Abstract
Bone fractures are not uncommon in paediatric age. However, when recurrent, an underlying clinical condition must not be excluded. We describe the case of a boy aged 7 years, referred for investigation of recurrent bone fractures. Personal and family histories were unremarkable. Physical examination was normal. Almost all primary bone disorders were excluded. Additional laboratory investigations ruled out the majority of secondary causes of bone fragility. Coeliac disease (CD) serologies, however, were positive, and duodenal biopsies confirmed this diagnosis (Marsh III B). On a gluten-free diet, he suffered no more fractures and the bone mineral density improved. CD was also confirmed in his asymptomatic older brother. It is essential to diagnose CD as early as possible in order to minimise the compromise in bone health and prevent other complications of the disease. First-degree relatives should always be screened for the disease, even asymptomatic ones.
Background
In the approach to children with recurrent fractures, primary bone diseases and secondary causes of bone fragility have to be evaluated. Coeliac disease (CD) can present in atypical or asymptomatic forms and should always be excluded since it constitutes a recognised cause of low mineral bone density and has an effective treatment: gluten-free diet (GFD). The diagnosis of the index case allowed his asymptomatic brother also to benefit from GFD. The adherence to GFD also has the additional advantage of preventing other manifestations of the disease.
Case presentation
A previously healthy Caucasian boy aged 7 years was referred for a paediatric appointment for investigation of recurrent bone fractures. In the past 2 years, he had three upper limb fractures: supracondylar fractures of right (figure 1) and left (figure 2) humerus and one of right medial humeral condyle (figure 3). All three episodes were caused by minor to moderate energy witnessed traumas at home or school, none of them related to sports practice. The fractures had healed after 4–5 weeks of cast immobilisation without sequelae. There were no symptoms of bone pain, abdominal cramps, urinary or bowel movement alterations, or other constitutional symptoms, or suggestive signs of child abuse.
Figure 1.
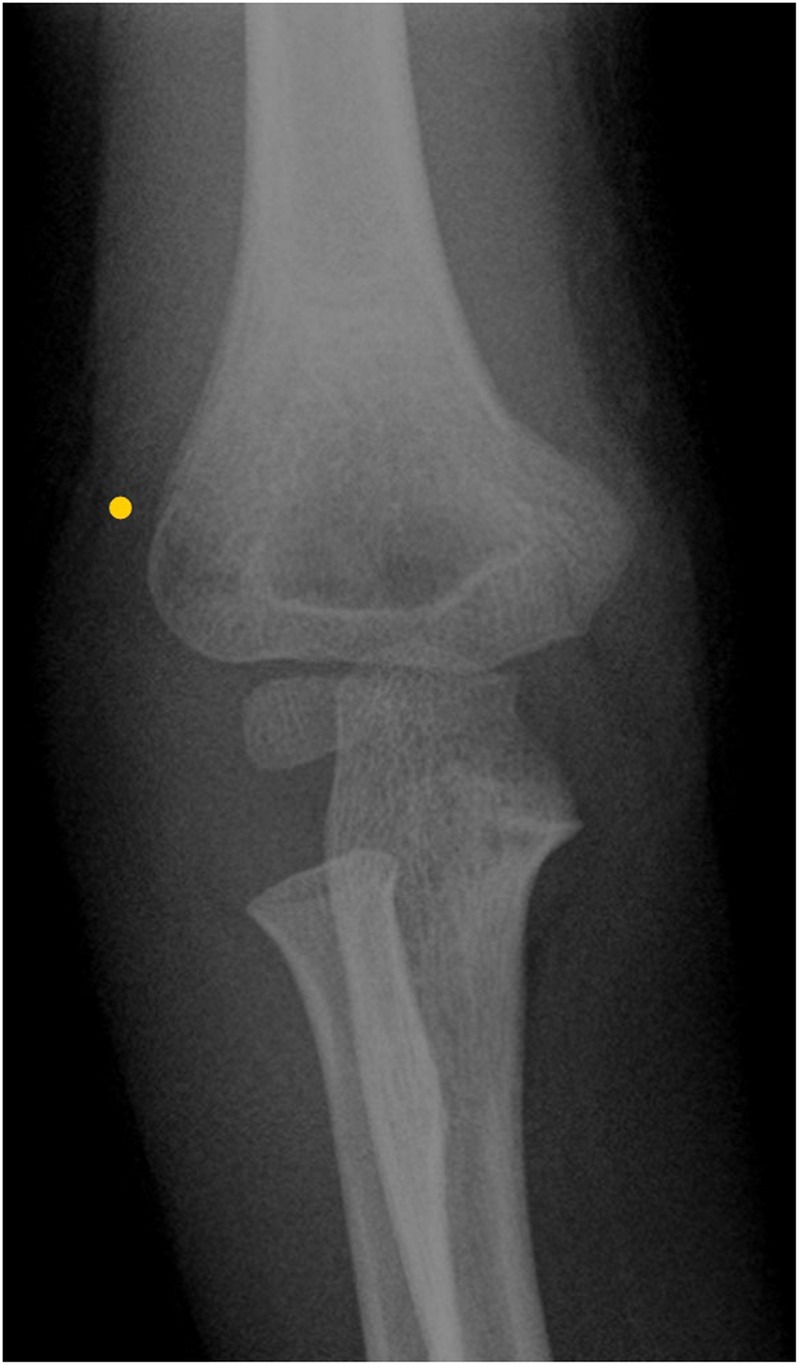
X-ray of the upper limb showing supracondylar fracture of the right humerus.
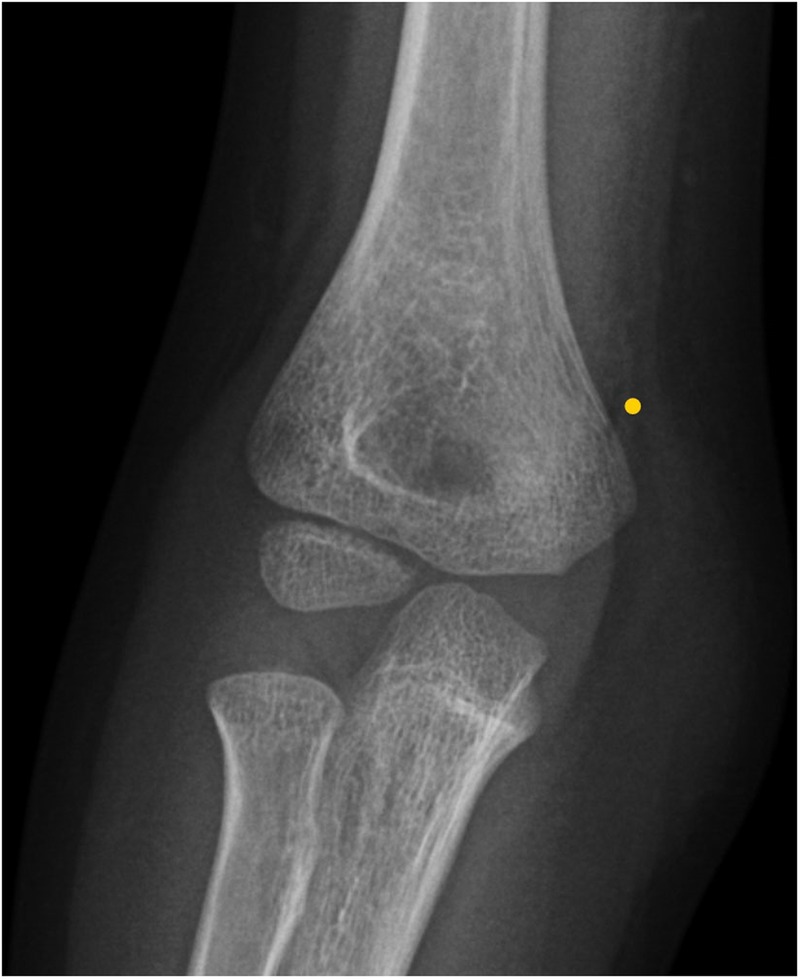
Figure 2.
