Abstract
Objective
Thyroid hormones are essential for normal brain development. The aim of this study is to assess if high concentration of thyroid stimulating hormone (TSH) that is below the clinical threshold (5–15 mIU/L) at neonatal screening is linked to psychomotor development impairments in the offspring at preschool age.
Design
A total of 284 Belgian preschool children 4–6 years old and their mothers were included in the study. The children were randomly selected from the total list of neonates screened in 2008, 2009 and 2010 by the Brussels newborn screening centre. The sampling was stratified by gender and TSH range (0.45–15 mIU/L). Infants with congenital hypothyroidism (>15 mIU/L), low birth weight and/or prematurity were excluded. Psychomotor development was assessed using the Charlop-Atwell scale of motor coordination. The iodine status of children was determined using median urinary iodine concentration. Socioeconomic, parental and child potential confounding factors were measured through a self-administered questionnaire.
Results
TSH level was not significantly associated with total motor score (average change in z-score per unit increase in TSH is 0.02 (−0.03, 0.07), p=0.351), objective motor score (p=0.794) and subjective motor score (p=0.124). No significant associations were found using multivariate regression model to control confounding factors.
Conclusions
Mild thyroid dysfunction in the newborn—reflected by an elevation of TSH that is below the clinical threshold (5–15 mIU/L)—was not associated with impaired psychomotor development at preschool age.
Keywords: psychomotor development, thyroid-stimulating hormone, preschool children, iodine deficiency, pregnancy
What is already known on this topic?
Thyroid hormones are essential for proper brain development.
A severe lack of thyroid hormones can lead to permanent intellectual disability and psychomotor deficits.
Little is known about the long-term consequences of mild thyroid dysfunction at birth on later psychomotor development.
What this study adds?
No significant association was found between neonatal TSH concentration and psychomotor scores at preschool age.
No impact of mild thyroid dysfunction at birth was found on psychomotor development of preschool children.
Introduction
Thyroid hormones, thyroxin (T4) and tri-iodothyronine (T3) are essential for fetal growth and optimal brain development in utero and after birth.1 During pregnancy, a sufficient production and transfer of thyroid hormones from the mother to the fetus is needed for healthy fetal brain development. Indeed thyroid hormones are involved in neural migration, neural differentiation, myelinisation, synaptogenesis and neurotransmission.2
Adequate iodine intake is essential for the production of thyroid hormones. Severe iodine deficiency can lead to congenital hypothyroidism in the neonate, a condition characterised by severe intellectual disability, dwarfism, deaf-mutism and spasticity.3 At mild to moderate levels, iodine deficiency can lead to subclinical thyroid dysfunction in the mother and the fetus and alter children's neurocognitive development.4 Epidemiological studies have shown that maternal hypothyroxinaemia or low maternal urinary excretion appearing during pregnancy could lead to subclinical impairments in verbal and non-verbal skills, perceptual and manipulative abilities, executive functioning skills and memory abilities.4
Besides intellectual disability, motor deficits are commonly observed in children with congenital hypothyroidism with alteration in gross and fine motor abilities, motor coordination and static balance.5–7 At the subclinical level, maternal hypothyroxinaemia has been found to be associated with altered gross motor abilities.8 9 Results from animal studies have shown that maternal thyroid hormones deficiency can alter cerebellum development and function and lead to motor coordination impairments in the offspring.10–12
Iodine deficiency is still present at mild levels in several European countries, including Belgium, and can lead to subclinical thyroid dysfunction in both the mother and the offspring.13 14 Little is known about the potential harmful effects of mild thyroid dysfunction at birth on later neurodevelopment of children. This is a public health concern and warrants further study in view of the sparse evidence available.
Thyroid hormone level is dependent on iodine nutrition when thyroid disease is excluded.15 The neonatal thyroid is very sensitive to variations of maternal iodine intake. This is the reason why thyroid stimulating hormone (TSH) concentration measured 3–5 days after birth has been proposed as an indicator of population iodine status using the percentage of newborns with a neonatal TSH above 5 mIU/L.15–19
To our knowledge, only two studies have investigated the association between high (>5 mIU/L) neonatal TSH as a marker of thyroid dysfunction and psychomotor development in young children.20 21
The aim of the present study is to assess the effect of mild thyroid dysfunction at birth—potentially secondary to maternal iodine deficiency—on psychomotor development at preschool age by investigating the association between neonatal TSH concentrations and psychomotor scores at preschool age.
Method
This study included 284 Belgian preschool children aged 4–6 years with a neonatal TSH concentration in the range 0.45–15 mIU/L (micro international U/L) from the PsychoTSH cohort study,22 23 which aimed to assess the association between neonatal TSH and cognitive, psychomotor and psychosocial development. The children were selected from the total list of newborns screened by the Brussels Newborn Screening Centre for Metabolic Disorders (Laboratoire de Pédiatrie, Université Libre de Bruxelles, Brussels) in 2008 (n=29 013), 2009 (n=29 602) and 2010 (n=30 126). The sampling was stratified by gender and TSH interval (0.45–1 mIU/L, 1–2 mIU/L, 2–3 mIU/L, 3–4 mIU/L, 4–5 mIU/L, 5–6 mIU/L, 6–7 mIU/L, 7–8 mIU/L, 8–9 mIU/L and 9–15 mIU/L). The sample size has been calculated for the relationship between TSH and cognitive score and is described elsewhere.22 23 Infants with congenital hypothyroidism (TSH concentration >15 mIU/L), prematurity (<37 weeks), low birth weight (<2500 g) and neurological disease were excluded from the sample.
