Abstract
Background
We sought to determine by meta-analysis the relationship between drinking alcohol and the risk of gastric cancer.
Material/Methods
A systematic Medline search was performed to identify all published reports of drinking alcohol and the associated risk of gastric cancer. Initially we retrieved 2,494 studies, but after applying inclusion and exclusion criteria, only ten studies were found to be eligible for our meta-analysis.
Results
Our meta-analysis showed that alcohol consumption elevated the risk of gastric cancer with an odds ratio (OR) of 1.39 (95% CI 1.20–1.61). Additionally, subgroup analysis showed that only a nested case-control report from Sweden did not support this observation. Subgroup analysis of moderate drinking and heavy drinking also confirmed that drinking alcohol increased the risk of gastric cancer. Publication bias analysis (Begg’s and Egger’s tests) showed p values were more than 0.05, suggesting that the 10 articles included in our analysis did not have a publication bias.
Conclusions
The results from this meta-analysis support the hypothesis that alcohol consumption can increase the risk of gastric cancer; suggesting that effective moderation of alcohol drinking may reduce the risk of gastric cancer.
MeSH Keywords: Alcohol Drinking, Case-Control Studies, Meta-Analysis, Stomach Neoplasms
Background
Gastric cancer, also known as stomach cancer, is one of the most frequent cancers in the world; almost two-thirds of gastric cancer cases and deaths occur in less developed regions such as China. The worldwide morbidity and mortality rate of gastric cancer has declined rapidly over the past few decades, likely due to the recognition of certain risk factors such as Helicobacter pylori and dietary and environmental risks factors [1]. However, the declining rate has been less dramatic in China compared with other countries. Although in China there is a lack of systematic national vital statistics, the results from retrospective sampling surveys of malignant tumors from 2004 to 2005 helped establish the mortality rate for gastric cancer in China, which ranked third in overall cancer mortality [2]. Interestingly, 42% of all gastric cancer cases worldwide are reported to occur in China [3].
It has been proposed that the development of gastric cancer is a multi-step process, although the exact influencing factors have not been elucidated. In the past, many researchers studying the cause of gastric cancer had contradictory hypotheses about the role of alcohol consumption in the development of gastric cancer. However, recent studies have confirmed that alcohol drinking can increase the risk of gastric cancer; and the main mechanism is likely related to the primary metabolites, acetaldehydes, that have a local toxic effect that increases the risk of gastric cancer [4–6].
However, it is still a matter of debate whether alcohol consumption elevates the risk of gastric cancer. We carried out this meta-analysis to explore the relationship between alcohol consumption and the development of gastric cancer. We retrieved studies published (both local and international) between 1995 and 2015, and then performed a comprehensive quantitative analysis to determine the relationship between alcohol consumption and gastric cancer. The aim of our study was to add to the understanding of and prevention of gastric cancer.
Material and Methods
Literature inclusion criteria and exclusion criteria
In this meta-analysis, we used the international PICOS format:
P: The case group was patients with gastric cancer; gastric cancer was diagnosed by pathology.
I: Alcohol drinking.
C: The control group was those persons with non-gastric cancer or healthy persons.
O: To explore whether drinking will increase the risk of stomach cancer or not.
S: Case-control study
Literature inclusion criteria
1) research method was a case-control study, 2) patients were diagnosed by histopathology, 3) study provided complete data, including loss data, 4) study was published between 1995 and 2015; 5) study controlled the main confounding factors, such as family history of cancer, race, height, smoking, and BMI.
Literature exclusion criteria
1) studu did not meet the inclusion criteria, 2) study had repetitive published data, duplicate data, or was a poor quality study, 3) raw data was not sufficient, and the case groups and the control groups total sample size was <80 cases, 4) same authors use the same case studies: only with the studies with the most samples and the latest published in the literature were used, and 5) animal experiments.
Literature retrieval strategies
We searched CBM, CNKI, Wanfang, VIP, PubMed, and Web of Science with MeSH terms “gastric cancer”, “alcohol drinking”, “case-control studies” following the Meta-analysis Of Observational Studies in Epidemiology guidelines to identify relevant studies in the published literature. The searched was performed for articles published between 1995 and 2015.
