Abstract
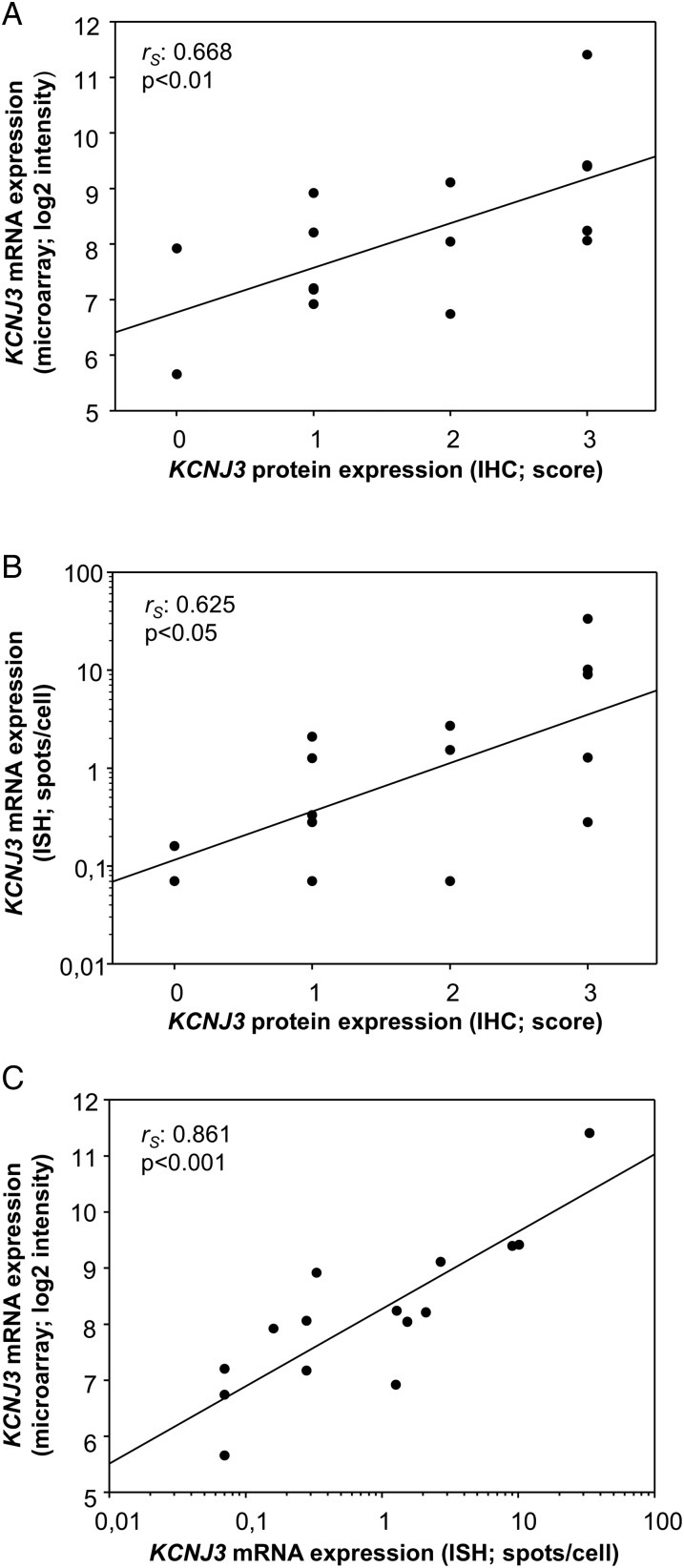
Increased expression levels of KCNJ3 have been correlated with lymph node metastases and poor prognosis in patients with breast cancer, suggesting a prognostic role of KCNJ3. We aimed to establish protocols for the detection of KCNJ3 in formalin-fixed, paraffin-embedded (FFPE) breast cancer tissue. Several antibodies were tested for sensitivity and specificity by western blot, followed by optimisation of the immunohistochemistry (IHC) procedure and establishment of KCNJ3 mRNA in situ hybridisation (ISH). Methods were validated by processing 15 FFPE breast cancer samples for which microarray data were available. Spearman's rank correlation analysis resulted in borderline significant correlation for IHC versus ISH (rS: 0.625; p<0.05) and IHC versus microarray (rS: 0.668; p<0.01), but in significant correlation for ISH versus microarray (rS: 0.861; p<0.001). The ISH method was superior to IHC, regarding robustness, sensitivity and specificity and will aid to further study expression levels of KCNJ3 in both malignant and physiological conditions.