TSH concentration was measured in dried blood spots on filter paper collected 3–5 days after birth using a commercial time-resolved fluoroimmunoassay (Autodelfia method).24 TSH values were analysed twice at 50 different TSH values in the range of 0–15 mIU/L to assess precision by determining the coefficient of variation. The interday and intraday coefficient of variation was below 20% in the range 0.9–15 mIU/L. For values <0.9 mIU/L, a weighted value of 0.45 mIU/L was given to include these data in statistical analysis.
Psychomotor development of children was assessed with the French version of the Charlop-Atwell Scale of Motor Coordination.25 26 This is an individually administered test evaluating gross motor coordination abilities of children aged 3½–6 years using six subtests. The French version of the test has good interobserver reliability, and internal and external validity.26 The scores comprise an objective subtest rating based on the accuracy of the performance and a subjective rating based on the quality of the performance. The test was performed at home with a psychologist who was blinded to the neonatal TSH value of the children.
A sample of urine was collected from the child in order to assess iodine status of the studied population. Samples were frozen at −80°C and urinary iodine excretion was measured by a modification of the Sandell-Kolthoff reaction with spectrophotometric detection27 at the Nuclear Medicine Department, Erasmus Hospital (Université Libre de Bruxelles, Brussels). During the home visit, actual body measurement of the child was taken. The body weight was determined using a SECA 815 or SECA 804 weight scale, the body height using a transportable SECA 214 stadiometer and the head circumference using a SECA 212 flexible measuring tape (seca GmbH. Co. kg, Germany).
Descriptive data, data on effect modifiers and covariates were collected from the screening centre (date of birth, date of blood sampling, pregnancy duration and body weight at birth), the health booklet of the child (body length at birth, head circumference at birth and Apgar score) and by a self-reported questionnaire filled in by the mothers.
The following information was assessed by the self-report questionnaire: prepregnancy body mass index and weight gain of the mother during pregnancy, thyroid disease, diabetes, drug intake and nutritional supplements intake during pregnancy, alcohol consumption, smoking habits, maternal age at birth, reproductive history, parity, gravidity, type of delivery, health condition of the newborn at birth, perinatal asphyxia, child's negative life events, child's chronic disease, child's lifetime nutritional supplement intake, maternal social support, marital discord and parent-child interactions, and maternal mental health. Maternal mental health was assessed using the General Health Questionnaire-12 items and vitality scale questionnaire of the Short Form Health Survey-36.
Statistical data analysis was performed using SAS statistical software V.9.3 (SAS Institute Cary, North Carolina, USA) for univariate analysis and Stata V.13 (StataCorp, College Station, Texas, USA) for multivariable analysis. Tests were two-sided and p values <0.05 was considered as statistically significant. Urinary iodine concentration and TSH values were expressed as median and IQR. Psychomotor scores were presented as z-scores and were standardised using the mean and SD by age group of the French population of reference.26 These scores were presented as mean and SD. Neonatal TSH values were analysed as ranks using 1 mIU/L interval or were classified in two groups: below 5 mIU/L and higher than 5 mIU/L.
Univariate associations between TSH concentration at birth and psychomotor scores were assessed using Pearson's correlation. To analyse the association between psychomotor scores and studied parameters, Student's t-test, analysis of variance (ANOVA) with Bonferroni correction and simple linear regression tests were performed.
Multivariable linear regression models were used to define the predictors of variation of psychomotor scores in children. All variables associated with psychomotor scores with a test p<0.20 were included in a stepwise backward selection procedure with a probability of entry of 0.10 and probability of exit of 0.15. The variables kept by this selection procedure were inserted in the final multiple linear regression model (except for the TSH value which was kept even without being selected). The normality of the distribution of residuals of each multivariable linear model was tested using a normal plot of residuals. Linearity and homoscedasticity of residuals were checked by analysing the plot of standardised residuals. Colinearity between predictors was tested using the test of variance inflation factor (VIF). Total VIF and individual VIF for each parameter in the model were around 1. Variables highly correlated with each other were not included together in the model. In addition, a second multivariable model was tested using a multiple imputation strategy to fill in missing values (4%) using the “mi” command in Stata V.13 (StataCorp, College Station, Texas, USA) with five imputations.
Results
The characteristics of the preschoolers are shown in table 1. The median TSH level was 3.7 mIU/L (1.81–5.9 (IQR), 0.45–13.9 (min–max)). The median urinary iodine concentration was 137 µg/L (88.2–233.6 (IQR)) indicating iodine sufficiency of the study population.
Table 1.