X-ray of the upper limb showing supracondylar fracture of the left humerus.
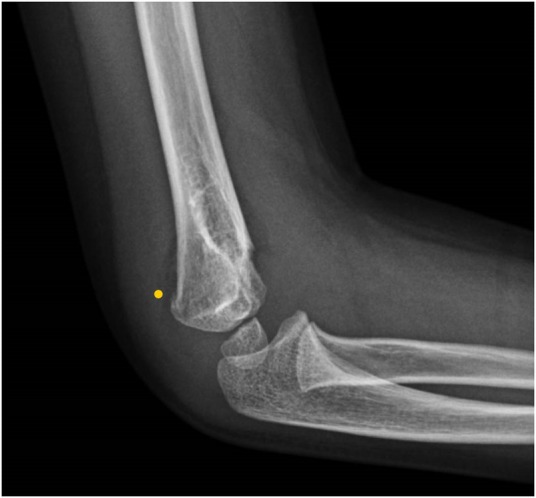
Figure 3.
X-ray of the right upper limb showing fracture of the medial humeral condyle.
His medical history was unremarkable and he did not take chronic medication. His weight and stature evolved regularly (P50–75 and P50, respectively) and he had an adequate intake of dairy products (600 mL of milk or yogurt per day). He exercised frequently, playing football three times a week.
There was no family history of recurrent fractures, joint hypermobility or other bone or autoimmune diseases.
A thorough clinical examination, with special attention to the musculoskeletal system and signs of chronic disease, was completely normal.
Investigations
The initial investigation included a comprehensive panel to evaluate bone mineral homeostasis, which showed normal levels of calcium, magnesium, phosphorus, thyroid-stimulating hormone, free thyroxine and parathyroid hormone. Alkaline phosphatase (ALP) was slightly reduced (160 UI/L, reference range (RR) 175–420) and 25-hydroxy-vitamin D was marginally above the lower limit (25.9 ng/mL, RR 20–100). Urine samples for urinalysis and evaluation of renal calcium excretion and renal tubular phosphate reabsorption were also analysed, with normal results.
Additionally, other parameters were evaluated to exclude other possible secondary causes of osteoporosis. The full blood count was normal and the erythrocyte sedimentation rate (ESR) was slightly elevated (16 mm/s; RR<13). Liver enzymes, renal function and serum albumin were normal. Coeliac serology showed positive antitissue transglutaminase IgA antibodies (156 RU/mL; positive >20), antiendomysial IgA (strongly positive) and antideamidated forms of gliadin peptides IgA (98.3 units; positive >25). These results prompted referral for a paediatric gastroenterologist. An upper digestive endoscopy with duodenal biopsies was performed, revealing specific findings compatible with the classification Marsh III B for CD.
Differential diagnosis
Recurrent bone fractures in children can be the result of either excessive force or reduced bone strength, due to primary bone disorders or secondary to other conditions.1 Child abuse must always be excluded by a thorough interview with a detailed fracture history and a careful clinical examination. Radiological exclusion of older fractures may also be considered in a case-by-case approach. The group of primary bone diseases that may result in repeated fractures includes genetic disorders (such as osteogenesis imperfecta and connective tissue diseases, as Ehler-Danlos and Marfan syndromes, among other diseases) and idiopathic juvenile osteoporosis.1–3 In the case described, there was no suggestive family history, no joint hypermobility, dental abnormalities, bone deformities, short stature or hearing impairment on physical examination and no suspicion of child abuse. Moreover, the healing time of the fractures was within the expected and the markers of bone mineral homeostasis were normal with the exception of low ALP, which can be reduced in CD.4
Careful physical examination and oriented complementary investigations also excluded secondary causes of bone fragility,1 2 such as infiltrative and inflammatory diseases (normal full blood count and ESR only slightly elevated); chronic renal disease and nephrotic syndrome; endocrine conditions, such as hyperthyroidism, diabetes mellitus and primary or secondary hyperparathyroidism and vitamin D deficiency. Iatrogenic causes were also excluded as the patient had no chronic medication intake.
