Abstract
BACKGROUND
Moderate to severe kidney disease increases risk for sudden cardiac death (SCD). Limited studies have evaluated how mild degrees of kidney dysfunction impact SCD risk.
OBJECTIVE
To evaluate the association of albuminuria, which is one of the earliest biomarkers of kidney injury, and SCD.
METHODS
The Reasons for Geographic and Racial Differences in Stroke (REGARDS) study is a prospective, population-based cohort of US adults. Associations between albuminuria, which is categorized using urinary albumin-to-creatinine ratio (ACR), estimated glomerular filtration rate (eGFR), and SCD were assessed independently and in combination.
RESULTS
After a median follow-up of 6.1 years, we identified 335 SCD events. Compared to participants with an ACR < 15 mg/g, those with higher levels had an elevated adjusted risk of SCD [ACR 15–30 mg/g, hazard ratio (HR) 1.53, 95% confidence interval (1.11, 2.11); ACR > 30 mg/g, HR 1.56, (1.17, 2.11)]. In contrast, compared to the eGFR > 90 ml/min/1.73m2 group, the adjusted risk of SCD was significantly elevated only among those with an eGFR < 45 ml/min/1.73m2 (HR 1.66, (1.06, 2.58)]. The eGFR < 45 ml/min/1.73m2 subgroup (n = 1,003) comprised 3.7% of REGARDS whereas ACR 15–30 mg/g (n = 3,089; 11.3%) and ACR > 30 mg/g (n = 4,040; 14.8%) were far more common. In the analysis that combined ACR and eGFR categories, albuminuria consistently identified individuals with an eGFR ≥ 60 ml/min/1.73m2 that were at significantly increased SCD risk.
CONCLUSION
Low levels of kidney injury as measured by ACR predict an increase in SCD risk.
Keywords: albuminuria, kidney function, sudden cardiac death, risk factor, population health
INTRODUCTION
Sudden cardiac death (SCD) is defined as an unexpected, pulseless condition attributable to a cardiac arrhythmia.1 The incidence of SCD in the United States ranges between 180,000 and 450,000 cases annually. Previous studies have demonstrated that a substantial proportion of cardiovascular-related deaths in advanced chronic kidney disease (CKD) or end stage renal disease (ESRD) are attributable to SCD.2–6 In addition, moderate to severe CKD is an established risk factor for bradyarrhythmias, ventricular tachycardia/fibrillation and SCD in populations with established cardiovascular disease.7–10 However, the majority of SCDs occur in the general population rather than among those with established cardiovascular disease or advanced CKD.11–14 In one prior population-based study of the elderly, increased cystatin C concentrations were associated with SCD risk15; however, limited studies have assessed systematically how modest levels of reduced kidney function impact SCD.
Albuminuria is one of the earliest biomarkers of kidney injury and largely represents endothelial injury at the level of the glomerular capillary wall. The subsequent passage of albumin into the urine, or albuminuria, is a risk factor for cardiovascular morbidity and mortality in persons with diabetes, hypertension, and among the general population.16–18 International guidelines have recommended including the urinary albumin-to-creatinine ratio (ACR) in the diagnosis and staging of CKD.19 Despite this growing recognition of the significance of albuminuria on cardiorenal risk, few, if any, studies have assessed the risk of SCD in relation to albuminuria. Combining information on albuminuria with estimated glomerular filtration rate (eGFR) based on current more sensitive equations that incorporate both creatinine and cystatin C20,21 may assist in the identification of broader populations with more mild levels of kidney dysfunction that may be at an elevated SCD risk. In the present investigation, we evaluated the absolute and relative risks of SCD across a broad spectrum of kidney health according to albuminuria and eGFR.
METHODS
Study Participants
The Reasons for Geographic and Racial Differences in Stroke (REGARDS) study is a population-based investigation of stroke incidence in black and white US adults aged 45 years and older.22 Trained personnel conducted computer-assisted telephone interviews to obtain information including participants’ sociodemographics, cardiovascular health profile, previous medical interventions, health behaviors (smoking, exercise, alcohol use), and measures of general health. Biometric data (blood pressure, electrocardiography, anthropometrics, fasting blood and urine samples) were collected by a health professional during an in-home visit. The REGARDS study protocol was approved by the institutional review boards at the participating centers, and all participants provided written informed consent.