Descriptive and demographic characteristics of the studied population according to gender
| (N=284) | Total | Male | Female | |||
|---|---|---|---|---|---|---|
| N | Per cent | N | Per cent | N | Per cent | |
| TSH level (mIU/L) | ||||||
| 1 | 41 | 14 | 22 | 8 | 19 | 7 |
| 2 | 35 | 12 | 21 | 7 | 14 | 5 |
| 3 | 35 | 12 | 20 | 7 | 15 | 5 |
| 4 | 35 | 12 | 16 | 6 | 19 | 7 |
| 5 | 35 | 12 | 20 | 7 | 15 | 5 |
| 6 | 36 | 13 | 19 | 7 | 17 | 6 |
| 7 | 33 | 12 | 20 | 7 | 13 | 5 |
| 8 | 12 | 4 | 9 | 3 | 3 | 1 |
| 9 | 13 | 5 | 6 | 2 | 7 | 2 |
| 10–15 | 9 | 3 | 5 | 2 | 4 | 1 |
| Age at testing | ||||||
| 4 | 211 | 75 | 118 | 42 | 93 | 33 |
| 5 | 65 | 23 | 36 | 13 | 29 | 10 |
| 6 | 7 | 2 | 4 | 1 | 3 | 1 |
| Maternity hospital | ||||||
| Brussels | 190 | 67 | 117 | 41 | 73 | 26 |
| Wallonia | 93 | 33 | 41 | 14 | 52 | 18 |
| Flanders | 1 | 0 | – | – | 1 | 0 |
| Home at examination | ||||||
| Brussels | 103 | 37 | 57 | 20 | 46 | 16 |
| Wallonia | 131 | 47 | 70 | 25 | 61 | 22 |
| Flanders | 45 | 16 | 28 | 10 | 17 | 6 |
| Children ethnicity | ||||||
| Europe (Caucasian) | 226 | 85 | 129 | 48 | 97 | 36 |
| Asian | 3 | 1 | 2 | 1 | 1 | 0 |
| Sub-Saharan Africa | 13 | 5 | 5 | 2 | 8 | 3 |
| North Africa | 25 | 9 | 14 | 5 | 11 | 4 |
| Mother ethnicity | ||||||
| Europe (Caucasian) | 226 | 83 | 130 | 48 | 96 | 35 |
| Asia | 5 | 2 | 2 | 1 | 3 | 1 |
| Sub-Saharan Africa | 15 | 6 | 6 | 2 | 9 | 3 |
| North Africa | 26 | 10 | 15 | 6 | 11 | 4 |
| Father ethnicity | ||||||
| Europe (Caucasian) | 228 | 84 | 129 | 48 | 99 | 37 |
| Asia | 2 | 1 | – | – | 2 | 1 |
| Sub-Saharan Africa | 14 | 5 | 5 | 2 | 9 | 3 |
| North Africa | 26 | 10 | 14 | 5 | 12 | 4 |
PsychoTSH study, Belgium, 2008–2014.
N, number of subjects; TSH, thyroid stimulating hormone.
The mean total motor score was −0.03 (1.1 (SD), −3.5–2.11 (min–max)), the mean objective motor score was 0.2 (1.1 (SD), −3.7–1.7 (min–max)), the mean subjective motor score was 0.28 (1.1 (SD), −2.9–2.5 (min–max)).
Univariate associations between psychomotor scores at preschool age and maternal, socioeconomic characteristics and markers of iodine status were tested. The results are shown in tables 2 and 3.
Table 2.
Association of psychomotor scores at preschool age with infant, maternal, socioeconomic characteristics and markers of iodine status: categorical variables
| Total | Total motor scale | Objective motor scale | Subjective motor scale | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Per cent | Mean | SD | p Value | Mean | SD | p Value | Mean | SD | p Value | |
| Children characteristics | |||||||||||
| Gender | <0.0001 | 0.0004 | <0.0001 | ||||||||
| Male | 158 | 54.60 | −0.29 | 1.19 | −0.43 | 1.17 | 0.02 | 1.09 | |||
| Female | 126 | 45.40 | 0.30 | 0.94 | 0.03 | 0.92 | 0.61 | 0.93 | |||
| Socioeconomic characteristics and maternal characteristics | |||||||||||
| Monthly income | 0.112 | 0.451 | 0.183 | ||||||||
| <€2000 | 33 | 12.67 | −0.51 | 1.17 | 0.15 | 1.12 | −0.27 | 1.23 | |||
| ≥€2000 | 240 | 87.33 | −0.19 | 1.07 | 0.30 | 1.05 | 0.01 | 1.10 | |||
| Mother education level | 0.262 | 0.939 | 0.513 | ||||||||
| No/primary | 10 | 3.28 | −0.46 | 1.28 | 0.18 | 1.44 | −0.20 | 1.39 | |||
| Lower high school | 20 | 7.21 | −0.58 | 1.23 | 0.17 | 1.30 | −0.33 | 1.38 | |||
| Upper high school | 44 | 16.39 | −0.31 | 1.14 | 0.28 | 1.05 | −0.07 | 1.14 | |||
| University or higher | 201 | 73.11 | −0.15 | 1.03 | 0.30 | 1.03 | 0.03 | 1.07 | |||