Finally, CD antibodies were positive and the histology of the duodenal biopsies confirmed the diagnosis. Although rare in paediatric patients, CD may present with low mineral bone density, associated with bone fragility and recurrent fractures, even in the absence of other suggestive signs and symptoms.
Treatment
GFD was introduced after confirmation of the diagnosis and the family received professional dietary counselling.
Outcome and follow-up
In the first appointment after the diagnosis, complementary analysis to investigate signs of intestinal malabsorption showed normal levels of ferritin, folic acid and vitamin B12. A dual-energy X-ray absorptiometry (DEXA) was also performed and showed a bone mineral density (BMD) Z-score of −1.2. A supplement of cholecalciferol was started in a 666 UI daily dosage and instructions on dietary measures were given to assure adequate intake of dairy products and adherence to GFD. During the first months, the therapeutic adhesion to GFD was suboptimal, but improved after reinforcing the dietary counselling. Coeliac antibodies became negative after 11 months.
During the 3.5-year-period of follow-up, he had no more bone fractures and his growth remains regular. DEXA was repeated 15 months after and revealed an improvement, with Z-score −1.
A short time after the diagnosis, and according to the guidelines from ESPGHAN5 for the diagnosis of CD, his brother aged 10 years, who was completely asymptomatic and without history of fractures, was screened for CD, with positive results: antibodies antitissue transglutaminase IgA (>2000 RU/mL; positive >20); antiendomysial IgA (strongly positive) and antideamidated forms of gliadin peptides IgA (305 units; positive >25). Duodenal biopsies confirmed the diagnosis of CD, classification Marsh III A/B. He started GFD without difficulties, remaining asymptomatic until the present time.
Discussion
In the approach to a child with recurrent bone fractures, the priority should be differentiating between a child with normal bone strength exposed to repeated or high-energy trauma and a child with an underlying primary or secondary bone fragility. Primary bone disorders are less probable in the absence of suggestive findings in medical history and physical examination, with the exception of idiopathic juvenile osteoporosis, which is a diagnosis of exclusion. The main secondary causes of bone fragility1 2 can almost all be excluded by medical examination and directed complementary investigations. Serologies for CD should always be included in this evaluation, since this disease constitutes a recognised cause of low mineral bone density.6
CD is an immune-mediated systemic disease induced by gluten peptides in genetically susceptible individuals. It may manifest with a variety of non-specific signs and symptoms or can even be asymptomatic. Even so, it is crucial to make the diagnosis as soon as possible due to the negative health consequences, especially in untreated cases.5 The classical presentation in paediatric age includes a variable combination of the following symptoms: chronic diarrhoea, anorexia, abdominal distension and pain, poor weight gain, vomiting and severe malnutrition when the diagnosis is delayed. Gastrointestinal symptoms are typically less evident in older children. Asymptomatic (silent) and atypical forms such as anaemia, delayed puberty, growth failure and decreased bone density have become more frequent, and the latter are more frequent in older children, adolescents and adults.5 7 This might result from an improved recognition of non-gastrointestinal forms of CD and the widespread use of serological screening.5 7 CD can be associated with many bone signs and symptoms, including pain, rickets, osteomalacia, osteopenia, osteoporosis, minimal trauma-related fractures or growth failure.8
In some studies, low BMD criteria (Z-score ≤−2.0) was found in 16–19% of the paediatric cases at the time of the diagnosis.9–12 Kuloglu et al12 identified 28 in 52 children with low BMD at the time of the diagnosis of CD. However, no case presenting with fractures was described. Hartman et al9 found 2 cases in 41 children with CD with minor trauma-related fractures occurring before the diagnosis. Sánchez et al13 described that, in a cohort of 265 patients with CD (65% diagnosed until the age of 16 years), 23% had experienced at least one fracture: in 66% of these, the fractures occurred before the diagnosis and 28% had more than one fracture. However, in this study, history of previous fractures was essentially related to the classic gastrointestinal presentation of CD. While these studies prove there is an obvious relation between fractures and CD, the initial clinical presentation of the disease with recurrent bone fractures is exceedingly rare, especially in paediatric age. Mulder et al14 and Rastogi et al15 published three cases of adult patients with CD with bone fractures as the initial presentation.
