Abstract
Dabigatran is a non-vitamin K antagonist oral anticoagulant that has been approved for atrial fibrillation and prevention of venous thromboembolism. Its use has been increasing in the USA since serum drug levels do not need monitoring. To date, no significant skin side effects have been reported other than 4 cases of non-specific skin lesion and 2 cases of leukocytoclastic vasculitis (LCV), which is a small vessel inflammatory disease that presents as palpable purpura in lower extremities. We present a man aged 57 years with chronic deep vein thrombosis who developed palpable purpura, petechiae, swelling in lower extremities, torso and distal upper extremities on the third day after dabigatran initiation. The present case highlights the potential risk for LCV with dabigatran use and provides insight into its management.
Background
Dabigatran is an oral direct thrombin inhibitor that was first approved in 2010 to prevent stroke and systemic embolism.1 Dabigatran was then approved for non-valvular atrial fibrillation, and clinical trials have shown that it is safe and effective in preventing venous thromboembolic events. It works by competitively and reversibly inhibiting thrombin via hydrophobic interaction at the active site of thrombin. More specifically, dabigatran interferes with free thrombin.2
Leukocytoclastic vasculitis (LCV) is a small vessel vasculitis that most commonly presents as palpable purpura in lower extremities.3 It is classified by histological features of immune complex deposits that result in acute inflammation. LCV can be caused by multiple aetiologies such as infection, drugs, chemicals including insecticide and petroleum products, food allergies, connective tissue diseases and malignant neoplasms.4 5 We describe a case of a patient aged 57 years who developed LCV 3 days after initiation of dabigatran for chronic deep vein thrombosis (DVT).
Case presentation
A man aged 57 years with a history of ulcerative colitis and recurrent DVTs with inferior vena cava filter was found to have persistent DVT on lower extremity ultrasound scan during haematology clinic visit. He was subsequently converted from warfarin to dabigatran. On the fifth day of dabigatran treatment, the patient presented to emergency department with lower extremity tingling, petechiae and a 2-day history of palpable purpura. The patient did not have pertinent family history and did not smoke tobacco or drink alcohol. Medication allergies included urticaria after omeprazole and ciprofloxacin use. Vitals on admission included BP 124/70, P 68, T 98.2, RR 16, O2 sat 98% RA and BMI 31. Physical examination was unremarkable for head, ears, eyes, nose and throat, cardiac, respiratory, abdomen, and neurological assessment. There was +2 non-pitting bilateral leg oedema with intact pulse. Skin examination findings were remarkable for palpable purpura and red macules involving the lower extremities, lower back, buttocks and lower arms with extension just superficial to the elbows (figure 1A, B).
Figure 1.
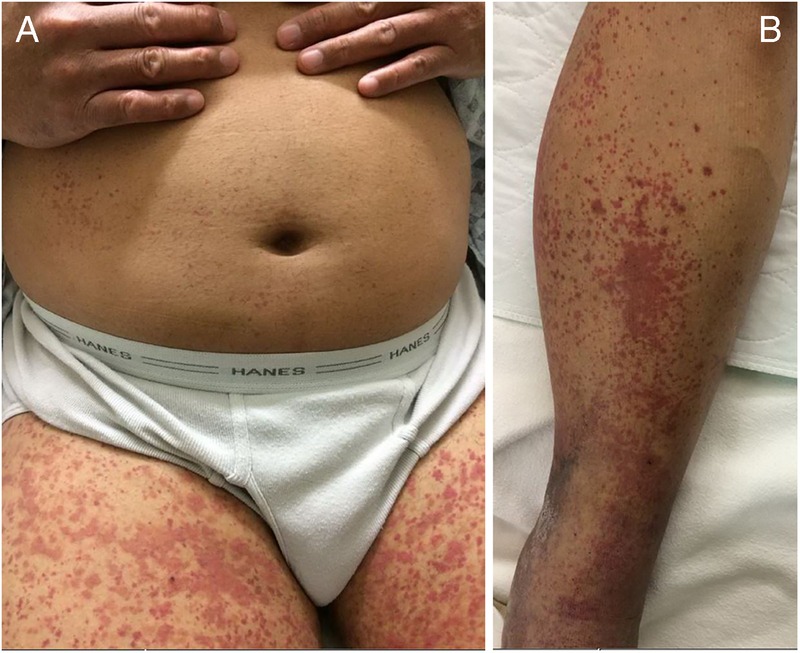
(A) Diffuse red to purple macules and papules on patient's bilateral proximal lower extremities. (B) Deep red to purple thin papules on bilateral distal lower extremities in the setting of lower extremity oedema.