| Delivery | 0.066 | 0.107 | 0.071 | ||||||||
| Normal | 213 | 75.73 | −0.21 | 1.04 | 0.26 | 1.03 | −0.03 | 1.07 | |||
| Caesarean | 39 | 13.92 | 0.00 | 1.22 | 0.59 | 1.22 | 0.26 | 1.30 | |||
| With vacuum | 27 | 10.03 | −0.63 | 1.24 | 0.06 | 1.10 | −0.39 | 1.27 | |||
| Parity—first child | 0.010 | 0.004 | 0.116 | ||||||||
| Yes | 121 | 43.37 | −0.23 | 1.17 | −0.44 | 1.13 | 0.17 | 1.08 | |||
| No | 158 | 56.63 | 0.13 | 1.08 | −0.06 | 1.04 | 0.37 | 1.05 | |||
| Smoking during pregnancy | 0.405 | 0.118 | 0.234 | ||||||||
| ≥10 cigarettes/day | 6 | 2.11 | −0.60 | 1.19 | −0.39 | 1.58 | −0.57 | 1.43 | |||
| <10 cigarettes/day or non-smoking | 278 | 97.89 | −0.22 | 1.09 | 0.30 | 1.05 | −0.02 | 1.12 | |||
| Alcohol during pregnancy | 0.172 | 0.978 | 0.392 | ||||||||
| Non-consumer | 188 | 68.08 | −0.28 | 1.11 | 0.29 | 1.10 | −0.06 | 1.17 | |||
| Still consuming | 89 | 31.92 | −0.09 | 1.04 | 0.29 | 1.01 | 0.06 | 1.04 | |||
| Markers of iodine status | |||||||||||
| Neonatal TSH level | 0.517 | 0.141 | 0.270 | ||||||||
| <5 | 181 | 64.42 | −0.26 | 1.02 | 0.21 | 0.98 | −0.09 | 1.03 | |||
| >5 | 103 | 35.58 | −0.17 | 1.21 | 0.40 | 1.19 | 0.07 | 1.28 | |||
| Vitamins during pregnancy | 0.705 | 0.527 | 0.898 | ||||||||
| Containing iodine | 48 | 43.59 | 0.06 | 1.17 | −0.07 | 1.04 | 0.25 | 1.19 | |||
| No vitamins | 57 | 56.41 | −0.01 | 0.97 | −0.19 | 0.97 | 0.27 | 0.93 | |||
| Urinary iodine concentration, lg/L | 0.544 | 0.398 | 0.648 | ||||||||
| <100 | 72 | 30.37 | 0.08 | 1.04 | −0.13 | 1.01 | 0.36 | 0.98 | |||
| 100–149 | 63 | 24.81 | −0.04 | 1.12 | −0.16 | 1.13 | 0.16 | 1.06 | |||
| 150–294 | 55 | 22.22 | 0.05 | 1.07 | −0.16 | 1.03 | 0.35 | 1.02 | |||
| ≥250 | 53 | 22.59 | −0.20 | 1.27 | −0.43 | 1.15 | 0.21 | 1.25 | |||
| Household salt | 0.097 | 0.181 | 0.135 | ||||||||
| Iodised salt | 84 | 34.53 | −0.10 | 1.16 | −0.25 | 1.10 | 0.17 | 1.11 | |||
| Non-iodised salt | 154 | 61.15 | 0.08 | 1.08 | −0.14 | 1.06 | 0.38 | 1.04 | |||
| No salt | 10 | 4.32 | −0.62 | 0.71 | −0.76 | 0.68 | −0.18 | 0.91 | |||
PsychoTSH study, Belgium, 2008–2014.
Significant associations (p values <0.05) are marked in bold. p Value from Student's t-test or analysis of variance (ANOVA).
N, number of subjects; TSH, thyroid stimulating hormone.
Table 3.
Association of psychomotor scores at preschool age with infant, maternal, socioeconomic characteristics and markers of iodine status: continuous variables
| Total motor scale | Objective motor scale | Subjective motor scale | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean/median* | SD/IQR* | Linear regression | |||||||||
| b | 95% CI | p Value | b | 95% CI | p Value | b | 95% CI | p Value | ||||
| TSH (mUI/L) | 284 | 3.7* | 1.8, 5.9* | 0.02 | −0.03 to 0.07 | 0.351 | 0.01 | −0.04 to 0.05 | 0.794 | 0.04 | −0.01 to 0.08 | 0.124 |
| Term pregnancy (week) | 278 | 39.3 | 1.6 | −0.05 | −0.13 to 0.03 | 0.215 | −0.05 | −0.13 to 0.03 | 0.215 | −0.05 | −0.12 to 0.03 | 0.226 |
| Birth weight (g) | 283 | 3406.7 | 428.3 | 0.00 | −0.00 to 0.00 | 0.652 | 0.00 | −0.00 to 0.00 | 0.498 | 0.00 | −0.00 to 0.00 | 0.817 |
| Birth length (cm) | 279 | 50.0 | 3.2 | −0.02 | −0.06 to 0.02 | 0.379 | −0.02 | −0.05 to 0.02 | 0.388 | −0.02 | −0.05 to 0.02 | 0.434 |
| Birth HC (cm) | 245 | 34.6 | 3.3 | −0.01 | −0.05 to 0.02 | 0.528 | −0.01 | −0.05 to 0.03 | 0.708 | −0.02 | −0.05 to 0.02 | 0.455 |
| Actual age (year) | 283 | 4.7 | 0.5 | −0.45 | −0.72 to −0.17 | 0.002 | −0.47 | −0.73 to −0.20 | 0.001 | −0.29 | −0.54 to −0.03 | 0.030 |