Material selection and extraction
The selection of the initial eligible studies and data extraction of eligible studies was performed independently by two authors (ZB and MK). A consultation with a third researcher was performed when a dispute occurred. All three researchers had expertise in clinical epidemiological methodology and related domain knowledge.
Extracting information, Excel spreadsheet, and fetching information
1) General information extracted was: title, first author, publication date, and region. 2) The characteristics of the research were: research type, number of cases and control group, crowd source, and distribution (proportion) of men and women. 3) Data characteristics were: capacity for alcohol, unit measure of alcohol consumption, relative risk (RR) and 95% confidence interval (CI) or odds ratio (OR) and 95% CI. If comparative data was not provided within the literature, it was obtained by statistical software.
Study participants were divided into three groups: 1) no drinking, 2) moderate drinking, and 3) heavy drinking.
Moderate drinking for women was defined as one standard cup of alcohol per day or 15 grams of alcohol per day. Moderate drinking for men was defined as two standard cups of alcohol per day or 30 grams of alcohol per day. The definition of a standard cup for drinking was 118 mL of beer or 355 mL of wine [7]. Heavy drinking was defined as a man or woman who drinks more than two standard cups a day (standard cup of alcohol content of about 15 grams of alcohol). Because units for alcohol consumption varied by study, we standardized the alcohol unit to grams per day, and converted all study data into grams per day for comparisons.
Statistical treatment
We used RevMan 5.0 and State 12.0 software to analyze the data, and to map forest and funnel plots. Data included: 1) alcohol consumption and gastric cancer risk, 2) non-alcohol consumption and gastric cancer risk, 3) the analysis of moderate alcohol consumption and gastric cancer, 4) the analysis of heavy alcohol consumption and gastric cancer, 5) heterogeneity test and subgroup analysis, 6) sensitivity analysis, 7) bias analysis, 8) the dose effect relationship of drinking and gastric cancer.
Results
In the literature
In total, 2,494 studies were retrieved; we excluded 2,484 studies by following the aforementioned exclusion and inclusion criteria. The eligible 10 case-control studies [8–17] including three studies from China, three studies from Sweden, one study from Brazil, one study from Korea, one study from Russia, and one study from the USA. A total of 19,302 persons were included in the 10 studies: case group (n=3,613) and control group (n=15,689). See Table 1. A flowchart depicting the study selection is shown in Figure 1.
Table 1.
Characteristic of studies included in meta-analysis.
| Study ID | Country | Study type | Study population | Cases | Controls |
|---|---|---|---|---|---|
| Bao 2001 | China | PCC | M+W | 311 | 1479 |
| Bu-Tian 1996 | China | PCC | M | 744 | 796 |
| Cheol 2011 | Korean | HCC | M+W | 445 | 370 |
| Hamada 2002 | Brazil | PCC | M+W | 96 | 192 |
| He 2012 | China | HCC | M+W | 212 | 158 |
| Lagergren 2000 | Sweden | PCC | M+W | 262 | 820 |
| Lindblad 2005 | Sweden | NCC | M+W | 522 | 10000 |
| Ye 1999 | Sweden | PCC | M+W | 504 | 1131 |
| Zaridze 2000 | Russia | HCC | M+W | 450 | 611 |
| Zhang 1996 | USA | HCC | M+W | 67 | 132 |
HCC – hospital case-control study; PCC – population case-control study; NCC – nested case-control study.
Figure 1.