Keywords: BREAST CANCER, IMMUNOHISTOCHEMISTRY, IN SITU HYBRIDISATION, ANTIBODIES, METHODOLOGY
Introduction
KCNJ3 encodes for G-protein activated inward rectifier potassium channel 1 (GIRK1; synonyms: KGA, Kir3.1). GIRKs are G-protein effectors that regulate cellular excitability and activity via neurotransmitters and hormones. Physiological roles of GIRKs include, among others, regulation of heartbeat, learning and memory functions.1 Most importantly, several lines of evidence demonstrate a correlation of increased KCNJ3 expression levels, breast cancer progression and patient’s prognosis. Stringer et al2 observed increased levels of KCNJ3 mRNA in primary invasive breast carcinomas when compared with corresponding normal breast tissue and found KCNJ3 mRNA levels positively correlating with the amount of metastatic lymph nodes. A study by Brevet et al3 supported these results by showing a correlation between GIRK1 protein expression and lymph node metastases as well as reduced overall survival of patients with tumours displaying high GIRK1 expression. The relevance of KCNJ3 in breast cancer is further underscored by Rezania et al,4 who demonstrated increased motility, invasiveness and angiogenesis of KCNJ3 overexpressing MCF-7 breast cancer cells compared with controls. Ko et al5 found KCNJ3 to be downregulated in p53 mutant breast cancer samples and upregulated in oestrogen receptor (ER) positive tumours. Our own findings6 also show a strong link between KCNJ3 expression and ER-positive tumour samples. Taken this evidence together, it seems worthwhile to study KCNJ3 expression in invasive breast carcinoma to validate it as a new prognostic biomarker for this disease. In order to pursue this goal, we aimed to establish methods for the detection of KCNJ3 gene products in formalin-fixed, paraffin-embedded (FFPE) breast cancer samples. Many commercially available antibodies are insufficiently tested in terms of sensitivity and specificity, which in turn can lead to misleading results, and also others experienced difficulties with anti-GIRK1 antibodies.7 Therefore, the aim of this study was to (a) critically test a panel of anti-GIRK1 antibodies for sensitivity and specificity, (b) to systematically optimise experimental immunohistochemistry (IHC) conditions, (c) to establish RNA in situ hybridisation (ISH) protocol as an antibody-independent and microarray-independent method for the detection of KCNJ3 mRNA in tumour samples, and (d) to determine the correlation of GIRK1 protein (IHC) with KCNJ3 mRNA (microarray and ISH) expression in human breast cancer in order to identify the best KCNJ3 detection method.
Materials and methods
Anti-GIRK1 antibodies
Ab#1: mouse monoclonal antibody from Abcam (#Ab11924); Ab#2: rabbit polyclonal antibody directed against the C-terminus of GIRK1 (generated by Kurt Schmidt, Institute of Pharmaceutical Sciences, University of Graz, Austria; described in ref. 8); Ab#3: rabbit polyclonal antibody directed against the N-terminus (N-T) of GIRK1 (generated by Kurt Schmidt); Ab#4: polyclonal goat antibody from Santa Cruz (#Sc-16131); Ab#5: rabbit polyclonal antibody from Alomone (#APC-005) and Ab#6: mouse monoclonal antibody from Alomone (#ALM-031). Sheep anti-rabbit/horseradish peroxidase (HRP), sheep anti-mouse/HRP (both kindly provided by Amir-Hassan Zarnani, Avicenna Research Institute, Tehran, Iran; both 1:10 000 dilution) and donkey anti-goat IgG-HRP antibodies (Santa Cruz Biotechnology, #Sc-2020; 1:5000 dilution) served as secondary antibodies.