| Actual weight (kg) | 280 | 18.6 | 3.0 | −0.05 | −0.10 to−0.01 | 0.013 | −0.05 | −0.09 to −0.01 | 0.011 | −0.04 | −0.08 to 0.00 | 0.051 |
| Actual height (cm) | 281 | 107.9 | 8.1 | −0.02 | −0.04 to−0.00 | 0.010 | −0.02 | −0.03 to −0.00 | 0.027 | −0.02 | −0.04 to −0.01 | 0.006 |
| Actual HC (cm) | 280 | 51.3 | 3.3 | −0.03 | −0.07 to 0.01 | 0.142 | −0.02 | −0.06 to 0.01 | 0.238 | −0.03 | −0.07 to 0.00 | 0.098 |
| Mother age (years) | 280 | 36.5 | 5.1 | 0.00 | −0.02 to 0.03 | 0.817 | 0.00 | −0.02 to 0.03 | 0.802 | 0.00 | −0.02 to 0.03 | 0.802 |
| Mother weight (kg) | 277 | 65.1 | 12.0 | 0.00 | −0.02 to 0.00 | 0.381 | 0.00 | −0.01 to 0.00 | 0.384 | 0.00 | −0.01 to 0.01 | 0.452 |
| Mother height (cm) | 275 | 165.8 | 6.2 | 0.00 | −0.02 to 0.02 | 0.951 | 0.01 | −0.01 to 0.03 | 0.621 | −0.01 | −0.03 to 0.01 | 0.475 |
| Weight gain during pregnancy (kg) | 261 | 13.3 | 5.6 | 0.00 | −0.02 to 0.02 | 0.969 | 0.00 | −0.02 to 0.03 | 0.868 | 0.00 | −0.02 to 0.02 | 0.954 |
| Nb of cigarettes during pregnancy | 17 | 5* | 5.0, 10.0* | −0.08 | −0.14 to −0.01 | 0.020 | −0.07 | −0.13 to 0.00 | 0.056 | −0.09 | −0.15 to −0.02 | 0.014 |
| Nb of people in the household | 274 | 3* | 3.0, 4.0* | 0.14 | 0.01 to 0.27 | 0.029 | 0.13 | 0.00 to 0.25 | 0.049 | 0.12 | −0.00 to 0.24 | 0.051 |
*Median and IQR are presented. Significant associations (p values <0.05) are marked in bold.
PsychoTSH study, Belgium, 2008–2014.
p value from univariate linear regression. HC, head circumference; N, number of subjects; Nb, number; TSH, thyroid stimulating hormone.
No significant association was found between psychomotor scores and the following factors: number of previous miscarriage, Apgar score at 5 min, health problems at birth, neonatal hospital attendance, breast feeding, child food allergy, child dietary supplement intake, child's negative life events, previous cognitive assessment, school attendance, child custody, child's fish and milk consumption, rural or urban residency, type of delivery, Graves' disease or Hashimoto's thyroiditis during pregnancy, hypothyroidism during pregnancy, diabetes during pregnancy, mother's social support, mother's score of psychological distress and mother's vitality index score (data not shown).
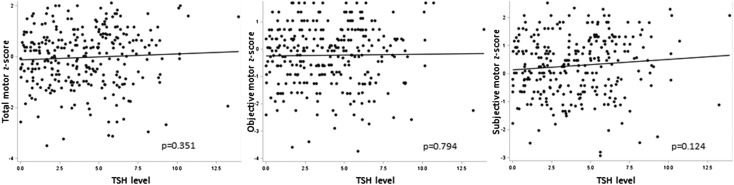
No differences in scores were found in children with TSH levels lower than 5 mIU/L compared withTSH levels higher than 5 mIU/L. In univariate linear analysis, TSH level was not significantly associated with total motor score (average change in z-score per unit increase in TSH is 0.02 (−0.03, 0.07), p=0.351), objective motor score (average change in z-score per unit increase in TSH is 0.01 (−0.04, 0.05), p=0.794) and subjective motor score (average change in z-score per unit increase in TSH is 0.04 (−0.01, 0.08), p=0.124) (see table 3 and figure 1). No significant associations were found between TSH levels and psychomotor scores in multiple linear regression analyses with correction of covariates (see table 4). No significant associations were found either when multivariable analyses were performed using multiple imputations to replace missing values (see table 4) or when stratified for day of collection and for year of birth (data not shown).
Figure 1.
Scatter plot of the association between thyroid stimulating hormone (TSH) concentration at birth and motor z-score at preschool age.
Table 4.