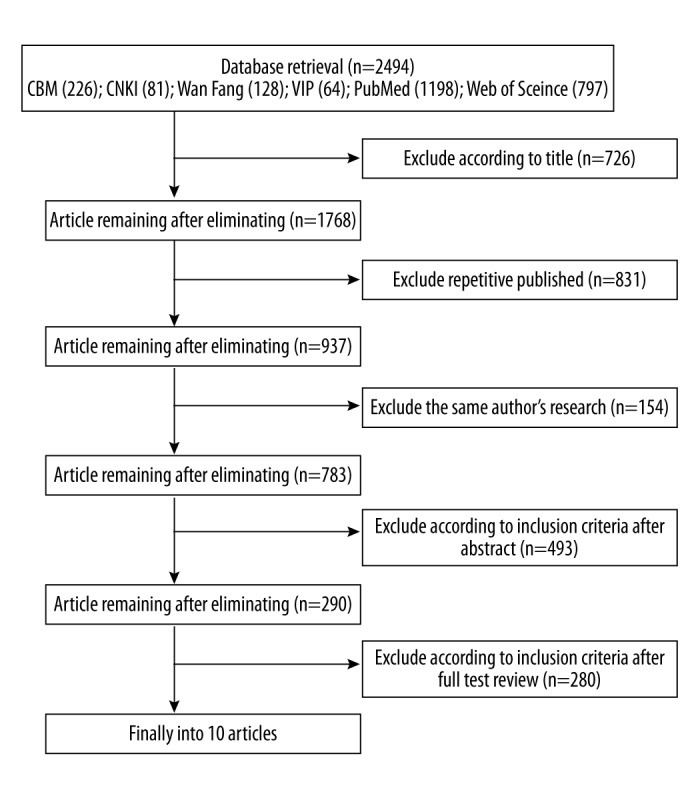
The results of literature retrieval.
Meta-analysis of drinking with gastric cancer
Alcohol drinking and gastric cancer risk: drinkers versus non-drinkers
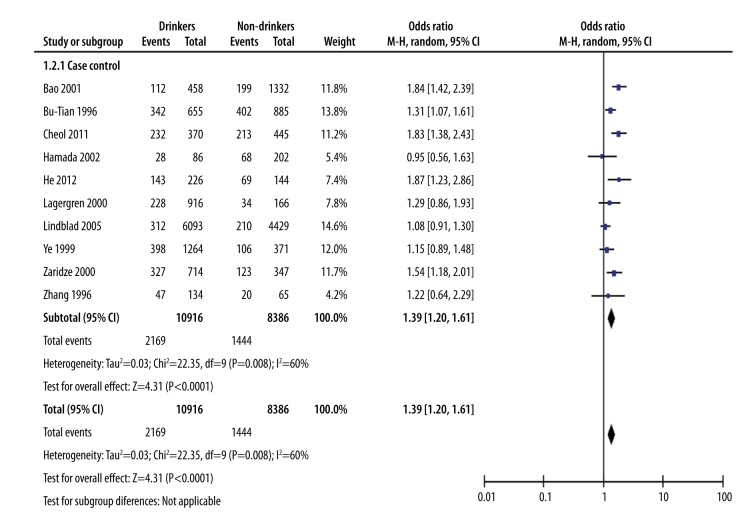
Because the studies were case-control studies, we use an odds ratio (OR) value to measure the effect quantity. To ensure the accuracy of the results and because of the high heterogeneity of the studies, we used the random effects model (in statistics a random effect model, also known as variance component model, is a type of hierarchical linear model). We used the Mantel-Haenszel (M-H) method to calculate the combined effect quantity. The combined effect quantity for drinkers versus non-drinkers had an OR value of 1.39 with 95% CI (1.20, 1.61). This suggests that alcohol drinking can increase the risk for gastric cancer. This may be because alcohol can act as a solvent, assisting other harmful chemicals to enter the cells lining in the upper digestive tract more easily. Heterogeneity test showed chi-square=22.35 and I2=60%. The large heterogeneity suggests the data was original. In order to determine the difference between moderate drinking and the risk of gastric cancer and heavy drinking and the risk of gastric cancer, we divided the study participants into three groups: control group (no drinking), moderate drinking group, and heavy drinking group. The difference between the three groups is shown in Figure 2.
Figure 2.
Alcohol drinking and gastric cancer risk: drinkers versus non-drinkers.