In CD, the pathophysiology of low BMD and increased risk of bone fractures is multifactorial.16 17 Intestinal malabsorption of iron, folic acid, calcium, zinc and the fat-soluble vitamins A, D, E and K can lead to anaemia and bone loss, often present in patients with CD.4 On the other hand, chronic intestinal inflammation causes an imbalance between the secretion of proinflammatory and inhibitory cytokines that reduces the RANKL/OPG ratio and leads to osteoclasts activation and osteoblasts inhibition.4 16 17 Further proposed mechanisms for increased risk of bone fractures include hormone-related disorders that are associated with CD, or GFD, which is often low in minerals, impacting on bone health.16 17
Although low BMD is frequently found at the diagnosis of CD, its correlation with the increased risk of bone fractures is not completely clear. Hartman et al9 reported that fracture risk is only minimally increased in patients with CD, while Heikkilä et al16 concluded they were 1.92 times more likely to have any fracture at some point in their lives. Additionally, the 2013 International Society for Clinical Densitometry position statement18 on paediatric osteoporosis criteria affirms that a BMD Z-score of >−2.0 does not preclude the possibility of skeletal fragility and increased risk of fracture, as observed in the case described above.
The description of the index case reinforces the incomplete knowledge about the physiopathology of bone disease in CD. The slight decrease in BMD and the history of multiple bone fractures were not completely explained by intake deficit or intestinal malabsorption. Other factors probably related to proinflammatory imbalance in bone homeostasis may have contributed to these atypical findings in CD.4
Snyder et al8 recently published recommendations to clarify the need of bone health surveillance in children with CD. According to these guidelines, BMD evaluation should not be performed routinely and must be reserved for the cases with severe malabsorption, prolonged delay in diagnosis or presentations suggestive of bone disease (bone pain, rickets, osteomalacia, tetany or minimal trauma-related fractures). It is further recommended that this evaluation should be performed at the time of diagnosis and every 1–2 years until the results normalise. In these cases, calcium, PO4, ALP and parathyroid hormone must also be evaluated at the time of diagnosis. In patients who do not adhere to GFD despite dietary counselling, the BMD should be evaluated during the follow-up. Vitamin D status can be assessed at the time of diagnosis (weak recommendation, due to lack of studies).
Initiation of GFD rapidly restores bone mass in almost all children and adolescents.8 This fact is supported by the bone disease-free interval during the 3.5-year period of follow-up of the index case. Since childhood and adolescence are crucial for reaching the peak of BMD, a delay in the diagnosis and lack of adhesion to a GFD should certainly be avoided.
This report also emphasises the importance of the screening guidelines recommended by ESPGHAN in asymptomatic children and adolescents with an increased risk for CD, namely first-degree relatives.5
Learning points.
In a child with recurrent bone fractures, it is crucial to differentiate between a child with normal bone strength exposed to repeated or high-energy trauma and a child with an underlying primary or secondary bone fragility.
Despite recurrent bone fractures being an exceedingly rare form of presentation of coeliac disease (CD), serologies for CD must always be included in the evaluation, since it constitutes a recognised cause of low mineral bone density and with an effective treatment. Gluten-free diet restores bone mineral density and prevents other complications of untreated CD.
Asymptomatic first-degree relatives of patients with CD should always be screened for the disease.
Acknowledgments
Dr. Mónica Oliva, who helped in guiding the initial investigation of the causes of recurrent bone fractures and Dr. Natalia Noronha for the collaboration in the translation of the manuscript.