Investigations
Punch biopsy demonstrated neutrophils in perivascular and interstitial pattern with associated karyorrhexis, fibrinoid necrosis and erythrocyte extravasation. Associated eosinophils and lymphocytes also were observed (figure 2A, B). Direct immunofluorescence was negative. Laboratory work-up included CBC with differential HIV, hepatitis B and C serology, blood culture, urinalysis with microscopy, complement and ANCA were negative or normal. Remarkable laboratory work-up included ESR at 63 mm/hour (normal: 0–15 mm/hour) and CRP 2.0 mg/dL (normal: <0.5 mg/dL), ANA of 1:160 (normal: <1:80) and CBC with eosinophilia at 590 (normal: 40–390). Chest X-ray was unremarkable. Drug-related LCV, other than dabigatran, was less likely, given that patient's only medication was warfarin which he took more than 5 years prior to initiating dabigatran. According to the American College of Rheumatology criteria, our patient met 5/5 criteria for, LCV (hypersensitivity vasculitis) including: (1) age of onset >16 years, (2) medication at disease onset, (3) palpable purpura, (4) maculopapular rash and (5) biopsy including histological change in venule and arteriole with perivascular or extravascular granulocyte accumulation.6 Three of five criteria correlate with a specificity of 83.9% and a sensitivity of 71%. Importantly, no additional organ involvement was noted on urinalysis or liver function tests.
Figure 2.
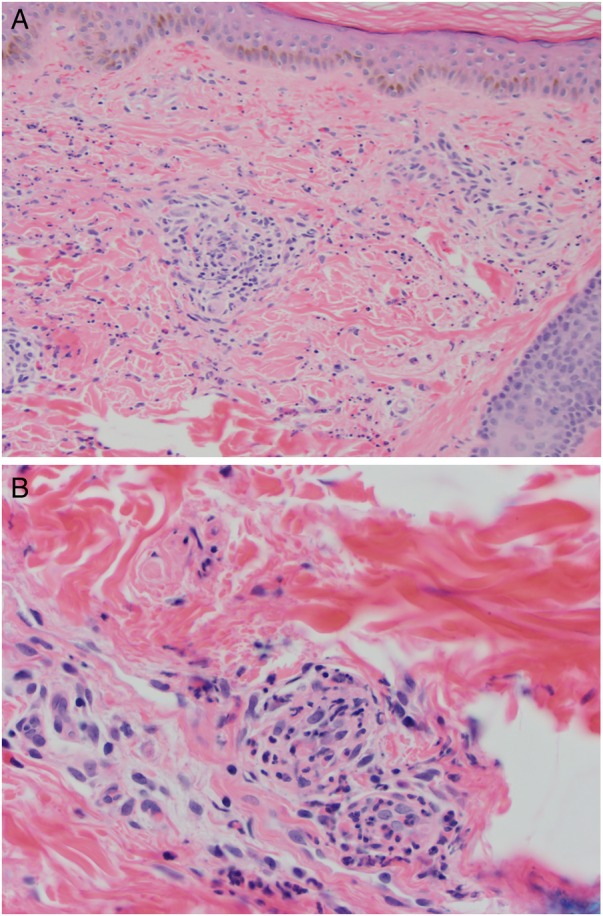
(A) Histopathology obtained from punch biopsy of thigh demonstrates neutrophils, eosinophils and lymphocytes centred on and involving blood vessels with associated haemorrhage (original magnification, 4×). (B) Higher power magnification highlights neutrophils with karyorrhexis, eosinophils and lymphocytes involving the blood vessels, consistent with leukocytoclastic vasculitis (original magnification, 40×, H&E).
Differential diagnosis
Other small to medium vasculitides including microscopic polyangiitis, granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis, cryoglobulinemic vasculitis, hypocomplementemic urticarial vasculitis need to be considered but were ruled out in our patient based on clinical and laboratory findings. Diagnosis of LCV secondary to medication (dabigatran) was made.
Treatment
Dabigatran was discontinued, and the patient was initially started on a therapeutic dose of enoxaparin with transition to rivaroxaban. For LCV, he was started on prednisone 40 mg daily with taper over 4 weeks, and Class I topical corticosteroid (halobetasol 0.5% cream) with ultimate resolution of his LCV.
Outcome and follow-up
After discontinuation of dabigatran and initiation of oral prednisone and topical steroids, the patient's symptoms including itching, bilateral leg pain and erythema significantly improved within 24–48 hours of hospitalisation. The patient was seen by his primary care provider 7-day postdischarge with resolution of his erythema, improvement in pain and swelling and moderate improvement in his purpura. At 1-month postdischarge, he had mild persistence of purpura of his anterior thighs but resolution at other locations.