Non-drinking: gastric cancer cases versus controls
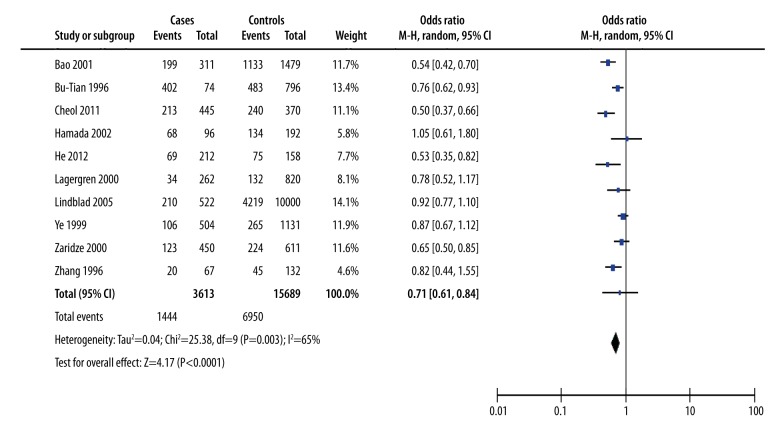
We performed M-H analysis to determine the difference between the non-drinking with the gastric cancer-cases group and the control group. The combined effect quantity OR value was 0.71 (95% CI=0.61–0.84), suggesting that non-drinking was a protective factor for gastric cancer. There was substantial heterogeneity between case-control studies (χ2=25.38, I2=65%) (Figure 3).
Figure 3.
Non-drinking with the gastric cancer: cases versus controls.
The analysis of moderate alcohol drinking with gastric cancer: drinkers versus non-drinkers
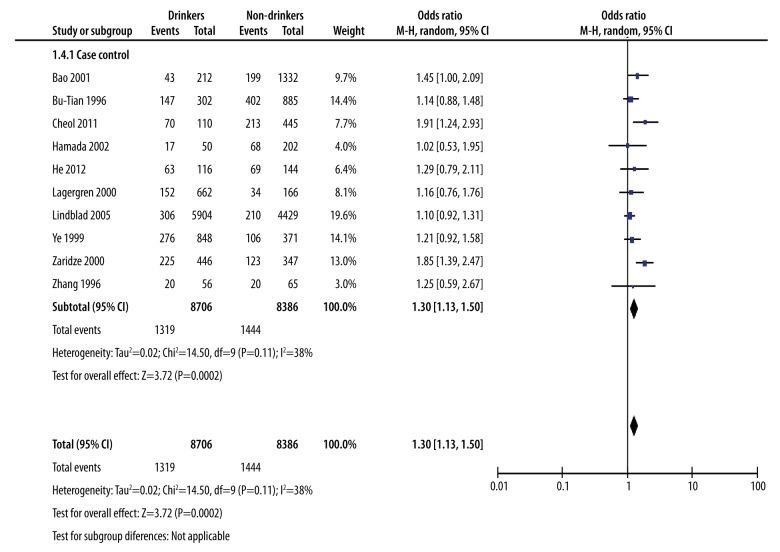
Based on alcohol consumption, we determined the risk of gastric cancer for moderate drinkers compared to non-drinkers. We used the M-H method of analysis. The combined effect quantity OR value was 1.30 with 95% CI (1.13, 1.50), indicating that the risk of gastric cancer in moderate drinkers was higher than non-drinkers. This suggests that moderate drinking could increase the risk of gastric cancer. The heterogeneity test showed chi square=14.50 and I2=38%, indicating the heterogeneity was acceptable (Figure 4).
Figure 4.
The analysis of moderate alcohol drinking with gastric cancer: drinker versus non-drinkers.