Footnotes
Contributors: FDC was involved in the diagnostic work-up and drafted the manuscript. CM and RF were responsible for the patients’ diagnosis and follow-up. SA made significant contributions to the content of the manuscript. SA, CM and RF were involved in critically reviewing the data. RF gave final approval of the version to be published.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Korula S, Titmuss AT, Biggin A et al. A practical approach to children with recurrent fractures. Endocr Dev 2015;28:210–25. 10.1159/000381047 [DOI] [PubMed] [Google Scholar]
- 2.Boyce AM, Gafni RI. Approach to the child with fractures. J Clin Endocrinol Metab 2011;96:1943–52. 10.1210/jc.2010-2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olney RC, Mazur JM, Pike LM et al. Healthy children with frequent fractures: how much evaluation is needed? Pediatrics 2008;121:890–7. 10.1542/peds.2007-2079 [DOI] [PubMed] [Google Scholar]
- 4.McCormick RK. Osteoporosis: integrating biomarkers and other diagnostic correlates into the management of bone fragility. Altern Med Rev 2007;12:113–45. [PubMed] [Google Scholar]
- 5.Husby S, Koletzko S, Korponay-Szabó IR et al. , ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastroenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012;54:136–60. 10.1097/MPG.0b013e31821a23d0 [DOI] [PubMed] [Google Scholar]
- 6.Lebwohl B, Michaëlsson K, Green PH et al. Persistent mucosal damage and risk of fracture in celiac disease. J Clin Endocrinol Metab 2014;99:609–16. 10.1210/jc.2013-3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garampazzi A, Rapa A, Mura S et al. Clinical pattern of celiac disease is still changing. J Pediatr Gastroenterol Nutr 2007;45:611–14. 10.1097/MPG.0b013e31814c3d79 [DOI] [PubMed] [Google Scholar]
- 8.Snyder J, Butzner JD, DeFelice AR et al. Evidence-informed expert recommendations for the management of celiac disease in children. Pediatrics 2016;138:e20153147 10.1542/peds.2015-3147 [DOI] [PubMed] [Google Scholar]
- 9.Hartman C, Hino B, Lerner A et al. Bone quantitative ultrasound and bone mineral density in children with celiac disease. J Pediatr Gastroenterol Nutr 2004;39:504–10. 10.1097/00005176-200411000-00011 [DOI] [PubMed] [Google Scholar]
- 10.Tau C, Mautalen C, De Rosa S et al. Bone mineral density in children with celiac disease. Effect of a gluten-free diet. Eur J Clin Nutr 2006;60:358–63. [DOI] [PubMed] [Google Scholar]
- 11.Turner J, Pellerin G, Mager D. Prevalence of metabolic bone disease in children with celiac disease is independent of symptoms at diagnosis. J Pediatr Gastroenterol Nutr 2009;49:589–93. 10.1097/MPG.0b013e31819ca18e [DOI] [PubMed] [Google Scholar]
- 12.Kuloğlu Z, Kirsaçlioğlu CT, Kansu A et al. Celiac disease: presentation of 109 children. Yonsei Med J 2009;50:617–23. 10.3349/ymj.2009.50.5.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sánchez MI, Mohaidle A, Baistrocchi A et al. Risk of fracture in celiac disease: gender, dietary compliance, or both? World J Gastroenterol 2011;17:3035–42. 10.3748/wjg.v17.i25.3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulder CJ, Cardile AP, Dickert J. Celiac disease presenting as severe osteopenia. Hawaii Med J 2011;70:242–4. [PMC free article] [PubMed] [Google Scholar]
- 15.Rastogi A, Bhadada SK, Bhansali A et al. Celiac disease: a missed cause of metabolic bone disease. Indian J Endocrinol Metab 2012;16:780–5. 10.4103/2230-8210.100674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heikkilä K, Pearce J, Mäki M et al. Celiac disease and bone fractures: a systematic review and meta-analysis. J Clin Endocrinol Metab 2015;100:25–34. 10.1210/jc.2014-1858 [DOI] [PubMed] [Google Scholar]
- 17.Krupa-Kozak U. Pathologic bone alterations in celiac disease: etiology, epidemiology, and treatment. Nutrition 2014;30:16–24. 10.1016/j.nut.2013.05.027 [DOI] [PubMed] [Google Scholar]
- 18.Bishop N, Arundel P, Clark E et al. ; International Society of Clinical Densitometry. Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD 2013 Pediatric Official Positions. J Clin Densitom 2014;17:275–80. 10.1016/j.jocd.2014.01.004 [DOI] [PubMed] [Google Scholar]


