sufficiently effective for the prediction of cancer in patients with BPH [34]. Hence, to investigate the clinical significance of EFEMP1 in PCa, we established the ROC curve to judge the diagnostic value and compared its accuracy with PSA in the early detection of this cancer. The results showed that EFEMP1 had a high diagnostic value, which was slightly better than the PSA in sensitivity and specificity. In addition, there was a significant inverse correlation between EFEMP1 expression and PSA levels. These findings provide the convincing evidence for the first time that the downregulation of EFEMP1 might serve as a novel molecular marker for the diagnosis of PCa. However, there are limitations in the present study. First, the sample size was small. Second, the patients were selected from the same hospital, which might affect the diagnostic evaluation. Thus, it is essential to plan and conduct a further validated study with larger sample size and including patients from multiple hospitals.
Conclusions
Our study indicates that EFEMP1 expression is lower in the serum of patients with PCa compared to non-cancerous individuals, and it participates in the development of the cancer. Moreover, EFEMP1 could serve as an independent diagnostic biomarker for PCa, with better diagnostic accuracy than PSA. However, future studies with a larger number of samples are needed to confirm these findings.
Footnotes
Source of support: Departmental sources
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Humphrey PA. Gleason grading and prognostic factors in carcinoma of the prostate. Mod Pathol. 2004;17(3):292–306. doi: 10.1038/modpathol.3800054. [DOI] [PubMed] [Google Scholar]
- 3.Abe S. Cytodiagnosis of lung cancer. A. The central (hilar) type. b. Small cell carcinoma. Rinsho Byori. 1986;34(Spec No 66):39–48. [in Japanese] [PubMed] [Google Scholar]
- 4.Kang BJ, Jeun M, Jang GH, et al. Diagnosis of prostate cancer via nanotechnological approach. Int J Nanomedicine. 2015;10:6555–69. doi: 10.2147/IJN.S91908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polascik TJ, Oesterling JE, Partin AW. Prostate specific antigen: A decade of discovery – what we have learned and where we are going. J Urol. 1999;162(2):293–306. doi: 10.1016/s0022-5347(05)68543-6. [DOI] [PubMed] [Google Scholar]
- 6.Oesterling JE. Prostate specific antigen: A critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. J Urol. 1991;145(5):907–23. doi: 10.1016/s0022-5347(17)38491-4. [DOI] [PubMed] [Google Scholar]
- 7.Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002;94(13):981–90. doi: 10.1093/jnci/94.13.981. [DOI] [PubMed] [Google Scholar]
- 8.Punglia RS, D’Amico AV, Catalona WJ, et al. Impact of age, benign prostatic hyperplasia, and cancer on prostate-specific antigen level. Cancer. 2006;106(7):1507–13. doi: 10.1002/cncr.21766. [DOI] [PubMed] [Google Scholar]
- 9.Duffy MJ. PSA as a marker for prostate cancer: a critical review. Ann Clin Biochem. 1996;33( Pt 6):511–19. doi: 10.1177/000456329603300604. [DOI] [PubMed] [Google Scholar]
- 10.Timpl R, Sasaki T, Kostka G, Chu ML. Fibulins: A versatile family of extracellular matrix proteins. Nat Rev Mol Cell Biol. 2003;4(6):479–89. doi: 10.1038/nrm1130. [DOI] [PubMed] [Google Scholar]
- 11.de Vega S, Iwamoto T, Yamada Y. Fibulins: Multiple roles in matrix structures and tissue functions. Cell Mol Life Sci. 2009;66(11–12):1890–902. doi: 10.1007/s00018-009-8632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lecka-Czernik B, Lumpkin CK, Jr, Goldstein S. An overexpressed gene transcript in senescent and quiescent human fibroblasts encoding a novel protein in the epidermal growth factor-like repeat family stimulates DNA synthesis. Mol Cell Biol. 1995;15(1):120–28. doi: 10.1128/mcb.15.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun BS, Zhu X, Clayton MM, et al. Identification of a protein isolated from senescent human cells that binds to hepatitis B virus X antigen. Hepatology. 1998;27(1):228–39. doi: 10.1002/hep.510270135. [DOI] [PubMed] [Google Scholar]
- 14.Weedon MN, Lango H, Lindgren CM, et al. Genome-wide association analysis identifies 20 loci that influence adult height. Nat Genet. 2008;40(5):575–83. doi: 10.1038/ng.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi N, Kostka G, Garbe JH, et al. A comparative analysis of the fibulin protein family. Biochemical characterization, binding interactions, and tissue localization. J Biol Chem. 2007;282(16):11805–16. doi: 10.1074/jbc.M611029200. [DOI] [PubMed] [Google Scholar]