The analysis of heavy alcohol drinking with gastric cancer: drinkers versus non-drinkers
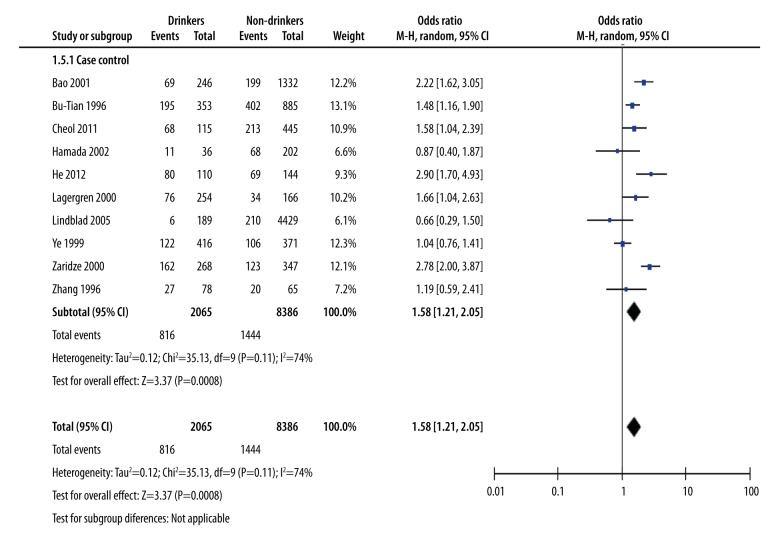
To further look at the associated strength between heavy drinking and risk of gastric cancer, we performed a meta-analysis of the heavy drinkers in the 10 studies. Based on the level of alcohol consumption, we determined the risk of gastric cancer by comparing heavy drinkers with non-drinkers and observe whether heavy drinking could increase the risk of gastric cancer. We used the M-H method of analysis. The combined effect quantity OR value was 1.58 with 95% CI (1.21, 2.05), indicating that the risk of gastric cancer in heavy drinkers was higher than non-drinkers. The data suggest that heavy drinking have an increased risk of gastric cancer. The heterogeneity test showed chi-square=35.13 and I2=74%. Because the heterogeneity was high, we also performed a sensitive analysis (Figure 5).
Figure 5.
Heavy alcohol drinking with gastric cancer: drinkers versus non-drinkers.
Heterogeneity test and subgroup analysis
We used RevMan software 5.0 to perform the heterogeneity inspection for study type, sample group, and region; we estimated whether the study populations had homogeneity. We applied hypothesis testing to examine whether the heterogeneity of multiple independent studies have statistically significant differences.
To further explain the heterogeneity, we performed subgroup analysis: the three study types were classified as: hospital-based case-control study, population-based case-control study, and nested case-control study. We divided the study participants into two groups based on the study population sex ratio: one group consisted of only men, and one group consisted of both men and women. We divided the case-control studies into three regional background groups: Chinese origin, Swedish origin, and other origin (see Table 2).
Table 2.
The subgroup analysis of meta-analysis between alcohols drinking with the risk of gastric cancer.
| Group | Number | OR | 95% CI | Heterogeneity test P value and I2 value | |
|---|---|---|---|---|---|
| Study type | |||||
| HCC | 4 | 1.66 | (1.40–1.97) | P=0.57 | I2=0% |
| PCC | 5 | 1.33 | (1.09–1.62) | P=0.07 | I2=54% |
| NCC | 1 | 1.08 | (0.91–1.30) | ||
| Sample sex | |||||
| Men | 1 | 1.31 | (1.07–1.61) | ||
| Men + Women | 9 | 1.40 | (1.17–1.67) | P=0.004 | I2=64% |
| Region | |||||
| China | 3 | 1.60 | (1.24–2.08) | P=0.08 | I2=60% |
| Sweden | 3 | 1.12 | (0.98–1.29) | P=0.74 | I2=0% |
| Others | 4 | 1.47 | (1.15–1.89) | P=0.1 | I2=41% |
Subgroup analysis for the nested case-control studies and the Swedish studies showed no correlation between alcohol drinking and gastric cancer development. The other group analysis showed similar results. This may be because the alcohol categories and alcohol capacity were different in different countries. The researchers from Sweden found non-significant differences between the case group and the control group.
The heterogeneity test showed that the heterogeneity of the case-control studies in crowd source, groups with men and women, and different regions of China were 54%, 64%, 60%, and 41%, respectively (Table 2).
Sensitive analysis
Sensitivity analysis found two studies, Lindblad et al. 2005 and Bao et al. 2001, that had high heterogeneity. When the Lindblad study was excluded, the heterogeneity I2 value was 45%. When the Bao study was excluded, the heterogeneity I2 value was 51%. The high heterogeneity in the Lindblad study may have been due to differences in region or drinking features. The high heterogeneity in the Bao study may have been due to the nested case-control study type (Table 3).