Cell culture
MCF-7 wild-type or stably overexpressing KCNJ3 and HEK-293 cells (both from ATCC) were cultured as described.4 8 HL-1 cells were purchased from William C. Claycomb and maintained as described.9 Cells were kept in a humidified atmosphere at 37°C and 5% CO2. Mycoplasma tests were negative, and short tandem repeat profiling proofed the cell lines to be authentic.
Patient samples
FFPE tissue samples of ER-positive primary invasive breast carcinomas were selected from a series of previously published breast cancers,10 for which microarray analysis (Affymetrix, GEO accession GSE17705) had been performed. Out of these, 15 tumour samples representing carcinomas with highest (n=5), intermediate (n=5) and lowest (n=5) KCNJ3 microarray expression levels were retrieved from the biobank of the Medical University of Graz. ER-positive samples were chosen, since a strong correlation between KCNJ3 expression and ER-positive breast cancer has been reported.5 6 All cases were annotated with detailed clinical and pathological information (see table 1 for patient characteristics). The use of the patient samples including the clinical data was approved by the ethics committee of the Medical University of Graz (24-081 ex 11/12).
Table 1.
Patient characteristics and KCNJ3 expression levels as determined by different methods
| Clinicopathological patient characteristics | Results of KCNJ3 expression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| # | AAD | Grade | pT | LN | pM | ER | PR | Microarray | IHC | RNA ISH |
| 01 | 78 | 2 | 2 | 3/20 | − | + | + | 11.407 | 3 | 33.33 |
| 02 | 60 | 2 | 2 | 5/20 | − | + | + | 9.417 | 3 | 10.11 |
| 03 | 56 | 2 | 2 | 3/23 | − | + | + | 9.393 | 3 | 9.01 |
| 04 | 71 | 2 | 2 | 0/29 | − | + | + | 9.109 | 2 | 2.69 |
| 05 | 68 | 3 | 2 | 0/18 | − | + | − | 8.917 | 1 | 0.33 |
| 06 | 68 | 2 | 1 | 11/18 | − | + | + | 8.239 | 3 | 1.28 |
| 07 | 43 | 2 | 3 | 2/8 | − | + | − | 8.207 | 1 | 2.10 |
| 08 | 50 | 1 | 4 | 0/11 | − | + | − | 8.061 | 3 | 0.28 |
| 09 | 70 | 3 | 2 | 0/17 | + | + | + | 8.042 | 2 | 1.53 |
| 10 | 63 | 2 | 2 | 0/14 | − | + | − | 7.919 | 0 | 0.16 |
| 11 | 62 | 3 | 2 | 9/27 | + | + | + | 7.205 | 1 | 0.07 |
| 12 | 50 | 1 | 1 | 3/16 | − | + | + | 7.174 | 1 | 0.28 |
| 13 | 63 | 2 | 1 | 0/0 | + | + | + | 6.918 | 1 | 1.26 |
| 14 | 53 | 2 | 4 | 16/19 | − | + | + | 6.740 | 2 | 0.07 |
| 15 | 70 | 2 | 2 | 3/14 | − | + | − | 5.656 | 0 | 0.07 |
#, patient sample number; −, negative; +, positive; AAD, age at diagnosis; ER, oestrogen receptor; IHC, immunohistochemical score of KCNJ3 protein expression; LN, lymph nodes (positive/total examined); Microarray, log2 intensities of KCNJ3 expression; pM, distant metastasis status; PR, progesterone receptor; pT, tumour size staging; RNA ISH, KCNJ3 RNA in situ hybridisation results as spots/cell.