- 16.Obaya AJ, Rua S, Moncada-Pazos A, Cal S. The dual role of fibulins in tumorigenesis. Cancer Lett. 2012;325(2):132–38. doi: 10.1016/j.canlet.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Mallick R, Patnaik SK, Wani S, Bansal A. A systematic review of esophageal microRNA markers for diagnosis and monitoring of Barrett’s esophagus. Dig Dis Sci. 2016;61(4):1039–50. doi: 10.1007/s10620-015-3959-3. [DOI] [PubMed] [Google Scholar]
- 18.Gualtero DF, Suarez Castillo A. Biomarkers in saliva for the detection of oral squamous cell carcinoma and their potential use for early diagnosis: A systematic review. Acta Odontol Scand. 2016;74(3):170–77. doi: 10.3109/00016357.2015.1110249. [DOI] [PubMed] [Google Scholar]
- 19.Tobin NP, Foukakis T, De Petris L, Bergh J. The importance of molecular markers for diagnosis and selection of targeted treatments in patients with cancer. J Intern Med. 2015;278(6):545–70. doi: 10.1111/joim.12429. [DOI] [PubMed] [Google Scholar]
- 20.Yue W, Dacic S, Sun Q, et al. Frequent inactivation of RAMP2, EFEMP1 and Dutt1 in lung cancer by promoter hypermethylation. Clin Cancer Res. 2007;13(15 Pt 1):4336–44. doi: 10.1158/1078-0432.CCR-07-0015. [DOI] [PubMed] [Google Scholar]
- 21.Nomoto S, Kanda M, Okamura Y, et al. Epidermal growth factor-containing fibulin-like extracellular matrix protein 1, EFEMP1, a novel tumor-suppressor gene detected in hepatocellular carcinoma using double combination array analysis. Ann Surg Oncol. 2010;17(3):923–32. doi: 10.1245/s10434-009-0790-0. [DOI] [PubMed] [Google Scholar]
- 22.Sadr-Nabavi A, Ramser J, Volkmann J, et al. Decreased expression of angiogenesis antagonist EFEMP1 in sporadic breast cancer is caused by aberrant promoter methylation and points to an impact of EFEMP1 as molecular biomarker. Int J Cancer. 2009;124(7):1727–35. doi: 10.1002/ijc.24108. [DOI] [PubMed] [Google Scholar]
- 23.Hwang CF, Chien CY, Huang SC, et al. Fibulin-3 is associated with tumour progression and a poor prognosis in nasopharyngeal carcinomas and inhibits cell migration and invasion via suppressed AKT activity. J Pathol. 2010;222(4):367–79. doi: 10.1002/path.2776. [DOI] [PubMed] [Google Scholar]
- 24.Tong JD, Jiao NL, Wang YX, et al. Downregulation of fibulin-3 gene by promoter methylation in colorectal cancer predicts adverse prognosis. Neoplasma. 2011;58(5):441–48. doi: 10.4149/neo_2011_05_441. [DOI] [PubMed] [Google Scholar]
- 25.Wang R, Zhang YW, Chen LB. Aberrant promoter methylation of FBLN-3 gene and clinicopathological significance in non-small cell lung carcinoma. Lung Cancer. 2010;69(2):239–44. doi: 10.1016/j.lungcan.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Hu B, Thirtamara-Rajamani KK, Sim H, Viapiano MS. Fibulin-3 is uniquely upregulated in malignant gliomas and promotes tumor cell motility and invasion. Mol Cancer Res. 2009;7(11):1756–70. doi: 10.1158/1541-7786.MCR-09-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seeliger H, Camaj P, Ischenko I, et al. EFEMP1 expression promotes in vivo tumor growth in human pancreatic adenocarcinoma. Mol Cancer Res. 2009;7(2):189–98. doi: 10.1158/1541-7786.MCR-08-0132. [DOI] [PubMed] [Google Scholar]
- 28.En-lin S, Sheng-guo C, Hua-qiao W. The expression of EFEMP1 in cervical carcinoma and its relationship with prognosis. Gynecol Oncol. 2010;117(3):417–22. doi: 10.1016/j.ygyno.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 29.Kim YJ, Yoon HY, Kim SK, et al. EFEMP1 as a novel DNA methylation marker for prostate cancer: Array-based DNA methylation and expression profiling. Clin Cancer Res. 2011;17(13):4523–30. doi: 10.1158/1078-0432.CCR-10-2817. [DOI] [PubMed] [Google Scholar]
- 30.Almeida M, Costa VL, Costa NR, et al. Epigenetic regulation of EFEMP1 in prostate cancer: Biological relevance and clinical potential. J Cell Mol Med. 2014;18(11):2287–97. doi: 10.1111/jcmm.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–28. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 32.Roehrborn CG, Boyle P, Gould AL, Waldstreicher J. Serum prostate-specific antigen as a predictor of prostate volume in men with benign prostatic hyperplasia. Urology. 1999;53(3):581–89. doi: 10.1016/s0090-4295(98)00655-4. [DOI] [PubMed] [Google Scholar]
- 33.Berger AP, Deibl M, Strasak A, et al. Large-scale study of clinical impact of PSA velocity: Long-term PSA kinetics as method of differentiating men with from those without prostate cancer. Urology. 2007;69(1):134–38. doi: 10.1016/j.urology.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Al-Azab R, Toi A, Lockwood G, et al. Prostate volume is strongest predictor of cancer diagnosis at transrectal ultrasound-guided prostate biopsy with prostate-specific antigen values between 2.0 and 9.0 ng/mL. Urology. 2007;69(1):103–7. doi: 10.1016/j.urology.2006.09.041. [DOI] [PubMed] [Google Scholar]