Discussion
Dabigatran is a new direct thrombin inhibitor that is used for chronic DVT and atrial fibrillation.7 To date, there are six case reports describing dabigatran-related skin adverse effects. In three cases, there was no further workup of the cutaneous side effects, and these could represent a morbilliform drug reaction and not LCV. Of the reported cases, one was a man aged 20 years with a history of atrial fibrillation who developed skin rash on his thigh and forearm after 2 weeks of dabigatran, the youngest case reported in the literature. The others were a man aged 78 years with atrial fibrillation having full body purpuric rash within 24 hours of dabigatran initiation, and a man aged 59 years with atrial flutter developing diffuse non-pruritic rash on torso and lower extremities after 5 days of dabigatran use.8–10 No biopsies were performed in these cases. An eczematous drug eruption also was reported in a woman aged 71 years with atrial fibrillation with palmar and plantar involvement.11 To the best of our knowledge, there have been only two reported cases of dabigatran-related LCV: Cakmak et al12 discussed a woman aged 74 years developing LCV within a week of dabigatran usage, and Potolidis et al13 reported a case in a man aged 70 years in less than a week of initiation of dabigatran. Both cases were treated with systemic steroids and were switched to other forms of anticoagulation including low molecular heparin and warfarin. In both cases, dabigatran was used for atrial fibrillation. In contrast with these two studies, the current report is the first case to discuss a patient younger than 60 years of age developing LCV after dabigatran use for chronic deep vein thrombosis. This case expands our knowledge about the potential risk of LCV with the use of dabigatran and management including discontinuation of the drug and the initiation of systemic and topical steroids.
Learning points.
Dabigatran is an oral direct thrombin inhibitor used for atrial fibrillation and preventing venous thromboembolism with uncommon cutaneous side effects.
Leukocytoclastic vasculitis (LCV) can be caused by infection, drugs, connective tissue diseases, malignancy, chemicals and food allergies.
The current case reports LCV after use of dabigatran for chronic deep vein thrombosis
Discontinuation of dabigatran and initiation of systemic and topical steroids are an effective strategy for the management of dabigatran-induced LCV.
Footnotes
Contributors: JA designed the manuscript and prepared the draft with important input from RG and JPL. KW provided overall revision and dermatological intellectual contribution.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Blommel ML, Blommel AL. Dabigatran etexilate: a novel oral direct thrombin inhibitor. Am J Health Syst Pharm 2011;16:1506–19. [DOI] [PubMed] [Google Scholar]
- 2.Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009;15(Suppl 1):9S–16S. 10.1177/1076029609343004 [DOI] [PubMed] [Google Scholar]
- 3.Sams WM, Thorne EG, Small P et al. Leukocytoclastic vasculitis. Arch Dermatol 1976;2:219–26. [PubMed] [Google Scholar]
- 4.Fiorentino DF. Cutaneous vasculitis. J Am Acad Dermatol 2003;3:311–40. [DOI] [PubMed] [Google Scholar]
- 5.Lotti T, Ghersetich I, Comacchi C et al. Cutaneous small-vessel vasculitis. J Am Acad Dermatol 1998;5:667–87. [DOI] [PubMed] [Google Scholar]
- 6.Hunder GG, Arend WP, Bloch DA et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis. Introduction. Arthritis Rheum 1990;8:1065–7. [DOI] [PubMed] [Google Scholar]
- 7.Pengo V, Crippa L, Falanga A et al. Questions and answers on the use of dabigatran and perspectives on the use of other new oral anticoagulants in patients with atrial fibrillation. A consensus document of the Italian Federation of Thrombosis Centers (FCSA). Thromb Haemost 2011;5: 868–76. [DOI] [PubMed] [Google Scholar]
- 8.Eid TJ, Shah SA. Dabigatran-induced rash. Am J Health Syst Pharm 2011;68:1489–90. 10.2146/ajhp110088 [DOI] [PubMed] [Google Scholar]
- 9.To K, Reynolds C, Spinler SA. Rash associated with dabigatran etrexilate. Pharmacotherapy 2013;33:e23–7. 10.1002/phar.1203 [DOI] [PubMed] [Google Scholar]
- 10.Whitehead H, Boyd JM, Blais DM et al. Drug induced exanthem following dabigatran. Ann Pharmacother 2011;45:e53 10.1345/aph.1Q317 [DOI] [PubMed] [Google Scholar]
- 11.Schleichert R, Goldner R, Dickfeld T. Palmoplantar pustular eruption due to dabigatran. Cutis 2016;97:e10–11. [PubMed] [Google Scholar]
- 12.Cakmak MA, Sahin S, Cinar N. Adverse skin reaction caused by dabigatran. Eur Rev Med Pharmacol Sci 2014;18:2595. [PubMed] [Google Scholar]
- 13.Potolidis E, Mandros C, Kotsa K et al. Dabigatran associated leukocytoclastic vasculitis. Case Rep Med 2015;2015:616109 10.1155/2015/616109 [DOI] [PMC free article] [PubMed] [Google Scholar]