Immunohistochemistry
FFPE sections of patient samples were mounted on glass slides together with formalin-fixed, agarose embedded HL-1 and HEK-293 cells which served as positive and negative on-slide controls.11 12 Staining conditions were optimised by systematic variation of staining conditions regarding heat-induced epitope retrieval, washing solutions and antibody dilution. A detailed description of the IHC protocol is given in online supplementary file 1. Cytoplasmic staining intensity in tumour tissue was scored by two independent investigators (SWJ and TB) using a semiquantitative four-tiered score with 0–3 corresponding to no staining (0), weak (1), intermediate (2) and strong (3) staining. Incubation without primary antibody served as additional negative control.
jclinpath-2016-203798supp1.pdf (110.2KB, pdf)
RNA in situ hybridisation
Sections of FFPE tissue (thickness 4 µm) were mounted on Superfrost Plus coated slides (Thermo Scientific) and invasive tumour areas were selected by trimming away peritumoural tissue devoid of cancer. The slides were processed according to manufacturer's instructions for the RNAscope 2.0 High Definition—BROWN kit (ACD). Three sections of each sample were stained with different probes: the KCNJ3 probe (#Hs-KCNJ3-tv1tv2), the negative control probe DapB (#310043) and the positive control probe POLR2A (#310451). For image analysis, a representative tumour region was selected for each tumour, and z-stacks comprising 10 images were captured at 40× magnification using a Zeiss Oberver.Z1 inverted microscope. Multiple adjacent single images (3×3 tiles) were acquired and aligned using the MosaiX module of the AxioVision software (Zeiss). Image sequences were stacked, and the SpotStudio software from ACD was used for detection of single cells, detection of spots and clusters and calculation of estimated number of spots per cell (see online supplementary file 2, eg, of image analysis). DapB and POLR2A probes served as technical quality controls that needed to fulfil the cut-off criteria (≤0.5 spots/cell for negative controls; ≥2.5 spots/cell for positive controls) in order to ensure technical specificity of the probes and to detect samples with highly degraded RNA.
jclinpath-2016-203798supp2.pdf (357.4KB, pdf)
Statistical analysis
Spearman's rank correlation analysis was performed to correlate the results of the different KCNJ3 detection methods. Analyses were performed using the SigmaPlot/SigmaStat V.12.5 software (Systat Software). Results with p<0.05 were considered statistically significant.
Results
Screening of different anti-GIRK1 antibodies for specificity and sensitivity
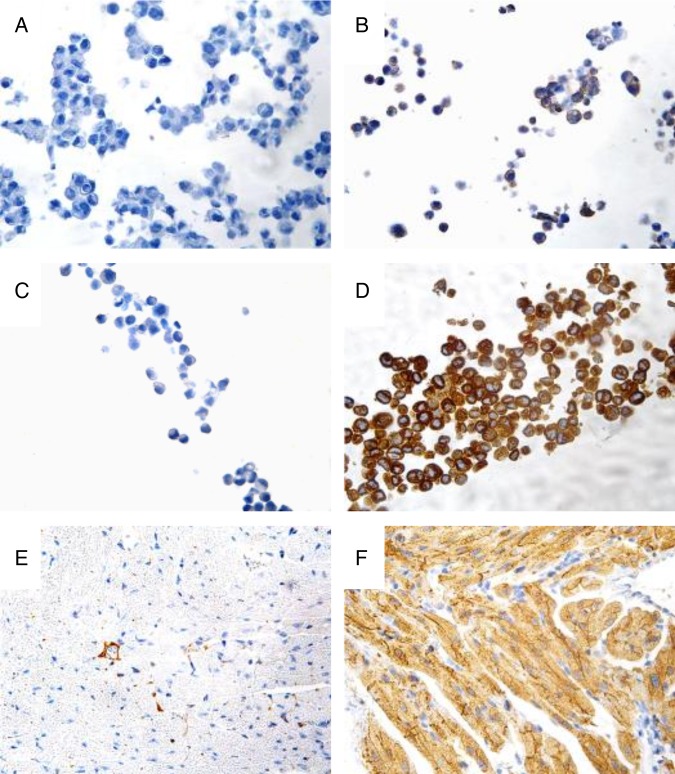
Specificity and sensitivity of the different anti-GIRK1 antibodies were first assessed by Western blot analysis as described in online supplementary file 3. In summary, Western blots recommended Ab#1 for best sensitivity and specificity, and it was therefore further tested for its suitability in IHC on formalin-fixed, agarose-embedded cell pellets and FFPE mouse tissue (figure 1). As expected, staining was absent in HEK-293 (figure 1A) and positive in HL-1 cells (figure 1B). Wild-type breast cancer MCF-7 cells were negative (figure 1C), in contrast to strong positivity of MCF-7 cells overexpressing KCNJ3 (figure 1D). Tissue sections from mouse atrium and ventricle served as additional positive and negative controls13 with expected negativity of GIRK1 staining in the ventricle in contrast to strong positivity of the atrial myocardium (figures 1E, F). In summary, Ab#1 yielded satisfactory results regarding specificity and sensitivity, and the protocol was further optimised for archived human FFPE breast cancer samples using patient sample #2 with high KCNJ3 mRNA expression levels (according to microarray data; table 1) as biological positive control (see online supplementary file 4 for details). The combination of heat-induced epitope retrieval at pH 9 (microwave), usage of Dako wash buffer and an antibody dilution of 1:50 of Ab#1 lead to best staining results with highest specificity and lowest background. This protocol was therefore used as standard protocol for the staining of the remaining patient samples.