Predictor Variables
Our primary predictors of interest were eGFR according to the 2012 Chronic Kidney Diseases Epidemiology Collaboration (CKD EPI) equation, which includes both creatinine and cystatin C, and urine albumin concentrations, which are expressed as the urinary albumin-to-creatinine ratio (ACR).20, 21, 23 Detailed descriptions of the covariates have been provided in prior publications from the REGARDS study24–26 and are reviewed in the Online Supplemental Methods.
Outcome definitions
Our definition for SCD was consistent with those proposed by an NHLBI-sponsored, expert panel: (a) unexpected death without an obvious extracardiac cause occurring with a rapid witnessed collapse;1 (b) for unwitnessed events, SCD is defined as an event that occurs within one hour after symptom onset or an unexpected death without obvious extracardiac cause that occurred within the previous 24 hours.1 All out-of-hospital cardiovascular deaths underwent further evaluation as a possible SCD event. These events could have occurred in the emergency room, but may not have been the consequence of acute trauma, intoxication, or the culmination of a terminal illness (cancer or end stage lung disease). Details of the adjudication protocol are provided in the Online Supplemental Methods.
Statistical Analysis
We first computed unadjusted incidence rates for SCD within categories of ACR and eGFR and plotted Kaplan-Meier curves using methods for censored data. Rates were reported according to each kidney measure independently. We analyzed associations with SCD separately for the ACR categories (< 15, 15–30, and > 30 mg/g) and for the eGFR categories (≥ 90, 60–89, 45–59, and < 45 ml/min/1.73m2). After confirming the proportionality of hazards, we constructed Cox proportional hazards regression models to estimate associations between each measure of kidney health and time to SCD. For each participant, the time at risk was defined from the baseline exam until the date of SCD, other cardiovascular death, non-cardiovascular death, censor due to loss to follow-up, or the end of available follow-up. We adjusted for confounding variables in a staged approach using regression models that have biological plausibility. We also adjusted for intervening MI and/or heart failure exacerbation hospitalization as time dependent covariates and performed a competing risk analysis.27 Additional details related to the statistical analyses are provided in the Online Supplemental Methods.
We also estimated the associations of combined eGFR and ACR categories with the risk of SCD. For this approach, given the multiple eGFR/ACR categories and relatively limited number of SCD events, we divided eGFR into 3 categories (≥ 90, 60–89, and < 60 ml/min/1.73m2). The 3 eGFR and 3 ACR categories were then combined to yield nine different exposure groups. The reference category was the group of participants with an eGFR ≥ 90 ml/min/1.73m2 and an ACR < 15 mg/g. A 2-sided p-value of 0.05 was considered statistically significant for all analyses.
RESULTS
This analysis included 27,296 black and white adults. The majority of REGARDS participants (74%) had an ACR < 15 mg/g. Approximately 11% had an ACR between 15 to 30 mg/g, and 15% of all individuals in this study had an ACR > 30 mg/g. Participants who had higher urinary ACR values were more likely to be older, black, less educated, and to have lower income (Table 1). In addition, they had a greater prevalence of cardiovascular risk factors. A lower eGFR demonstrated similar patterns among these baseline characteristics (Online Supplemental Table 1).