Multiple linear regressions of factors explaining variation in psychomotor scores at preschool age
| Model 1 (analysis restricted to complete data) | ||||||
|---|---|---|---|---|---|---|
| Total motor scale | Objective motor scale | Subjective motor scale | ||||
| R2=13% (N=275) | p Value | R2=13% (N=272) | p Value | R2=10% (N=283) | p Value | |
| b (95% CI) | b (95% CI) | b (95% CI) | ||||
| TSH (mIU/L) | 0.04 (−0.01 to 0.09) | 0.103 | 0.02 (−0.02 to 0.07) | 0.255 | 0.03 (−0.01 to 0.08) | 0.107 |
| Gender | <0.001 | <0.001 | <0.001 | |||
| Male | Ref | Ref | Ref | |||
| Female | 0.61 (0.36 to 0.87) | 0.46 (0.21 to 0.71) | 0.59 (0.34 to 0.83) | |||
| Age at testing (year) | −0.41 (−0.66 to −0.15) | 0.002 | −0.33 (−0.59 to −0.07) | 0.013 | −0.27 (−0.51 to −0.04) | 0.022 |
| Parity | −0.17 (−0.31 to −0.03) | 0.016 | −0.23 (−0.36 to −0.91) | 0.001 | * | |
| Height at testing (cm) | * | −0.01 (−0.03 to 0.00) | 0.055 | * | ||
| Model 2 (with multiple imputation of missing values) | ||||||
| Total motor scale | Objective motor scale | Subjective motor scale | ||||
| N=284 | p Value | N=284 | p Value | N=284 | p Value | |
| b (95% CI) | b (95% CI) | b (95% CI) | ||||
| TSH (mIU/L) | 0.03 (−0.02 to 0.08) | 0.235 | 0.02 (−0.03 to 0.06) | 0.490 | 0.04 (0.01 to 0.10) | 0.108 |
| Gender | <0.001 | <0.001 | <0.001 | |||
| Male | Ref | Ref | Ref | |||
| Female | 0.58 (0.33 to –0.83) | 0.44 (0.19 to 0.68) | 0.59 (0.35 to 0.82) | |||
| Age at testing (year) | −0.41 (−0.66 to −0.16) | 0.001 | −0.35 (−0.60 to −0.10) | 0.007 | −0.28 (−0.51 to −0.4) | 0.022 |
| Parity | −0.17 (−0.31, to −0.03) | 0.018 | −0.21 (−0.35 to −0.07) | 0.003 | * | |
| Height at testing (cm) | * | −0.01 (−0.03 to 0.01) | 0.077 | * | ||
PsychoTSH study, Belgium, 2008–2014.
Significant associations (p values <0.05) are marked in bold.
*Variable not included in the multivariable model.
N, number of subjects; TSH, thyroid stimulating hormone.
Table 4 shows the results of multiple linear regressions assessing parameters explaining variation of psychomotor scores. Female gender was associated with higher psychomotor scores (total score, p<0.001, 95% CI 0.36 to 0.87, objective score, p<0.001, 95% CI 0.21 to 0.71, subjective score p<0.001, 95% CI 0.34 to 0.83). A negative association was found between psychomotor scores and age at testing (total score, p=0.002, 95% CI −0.66 to −0.15, objective score, p=0.013, 95% CI −0.59 to −0.07, subjective score=0.022, 95% CI −0.51 to −0.04) and number of previous births (total score, p=0.016, 95% CI −0.31 to −0.03, objective score, p=0.001, 95% CI −0.36 to −0.91).
Discussion
In this study, we aimed to assess if elevation of TSH concentration measured 3–5 days after birth—used as a marker of mild thyroid dysfunction potentially due to maternal iodine deficiency during late pregnancy—is associated with lower psychomotor scores at preschool age. We found no association between TSH levels and psychomotor scores both in univariate and multivariable analyses.
To our knowledge, only two studies have investigated psychomotor performance of children born with an elevated TSH and those had several limitations. In Italy, a retrospective cohort study was conducted in a group of 102 infants born between 26 weeks and 32 weeks. They found that preterm newborns with a neonatal TSH value above 4.3 mIU/L had a suboptimal motor outcome at 18 months.21 As preterm birth is known to increase TSH levels and to be related to suboptimal motor outcome22 the results of this study are not applicable to children born at term. The second study is an Iranian research which assessed the effect of transient neonatal hyperthyrotropinaemia (TNH) on psychomotor performance with Bender-Gestalt test on a sample of children 9 years of age.20 They evaluated 18 children with TNH in comparison with 19 children without thyroid problems. They found no significant difference in psychomotor scores between the two groups. In addition to its very small sample size and to the lack of control of covariates, the results of this study are not comparable to those of the present study because children had much higher TSH values (23.4±8.3) than those included in the present study.
Several studies have investigated the effect of maternal hypothyroxinaemia during pregnancy on psychomotor development, most of them showed subtle motor impairments when hypothyroxinaemia appears during pregnancy.4 However, two studies found no association between hypothyroxinaemia during pregnancy and motor scores. One of them was a Spanish study which investigated motor development of McCarthy Scales of Children's Abilities of 147 children aged between 38 months and 60 months.28 The other investigated motor development in a population-based cohort of 1761 children with Bayley Scales of Infant Development at 2 years of age.29 One animal study assessing the impact of the severity of iodine deficiency on cerebellum development found no impact of mild iodine deficiency exposure on the cerebellum Purkinje cells.30
The present study has assessed psychomotor outcomes of children born at term with elevated TSH at birth with the exclusion of children of low birth weight and infants with congenital hypothyroidism (>15 mIU/L). In this study the sample was stratified by TSH level and by gender to ensure that the whole range of TSH values are included. In addition, psychomotor assessment of the children was done by psychologists blinded about the TSH value of the children. The assessment was performed at preschool age, reducing the time between TSH testing and psychomotor testing. For a sample size of 284, with an α error of 0.05 and a power of 0.95, the minimum score difference that we were able to detect is a difference of 3.12 points. This seems reasonable to detect children's motor development difference at the population level.
Elevated TSH concentration at birth has been considered in this study as a potential indicator of maternal iodine deficiency during late pregnancy. However, several other factors may affect TSH concentration such as maternal disease, maternal drugs intake, type of delivery and birth conditions, TSH assay used and timing of TSH determination.4 31 Future studies should assess the neurodevelopmental impact of mild iodine deficiency using a prospective design and measuring urinary iodine excretion in the mothers as well as thyroid parameters at different stages of pregnancy starting, if possible, before the conception. Many studies started from the first trimester and disabilities were, most of the times, found when MID appeared before mid-gestation. In addition, neurodevelopmental performance should be assessed at different stages of development, starting earlier than 4–5 years.