Figure 1.
Performance of Ab#1 for immunohistochemistry on cell lines and formalin-fixed, paraffin-embedded mouse heart. (A) GIRK1 immunohistochemistry on formalin-fixed, agarose-embedded HEK-293 cells (negative control), (B) HL-1 cells (positive control), (C) MCF-7 cells and (D) MCF-7 cells overexpressing KCNJ3. (E) GIRK1 immunohistochemistry on FFPE mouse ventricle and (F) atrium. Micrographs were taken at 40× magnification.
jclinpath-2016-203798supp3.pdf (341.6KB, pdf)
jclinpath-2016-203798supp4.pdf (369.5KB, pdf)
IHC staining results of FFPE breast cancer samples
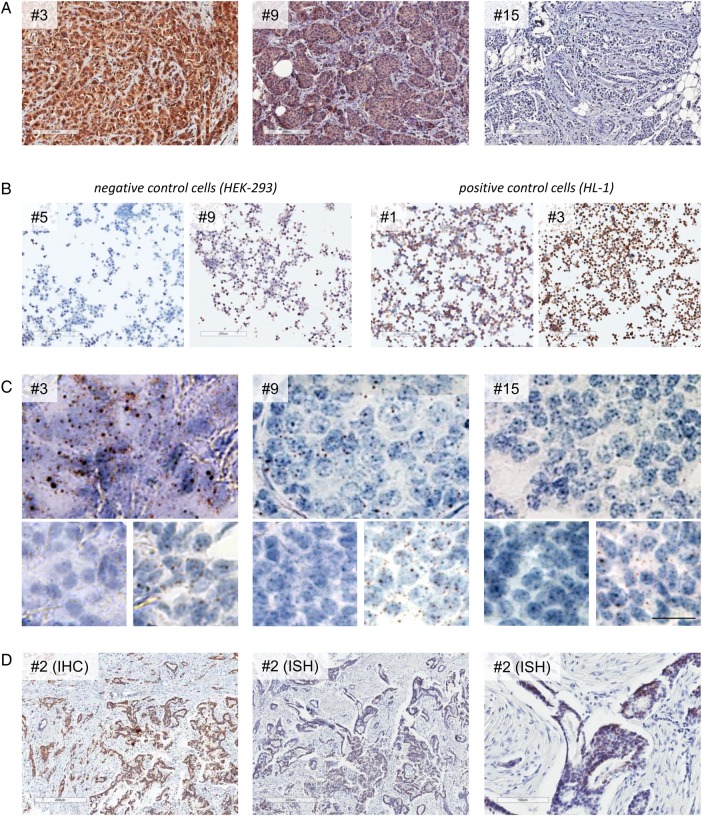
Subsequently, GIRK1 IHC was performed on 15 patient samples with different levels of KCNJ3 mRNA expression as assessed by microarray analysis (see table 1 for patient characteristics, microarray data and staining results). Representative images of patient samples with high, intermediate and low KCNJ3 expression are displayed in figure 2A. On each IHC slide, formalin-fixed, agarose-embedded HEK-293 and HL-1 cells were mounted as on-slide controls. Assessment of these controls revealed that antibody staining was not consistent, even in same runs, as shown exemplarily in figure 2B. However, positive staining was found in tumour cells only, but not in tumour stroma, inflammatory cells or normal structures including mammary ducts (figure 2D, left). IHC staining results correlated significantly with the microarray data (p<0.01), with a correlation coefficient of rS=0.668 (figure 3A).