Table 1.
Baseline characteristics of REGARDS participants according to albumin to creatinine ratio
| ACR Category, mg/g | ||||
|---|---|---|---|---|
| Characteristics | ACR < 15 (n=20,167) |
ACR = 15– 30 (n=3,089) |
ACR >30 (n=4,040) |
P value |
| Age (years)±SD | 64±9 | 67±10 | 67±10 | <0.001 |
| Women n(%) | 11058 (55) | 1776 (58) | 2004 (50) | <0.001 |
| African American n(%) |
7617 (38) | 1254 (41) | 2069 (51) | <0.001 |
| Education <high school graduation n(%) |
2103 (10) | 470 (15) | 744 (18) | <0.001 |
| Income <$35,000† n(%) |
7770 (39) | 1448 (47) | 2085 (52) | <0.001 |
| Body mass index‡ (kg/m2)±SD |
29.0±5.9 | 29.5±6.6 | 30.3±6.7 | <0.001 |
| Systolic BP (mmHg)±SD |
125±15 | 131±18 | 135±19 | <0.001 |
| Diastolic BP (mm Hg)±SD |
76±9 | 78±10 | 79±11 | <0.001 |
| Lifestyle habits | ||||
| Current smoker n(%) |
2686 (13) | 457 (15) | 744 (19) | <0.001 |
| Exercise (none) n(%) |
6250 (31) | 1142 (38) | 1657 (42) | <0.001 |
| Comorbidities | ||||
| Coronary heart disease n(%) |
3031 (15) | 654 (21) | 1079 (27) | <0.001 |
| Diabetes n(%) | 3085 (15) | 872 (28) | 1691 (42) | <0.001 |
| Hypertension n(%) |
10789 (54) | 2111 (69) | 3124 (78) | <0.001 |
| Atrial fibrillation n(%) |
1505 (8) | 327 (11) | 496 (13) | <0.001 |
| ECG Variables | ||||
| QRS Interval (ms)±SD |
88±15 | 90±18 | 91±19 | <0.001 |
| Corrected QT interval (ms)±SD 406±23 |
410±25 | 410±25 | <0.001 | |
| Heart rate (beats per minute)±SD |
66±11 | 68±12 | 69±13 | <0.001 |
| Left ventricular hypertrophy n (%) |
1638 (8) | 368 (12) | 588 (15) | <0.001 |
| Laboratory Parameters | ||||
| Glomerular Filtration Rate, ml/min/1.73m2 | <0.001 | |||
| ≥90 n(%) | 9596 (48) | 1315 (43) | 1380 (34) | |
| 60–89 n(%) | 9044 (45) | 1384 (45) | 1590 (39) | |
| 45–59 n(%) | 1156 (6) | 279 (9) | 549 (14) | |
| <45 n(%) | 371 (2) | 111 (4) | 521 (13) | |
| Total cholesterol (mg/dL)±SD |
191±41 | 190±43 | 193±39 | <0.001 |
| HDL (mg/dL)±SD |
52±16 | 52±17 | 50±16 | <0.001 |
| Medications | ||||
| Statins n(%) | 5995 (30) | 1049 (34) | 1554 (39) | <0.001 |
|
Antihypertensives n(%) |
11030 (55) | 2087 (68) | 3059 (76) | <0.001 |
| ACEI/ARB n(%) |
6464 (32) | 1275 (41) | 2011 (50) | <0.001 |
| Digoxin n(%) | 350 (2) | 127 (4) | 199 (5) | <0.001 |
Indicates annual family income
BMI was calculated on weight in kilograms divided by height in meters squared
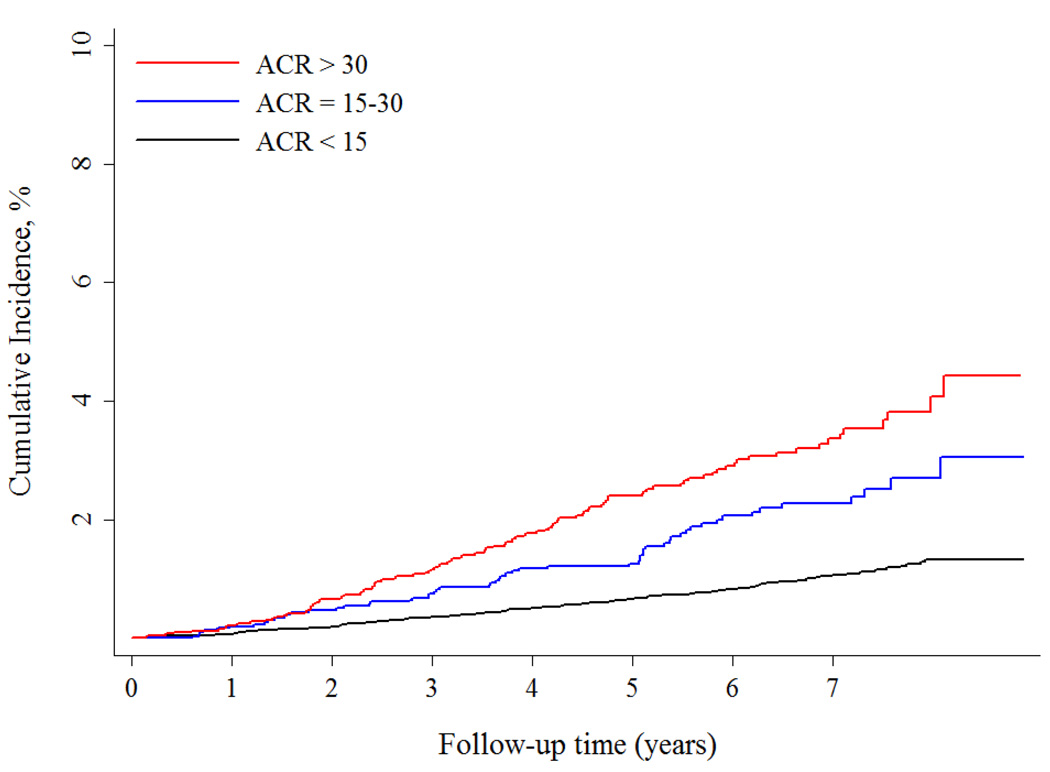
Over a median follow-up of 6.1 [interquartile range 4.6 – 7.3] years, the incidence of SCD increased across ACR groups and ranged from 0.14% per year among individuals with an ACR < 15 mg/g to 0.4% per year among those with an ACR > 30 mg/g (Figure 1A). Adjustment for demographics, cardiovascular disease risk factors and comorbid conditions had the greatest impact in attenuating the association between higher ACR categories and SCD risk (Table 2). However, compared with the ACR < 15 mg/g group, ACR levels of 15–30 mg/g and > 30 mg/g were associated with over a 1.5 fold increased, independent risk of SCD. Further adjustment for eGFR, intervening MI and heart failure hospitalizations and competing risk of non-SCD resulted in only minor attenuation of risk; higher ACR remained an independent risk factor for SCD.