The use of neonatal TSH at birth for monitoring iodine status has been questioned. Indeed, several studies have shown that percentage of TSH >5 mIU/L collected after birth has failed to detect mild iodine deficiency.32–34 Further studies are needed to assess the association between maternal urinary iodine and neonatal TSH levels in order to define a relevant cut-off point in TSH concentration to define mild iodine deficiency.
In conclusion, the present study found no association between neonatal TSH levels within the range of 0.45–15 mIU/L and psychomotor development of preschool children in Belgium.
Acknowledgments
The authors thank the Brussels Newborn Screening Centre for Metabolic Disorders of the ULB for providing the screening data. The authors also thank all the children and mothers who participated.
Footnotes
Contributors: All authors contributed to the conception and the design of the study, the data interpretation, and the revision of the article. CT wrote the manuscript, performed the data collection and the data analysis. All authors helped in the interpretation of the results and helped to write the manuscript. All the authors approved the final version of the article.
Funding The ‘Fonds de la Recherche Scientifique Medicale’ (Grant number: 3.4572.11) and the ‘Belgian Federal Science Policy Office’.
Competing interests: None declared.
Patient consent: Parental/guardian consent obtained.
Ethics approval: The study protocol and consent form were approved by the Ethical Committee of the Erasmus hospital (Université Libre de Bruxelles, Brussels) in accordance with the Code of Ethics of the World Medical Association for experiments involving humans (Declaration of Helsinki). This study was also approved by the Belgian Commission for the Protection of the Privacy Reference: RN29/2012.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Delange F. The disorders induced by iodine deficiency. Thyroid 1994;4:107–28. 10.1089/thy.1994.4.107 [DOI] [PubMed] [Google Scholar]
- 2.Bernal J. Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab 2007;3:249–59. 10.1038/ncpendmet0424 [DOI] [PubMed] [Google Scholar]
- 3.Zimmermann MB. Iodine deficiency in pregnancy and the effects of maternal iodine supplementation on the offspring: a review. Am J Clin Nutr 2009;89:668S–72S. 10.3945/ajcn.2008.26811C [DOI] [PubMed] [Google Scholar]
- 4.Trumpff C, De Schepper J, Tafforeau J, et al. . Mild iodine deficiency in pregnancy in Europe and its consequences for cognitive and psychomotor development of children: a review. J Trace Elem Med Biol 2013;27:174–83. 10.1016/j.jtemb.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 5.Oerbeck B, Sundet K, Kase BF, et al. . Congenital hypothyroidism: influence of disease severity and L-thyroxine treatment on intellectual, motor, and school-associated outcomes in young adults. Pediatrics 2003;112:923–30. 10.1542/peds.112.4.923 [DOI] [PubMed] [Google Scholar]
- 6.Kempers MJ, van d er Sluijs Veer L, Nijhuis-van der Sanden MW, et al. . Intellectual and motor development of young adults with congenital hypothyroidism diagnosed by neonatal screening. J Clin Endocrinol Metab 2006;91:418–24. 10.1210/jc.2005-1209 [DOI] [PubMed] [Google Scholar]
- 7.Kooistra L, Laane C, Vulsma T, et al. . Motor and cognitive development in children with congenital hypothyroidism: a long-term evaluation of the effects of neonatal treatment. J Pediatr 1994;124:903–9. 10.1016/S0022-3476(05)83178-6 [DOI] [PubMed] [Google Scholar]
- 8.Pop VJ, Kuijpens JL, van Baar AL, et al. . Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 1999;50:149–55. 10.1046/j.1365-2265.1999.00639.x [DOI] [PubMed] [Google Scholar]
- 9.Pop VJ, Brouwers EP, Vader HL, et al. . Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 2003;59:282–8. 10.1046/j.1365-2265.2003.01822.x [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Wang Y, Dong J, et al. . Developmental hypothyroxinaemia and hypothyroidism limit dendritic growth of cerebellar Purkinje cells in rat offspring: involvement of microtubule-associated protein 2 (MAP2) and stathmin. Neuropathol Appl Neurobiol 2014;40:398–415. 10.1111/nan.12074 [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Wang Y, Dong J, et al. . Developmental hypothyroxinemia and hypothyroidism reduce proliferation of cerebellar granule neuron precursors in rat offspring by downregulation of the sonic hedgehog signaling pathway. Mol Neurobiol 2014;49:1143–52. 10.1007/s12035-013-8587-3 [DOI] [PubMed] [Google Scholar]
- 12.Harry GJ, Hooth MJ, Vallant M, et al. . Developmental neurotoxicity of 3,3′,4,4′-tetrachloroazobenzene with thyroxine deficit: Sensitivity of glia and dentate granule neurons in the absence of behavioral changes. Toxics 2014; 2:496–532. 10.3390/toxics2030496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersson M, de Benoist B, Darnton-Hill I, et al. , eds. Iodine deficiency in Europe: a continuing public health problem. Switzerland: WHO Press, World Health Organization; 2007:1–86. [Google Scholar]