Figure 2.
Comparison of KCNJ3 protein (GIRK1) and mRNA expression in formalin-fixed, paraffin-embedded samples of patients with human breast cancer. (A) GIRK1 immunohistochemistry (IHC) using the optimised protocol for Ab#1. Generic micrographs for patients with high (#3), intermediate (#9) and low (#15) GIRK1 protein expression are shown. (B) Two examples of on-slide negative control cells (HEK-293 on slides of samples #5 and #9) and positive control cells (HL-1 on slides of samples #1 and #3). (C) KCNJ3 RNA in situ hybridisation (ISH) results of the same samples as used in (A). Top panel: KCNJ3 probe. Lower panel left: DapB probe (negative control); right: POLR2A probe (positive control). Scale bar: 20 µm; all images shown at identical magnification. (D) Positive KCNJ3 signals are present in tumour cells but not in non-neoplastic cells. Left: GIRK1 IHC of patient sample #2; middle: KCNJ3 RNA ISH of patient sample #2; right: detail of middle image.
Figure 3.
Correlation of KCNJ3 gene product expression as assessed by different methods. (A) Scatter plot of KCNJ3 expression levels in 15 patients with breast cancer as assessed by microarray (log2 intensity) and immunohistochemistry (IHC) score. (B) RNA in situ hybridisation (ISH; spots/cell) versus IHC (score) is plotted. (C) Log2 intensity of microarray versus RNA ISH (spots/cell) is plotted. Spearman's rank correlation coefficients (rS) and p values are given for each plot.
RNA ISH staining results of FFPE breast cancer samples
Further, we aimed to establish a protocol for RNA ISH in order to quantitate KCNJ3 mRNA directly in FFPE sections and to correlate the results with microarray data and IHC staining. To achieve maximum technical concordance between microarray and RNA ISH data, the specific KCNJ3 probes for ISH covered RNA regions identical to the Affymetrix microarray assay. Technical negative and positive controls all met the given cut-off criteria, and samples were therefore regarded as successfully stained. In addition, sample #6 was used as biological positive control in each run (see online supplementary file 5). Results are given in table 1, and representative images of patient samples and their respective controls are displayed in figure 2C. In concordance with the IHC results, KCNJ3 mRNA expression was present only in tumour cells, but not in surrounding normal cells (figure 2D, middle and right). RNA ISH results correlated positively with IHC results (p<0.05, rS=0.625; figure 3B; see also table 1), and correlation with microarray data was highly significant (p<0.001, rS=0.861; figure 3C).