Table 3.
Sensitive analysis.
| Literature rejection | Chi2 | I2 | OR (95%CI) |
|---|---|---|---|
| Lindblad 2005 | 14.62 | 45% | 1.45 (1.26–1.68) |
| Bao 2001 | 16.3 | 51% | 1.33 (1.15–1.54) |
Bias analysis
We used STATA software to analyze the publication bias for the 10 articles, Begg’s test shows that p value was higher than 0.05, indicating that there was no significant publication bias observed in the selected studies (the Begg’s funnel plot was symmetrical (Figure 6).
Figure 6.
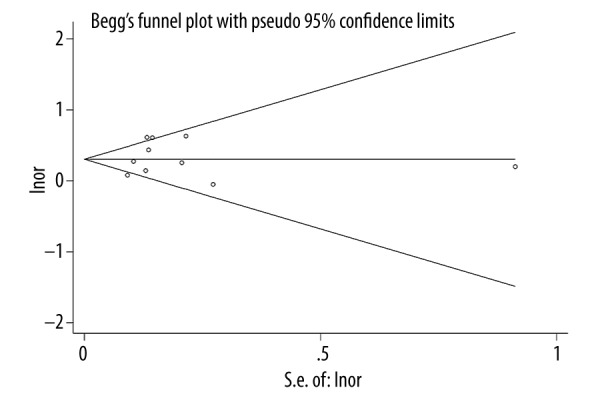
Funnel figure of publication bias.
Dose-response relationship between alcohol consumption and gastric cancer risk
We used STATA software to analyze the dose-response relationship between alcohol consumption and gastric cancer risk. We found a significantly increased risk at any level of alcohol intake, with a minimum at 0 grams per day; the curve was <1 gram per day to 85 grams per day (Figure 7).
Figure 7.
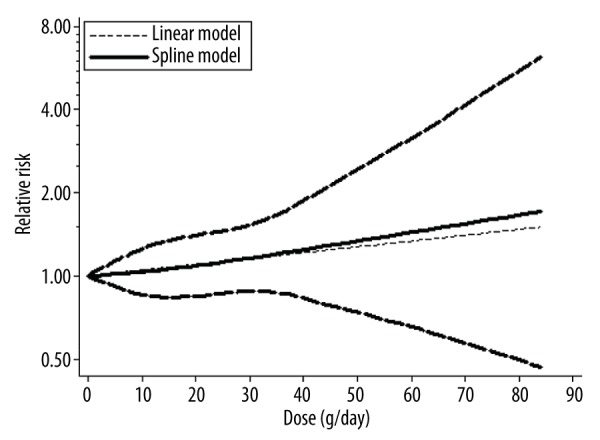
Dose response relationship between alcohol consumption and gastric cancer risk.
Discussion
Gastric cancer is a common cancer worldwide. Although incidences of gastric cancer are declining, it is still a threat to people’s health. Alcohol is a commonly consumed drink. The relationship between drinking alcohol and the risk of gastric cancer is biologically plausible; ethanol is fat soluble and might cause damage to the gastric mucosa. Its metabolite acetaldehyde can have a local toxic effect which may be related to the occurrence of gastric cancer [18]. The pathogenesis of ethanol on gastric mucosal damage is associated with disrupting the balance of gastric mucosal defense and external invasion [19–22]. However, whether drinking alcohol can cause gastric cancer has been inconsistently reported. In this study, we present a meta-analysis of the literature published over the past twenty years on the role of alcohol drinking related to the development of gastric cancer. Our results demonstrated that alcohol consumption increased the risk gastric cancer even at lower levels of alcohol consumption.