- 14.de Benoist B, McLean E, Andersson M, et al. . Iodine deficiency in 2007: global progress since 2003. Food Nutr Bull 2008;29:195–202. 10.1177/156482650802900305 [DOI] [PubMed] [Google Scholar]
- 15.Delange F. Screening for congenital hypothyroidism used as an indicator of the degree of iodine deficiency and of its control. Thyroid 1998;8:1185–92. 10.1089/thy.1998.8.1185 [DOI] [PubMed] [Google Scholar]
- 16.WHO, UNICEF, ICCIDD. Indicators for assessing iodine deficiency disorders and their control through salt iodisation. WHO/NUT/94.6. Geneva: WHO, 1994. [Google Scholar]
- 17.Delange F. Neonatal thyroid screening as a monitoring tool for the control of iodine deficiency. Acta Paediatr Suppl 1999;88:21–4. 10.1111/j.1651-2227.1999.tb01150.x [DOI] [PubMed] [Google Scholar]
- 18.Delange F. Neonatal screening for congenital hypothyroidism: results and perspectives. Horm Res 1997;48:51–61. 10.1159/000185485 [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann MB, Aeberli I, Torresani T, et al. . Increasing the iodine concentration in the Swiss iodized salt program markedly improved iodine status in pregnant women and children: a 5-y prospective national study. Am J Clin Nutr 2005;82:388–92. [DOI] [PubMed] [Google Scholar]
- 20.Azizi F, Afkhami M, Sarshar A, et al. . Effects of transient neonatal hyperthyrotropinemia on intellectual quotient and psychomotor performance. Int J Vitam Nutr Res 2001;71:70–3. 10.1024/0300-9831.71.1.70 [DOI] [PubMed] [Google Scholar]
- 21.Belcari F, Placidi G, Guzzetta A, et al. . Thyroid-stimulating hormone levels in the first days of life and perinatal factors associated with sub-optimal neuromotor outcome in pre-term infants. J Endocrinol Invest 2011;34:e308–13. 10.3275/7795 [DOI] [PubMed] [Google Scholar]
- 22.Trumpff C, Vanderfaeillie J, Vercruysse N, et al. . Protocol of the PSYCHOTSH study: association between neonatal thyroid stimulating hormone concentration and intellectual, psychomotor and psychosocial development at 4–5 year of age: a retrospective cohort study. Arch Public Health 2014;72:27 10.1186/2049-3258-72-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trumpff C, De Schepper J, Vanderfaeillie J, et al. . Thyroid-stimulating hormone (TSH) concentration at birth in Belgian neonates and cognitive development at preschool age. Nutrients 2015;7:9018–32. 10.3390/nu7115450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soini E, Kojola H. Time-resolved fluorometer for lanthanide chelates—a new generation of nonisotopic immunoassays. Clin Chem 1983;29:65–8. [PubMed] [Google Scholar]
- 25.Charlop M, Atwell CW. The Charlop-Atwell scale of motor coordination: a quick and easy assessment of young children. Percept Mot Skills 1980;50:1291–308. 10.2466/pms.1980.50.3c.1291 [DOI] [PubMed] [Google Scholar]
- 26.Albaret JM, Noack N. Manuel de l’échelle de coordinations lotrices de Charlop-Atwell. Paris: Editions du Centre de Psychologie appliquée, 1994. [Google Scholar]
- 27.Pino S, Fang SL, Braverman LE. Ammonium persulfate: a safe alternative oxidizing reagent for measuring urinary iodine. Clin Chem 1996;42:239–43. [PubMed] [Google Scholar]
- 28.Suárez-Rodríguez M, Azcona-San Julián C, Alzina de Aguilar V.. Hypothyroxinemia during pregnancy: the effect on neurodevelopment in the child. Int J Dev Neurosci 2012;30:435–8. 10.1016/j.ijdevneu.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 29.Julvez J, Alvarez-Pedrerol M, Rebagliato M, et al. . Thyroxine levels during pregnancy in healthy women and early child neurodevelopment. Epidemiology 2013;24:150–7. 10.1097/EDE.0b013e318276ccd3 [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Sun YN, Li YM, et al. . Effect of different iodine nutrition on cerebellum Pcp-2 in rat offspring during lactation. Biol Trace Elem Res 2011;143:1629–39. 10.1007/s12011-011-8991-3 [DOI] [PubMed] [Google Scholar]
- 31.Trumpff C, Vandevijvere S, Moreno-Reyes R, et al. . Neonatal thyroid-stimulating hormone level is influenced by neonatal, maternal, and pregnancy factors. Nutr Res 2015;35:975–81. 10.1016/j.nutres.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 32.Burns R, Mayne PD, O'Herlihy C, et al. . Can neonatal TSH screening reflect trends in population iodine intake? Thyroid 2008;18:883–8. 10.1089/thy.2008.0036 [DOI] [PubMed] [Google Scholar]
- 33.Rajatanavin R. Iodine deficiency in pregnant women and neonates in Thailand. Public Health Nutr 2007;10:1602–5. 10.1017/S1368980007360990 [DOI] [PubMed] [Google Scholar]
- 34.Gruñeiro-Papendieck L, Chiesa A, Mendez V, et al. . Neonatal TSH levels as an index of iodine sufficiency: differences related to time of screening sampling and methodology. Horm Res 2004;62:272–6. 10.1159/000081786 [DOI] [PubMed] [Google Scholar]