jclinpath-2016-203798supp5.pdf (210KB, pdf)
Discussion
Here we report two methods for the detection of KCNJ3 in archived FFPE human breast cancer tissue. Our study shows that accurate immunohistochemical detection of GIRK1 in human tissue is challenging due to low analytical specificity and/or sensitivity of available antibodies. Liang et al7 arrived at similar conclusions, aiming to assess GIRK1 and GIRK4 protein expression by immunofluorescence. While the anti-GIRK4 antibody used proved sufficiently specific and sensitive in their study, none of the anti-GIRK1 antibodies tested (comprising also Ab#5 evaluated in the current study) was suitable due to the high background staining in negative controls.7 This was supported by our finding that some samples with low KCNJ3 mRNA expression still displayed elevated protein expression in IHC, leading to unconvincing correlation coefficients when IHC results were compared with microarray and ISH data. It should be stated at this point that discrepancies between microarray and IHC data might occur, since mRNA and protein expression levels might differ.14 However, our data point towards differences in performance and robustness of the detection methods used and not towards biological reasons for highly differential mRNA and protein expression. Remarkably, few studies succeeded in immunohistochemical staining of GIRK1 on FFPE human tissue using Ab#48 and Ab#5.3 15 16 In retrospect, these previous studies might have overestimated GIRK1 expression, since anti-GIRK1 antibodies might lead to unspecific background staining. In addition to the IHC, we aimed to establish an antibody-independent method to detect KCNJ3 expression in human FFPE tissue that would allow to visualise mRNA expression in situ. The RNA ISH technique described here offers both high specificity and sensitivity with virtually no background due to its specific probe design. This methodological design offers the possibility to reliably detect mRNA even in archived FFPE tissue with poor RNA quality.17 Evaluation of KCNJ3 staining patterns demonstrated exclusive expression in neoplastic tumour epithelium, but not in the tumour stroma or in normal mammary parenchyma. The correlation of KCNJ3 mRNA levels assessed by ISH with the corresponding microarray data was excellent (rS=0.861, p<0.001), indicating superiority of the RNA ISH method to IHC regarding robustness and reliability, even in FFPE tissue samples stored for up to 25 years. The ISH method presents a sensitive and specific detection technique that is an indispensable tool to efficiently investigate larger patient cohorts in order to derive possible prognostic and predictive information of KCNJ3 in breast cancer. In general, ion channels are gaining increasing interest as new targets in cancer development and metastasis.18 Among these, potassium channels have shown to promote proliferation and apoptosis and to be involved in stemness of cancer cells.19 20 Several studies showed an upregulation of KCNJ3 in breast cancer, but its function in cancer cell behaviour needs to be further studied.2 3 15 We are confident that the comprehensive characterisation of KCNJ3 detection techniques presented here will aid in further clarification of expression patterns, function and relevance of this intriguing cellular target in malignant diseases, and in physiological conditions.
Take home messages.
Establishment of GIRK1 immunohistochemistry is challenging due to low specificity and/or sensitivity of available anti-GIRK1 antibodies.
RNA in situ hybridisation is superior to IHC in detecting KCNJ3 in human tissue.
The KCNJ3 RNA ISH method presented here is robust, reliable, highly sensitive and specific.
Acknowledgments
The authors thank Kurt Schmidt (Institute of Pharmaceutical Sciences, University of Graz) for providing Ab#2 and Ab#3, Amir-Hassan Zarnani (Avicenna Research Institute, Tehran, Iran) for providing secondary HRP-coupled antibodies, Astrid Gorischek (Institute of Biophysics, Medical University of Graz) for oocyte preparation and injection, Ernst Malle (Institute of Molecular Biology and Biochemistry, Medical University of Graz) for providing FFPE mouse tissue and Amin El-Heliebi (Institute of Cell Biology, Histology and Embryology, Medical University of Graz) for RNA ISH image acquisition.
Footnotes
Handling editor: Cheok Soon Lee
Contributors: SK, SWJ, PR, WS and TB concepted and designed the study; SK, EW, SE and SR performed experiments; SK, SWJ, PR, MP, WS and TB analysed and interpreted the data; SK, SWJ, MP, WS and TB drafted the manuscript.
Funding: This project was funded by the Austrian Science Fund (projects KLIF-182 and P22974) within the Doctoral School ‘Translational Molecular and Cellular Biosciences’ at the Medical University of Graz.
Competing interests: None declared.