A total of 10 articles were included in this meta-analysis. We used RevMan 5.0 and State 12.0 software to analyze the data; the combined effect quantity OR value was 1.39, with 95% CI (1.20, 1.61). This showed that alcohol can increase the risk of gastric cancer. In order to further observe the different level of alcohol consumption associated with the risk of gastric cancer, we divided the study participants into three groups (no drinking, moderate drinking, and heavy drinking). We used the M-H method to calculate the combined effect quantity OR value. The combined effect quantity OR for non-drinking was 0.71, with 95% CI (0.61, 0.84). This indicated that nondrinking does not increase the risk of gastric cancer; The combined effect quantity OR for moderate drinking was 1.30 with 95% CI (1.13, 1.50). The combined effect quantity OR for heavy drinking was 1.58, with 95% CI (1.21, 2.05), which indicated that moderate drinking and heavy drinking can increase the risk of gastric cancer. In order to explain the heterogeneity, we performed subgroup analysis and bias analysis. The subgroup analysis showed that only the nested case-control study and research from Sweden did not support alcohol drinking as a risk of gastric cancer. The remaining research found that alcohol consumption can increase the risk of gastric cancer. Begg’s test showed a p value >0.05, which indicated that there was no publication bias in the 10 studies. This dose effect relationship of drinking and gastric cancer risk showed significantly increased risk at any level of alcohol intake.
Conclusions
This meta-analysis includes 10 case control studies on alcohol consumption and gastric cancer risk. This met-analysis confirmed that alcohol consumption can increase the risk of gastric cancer even at lower levels of alcohol consumption. As this meta-analysis had one study type, it had high homogeneity. Hence, the meta-analysis was not affected by a variety of research study types. However, the meta-analysis results may support one-sidedness with exclusion of cohort studies and only the retention of case-control studies. Our results were different from Tramacere et al. [23,24]. This may be due to differences in the study populations, regions, alcohol usage, alcohol type, or research methods. Therefore, a more rigorous scientific study is needed to continue to explore the relationship between alcohol drinking and gastric cancer risk.
Footnotes
Compliance with ethical standards
Conflict of interest: All the authors declare that they have no conflict of interest.
Ethical approval: This article does not contain any studies with human participants performed by any of the authors.
Source of support: Departmental sources
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2015. Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Whelan SL, Ferlay WJ, et al. Cancer incidence in five continents. VIII. France: IARC Scientific Publication No. 155; [Google Scholar]
- 3.Zou XN, Duan JJ, Huangfu XM, et al. [Analysis of stomach cancer mortality in the national retrospective sampling survey of death causes in China, 2004–2005]. Zhonghua Yufang Yixue Zazhi. 2010;44:390–97. [in Chinese] [PubMed] [Google Scholar]
- 4.Brzozowski T, Konturek PC. Implications of reactive oxygen species and cytokines in gastroprotection against stress-induced gastric damage by nitric oxide releasingaspirin. Int J Colorectal Dis. 2003;18:320–29. doi: 10.1007/s00384-002-0451-2. [DOI] [PubMed] [Google Scholar]
- 5.Kwiecien S, Brzozowski T, Konturek SJ. Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J Physiol Pharmacol. 2002;53:39–50. [PubMed] [Google Scholar]
- 6.Huh K, Kwon TH, Shin US, et al. Inhibitory effects of DA-9601 on ethanol-induced gastro-hemorrhagic lesions and gastric xanthine oxidase activity in rats. J Ethnopharmacol. 2003;88:269–73. doi: 10.1016/s0378-8741(03)00235-6. [DOI] [PubMed] [Google Scholar]