Ethics approval: Ethics committee of the Medical University of Graz (24-081 ex 11/12).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Lüscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci 2010;11:301–15. 10.1038/nrn2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stringer BK, Cooper AG, Shepard SB. Overexpression of the G-protein inwardly rectifying potassium channel 1 (GIRK1) in primary breast carcinomas correlates with axillary lymph node metastasis. Cancer Res 2001;61:582–8. [PubMed] [Google Scholar]
- 3.Brevet M, Ahidouch A, Sevestre H, et al. Expression of K+ channels in normal and cancerous human breast. Histol Histopathol 2008;23:965–72. [DOI] [PubMed] [Google Scholar]
- 4.Rezania S, Li C, Kammerer S, et al. G-protein activated inwardly rectifying potassium channels control motility of breast cancer cells. Biophys J 2014;106:543a 10.1016/j.bpj.2013.11.3026 [DOI] [Google Scholar]
- 5.Ko JH, Ko EA, Gu W, et al. Expression profiling of ion channel genes predicts clinical outcome in breast cancer. Mol Cancer 2013;12:106 10.1186/1476-4598-12-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kammerer S, Sokolowski A, Hackl H, et al. 65PGIRK1 overexpression correlates with ER positive breast cancer subtypes and is associated with poor prognosis. Ann Oncol 2015;26:iii22 10.1093/annonc/mdv117.27 [DOI] [Google Scholar]
- 7.Liang B, Nissen JD, Laursen M, et al. G-protein-coupled inward rectifier potassium current contributes to ventricular repolarization. Cardiovasc Res 2014;101:175–84. 10.1093/cvr/cvt240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner V, Stadelmeyer E, Riederer M, et al. Cloning and characterisation of GIRK1 variants resulting from alternative RNA editing of the KCNJ3 gene transcript in a human breast cancer cell line. J Cell Biochem 2010;110:598–608. 10.1002/jcb.22564 [DOI] [PubMed] [Google Scholar]
- 9.Scheruebel S, Koyani CN, Hallström S, et al. I(f) blocking potency of ivabradine is preserved under elevated endotoxin levels in human atrial myocytes. J Mol Cell Cardiol 2014;72:64–73. 10.1016/j.yjmcc.2014.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Symmans WF, Hatzis C, Sotiriou C, et al. Genomic index of sensitivity to endocrine therapy for breast cancer. J Clin Oncol 2010;28:4111–19. 10.1200/JCO.2010.28.4273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nobles M, Sebastian S, Tinker A. HL-1 cells express an inwardly rectifying K+ current activated via muscarinic receptors comparable to that in mouse atrial myocytes. Pflugers Arch 2010;460:99–108. 10.1007/s00424-010-0799-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krapivinsky G, Gordon EA, Wickman K, et al. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K(+)-channel proteins. Nature 1995;374:135–41. 10.1038/374135a0 [DOI] [PubMed] [Google Scholar]
- 13.Dascal N. Signalling via the G protein-activated K+ channels. Cell Signal 1997;9:551–73. 10.1016/S0898-6568(97)00095-8 [DOI] [PubMed] [Google Scholar]
- 14.Kosti I, Jain N, Aran D, et al. Cross-tissue analysis of gene and protein expression in normal and cancer tissues. Sci Rep 2016;6:24799 10.1038/srep24799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brevet M, Fucks D, Chatelain D, et al. Deregulation of 2 potassium channels in pancreas adenocarcinomas: implication of KV1.3 gene promoter methylation. Pancreas 2009;38:649–54. 10.1097/MPA.0b013e3181a56ebf [DOI] [PubMed] [Google Scholar]
- 16.Nockemann D, Rouault M, Labuz D, et al. The K(+) channel GIRK2 is both necessary and sufficient for peripheral opioid-mediated analgesia. EMBO Mol Med 2013;5:1263–77. 10.1002/emmm.201201980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang F, Flanagan J, Su N, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn 2012;14:22–9. 10.1016/j.jmoldx.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prevarskaya N, Skryma R, Shuba Y. Ion channels and the hallmarks of cancer. Trends Mol Med 2010;16:107–21. 10.1016/j.molmed.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 19.Yang M, Brackenbury WJ. Membrane potential and cancer progression. Front Physiol 2013;4:185 10.3389/fphys.2013.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruggieri P, Mangino G, Fioretti B, et al. The inhibition of KCa3.1 channels activity reduces cell motility in glioblastoma derived cancer stem cells. PLoS ONE 2012;7:e47825 10.1371/journal.pone.0047825 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jclinpath-2016-203798supp1.pdf (110.2KB, pdf)
jclinpath-2016-203798supp2.pdf (357.4KB, pdf)
jclinpath-2016-203798supp3.pdf (341.6KB, pdf)
jclinpath-2016-203798supp4.pdf (369.5KB, pdf)
jclinpath-2016-203798supp5.pdf (210KB, pdf)