- 7.Cao Y, Willett WC, Rimm EB, et al. Light to moderate intake of alcohol, drinking patterns, and risk of cancer: Results from two prospective US cohort studies. BMJ. 2015;18(351):h4238. doi: 10.1136/bmj.h4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao P, Tao M. A case-control study of smoking, alcohol consumption and stomach cancer. TUMOR (Shanghai) 2001;9:334–38. [Google Scholar]
- 9.Ji BT, Chow WH, Yang G, et al. The influence of cigarette smoking, alcohol, andgreen tea consumption on the risk of carcinoma of the cardia and distal stomachin Shanghai, China. Cancer. 1996;77:2449–57. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2449::AID-CNCR6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 10.Shin CM, Kim N, Cho SI, et al. Association between alcohol intake and risk for gastric cancer with regard to ALDH2 genotype in the Korean population. Int J Epidemiol. 2011;40:1047–55. doi: 10.1093/ije/dyr067. [DOI] [PubMed] [Google Scholar]
- 11.Hamada GS, Kowalski LP, Nishimoto IN, et al. Risk factors for stomach cancer inBrazil (II): A case-control study among Japanese Brazilians in Sao Paulo. Jpn J Clin Oncol. 2002;32:284–90. doi: 10.1093/jjco/hyf061. [DOI] [PubMed] [Google Scholar]
- 12.He H, Chi J. Acase-control study of smoking-alcohol consumption and the occurrence of stomach cancer. Chinese J Disease Control. 2012;10:20–28. [Google Scholar]
- 13.Lagergren J, Bergstrom R, Lindgren A, Nyren O. The role of tobacco, snuff and alcohol use in the etiology of cancer of the esophagus and gastric cardia. Int J Cancer. 2000;85:340–46. [PubMed] [Google Scholar]
- 14.Lindblad M, Rodriguez LA, Lagergren J. Body mass, tobacco and alcohol andrisk of esophageal, gastric cardia, and gastric non-cardia adenocarcinoma among men and women in a nested case-control study. Cancer Causes Control. 2005;16:285–94. doi: 10.1007/s10552-004-3485-7. [DOI] [PubMed] [Google Scholar]
- 15.Ye W, Ekstrom AM, Hansson LE, et al. Tobacco, alcohol and the risk of gastric cancer by sub-site and histologic type. Int J Cancer. 1999;83:223–29. doi: 10.1002/(sici)1097-0215(19991008)83:2<223::aid-ijc13>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 16.Zaridze D, Borisova E, Maximovitch D, Chkhikvadze V. Alcohol consumption, smoking and risk of gastric cancer: Case-control study from Moscow, Russia. Cancer Causes Control. 2000;11:363–71. doi: 10.1023/a:1008907924938. [DOI] [PubMed] [Google Scholar]
- 17.Zhang ZF, Kurtz RC, Sun M, et al. Adenocarcinomas of the esophagus and gastriccardia: medical conditions, tobacco, alcohol, and socioeconomic factors. Cancer Epidemiol Biomarkers Prev. 1996;5:761–68. [PubMed] [Google Scholar]
- 18.Bao P, Tao M, Liu D, et al. A case-control study of smoking, alcohol consumption and the occurrence of stomach cancer. Cancer. :2001–2005. [Google Scholar]
- 19.Wu AH, Wan P, Bernstein L. A multiethnic population-based study of smoking, alcohol and body size and risk of adenocarcinomas of the stomach andesophagus (United States) Cancer Causes Control. 2001;12:721–32. doi: 10.1023/a:1011290704728. [DOI] [PubMed] [Google Scholar]
- 20.Freedman ND, Abnet CC, Leitzmann MF, et al. A prospective study of tobacco,alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol. 2007;165:1424–33. doi: 10.1093/aje/kwm051. [DOI] [PubMed] [Google Scholar]
- 21.Steevens J, Schouten LJ, Goldbohm RA, van den Brandt PA. Alcohol consumption, cigarette smoking and risk of subtypes of esophageal and gastriccancer: A prospective cohort study. Gut. 2010;59:39–48. doi: 10.1136/gut.2009.191080. [DOI] [PubMed] [Google Scholar]
- 22.Barstad B, Sorensen TI, Tjonneland A, et al. Intake of wine, beer and spirits and risk of gastric cancer. Eur J Cancer Prev. 2005;14:239–43. doi: 10.1097/00008469-200506000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Tramacere I, Pelucchi C, Bagnardi V, et al. A meta-analysis on alcohol drinking and esophageal andgastric cardia adenocarcinoma risk. Ann Oncol. 2013;23:287–97. doi: 10.1093/annonc/mdr136. [DOI] [PubMed] [Google Scholar]
- 24.Tramacere I, Negri E, Pelucchi C, et al. A meta-analysis on alcohol drinking and gastric cancer risk. Ann Oncol. 2013;23:28–36. doi: 10.1093/annonc/mdr135. [DOI] [PubMed] [Google Scholar]