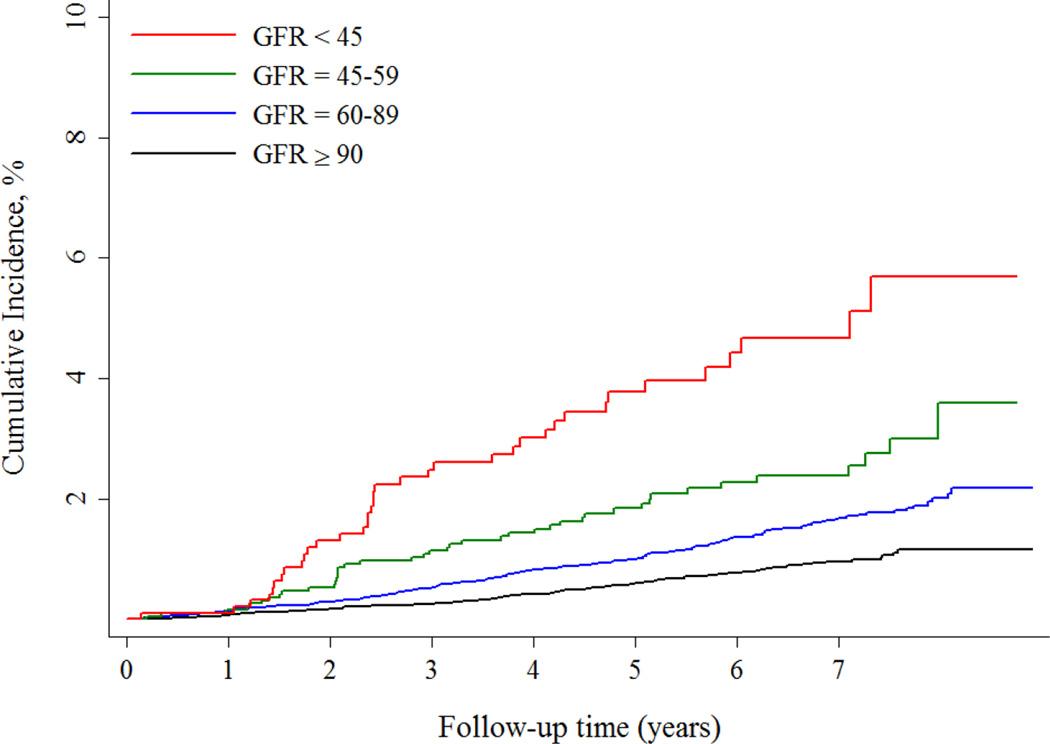
Figure 1.
A Risk of Sudden Cardiac Death across ACR categories
B Risk of Sudden Cardiac Death across eGFR categories
Table 2.
Association of Urinary Albumin to Creatinine Ratio with Sudden Cardiac Death
| ACR Range | |||
|---|---|---|---|
| ACR <15 mg/g | ACR 15–30 mg/g | ACR >30 mg/g | |
| Annual Event Rate (#events/#at risk) |
0.14% (173/20,167) |
0.26% (57/3,089) | 0.40% (105/4,040) |
| Unadjusted; HR (95% Cl) | 1.00 (ref) | 2.24 (1.66, 3.03) | 3.37 (2.64, 4.29) |
| Adjusted for demographics†; HR (95% CI) |
1.00 (ref) | 1.90 (1.40, 2.56) | 2.56 (2.00, 3.29) |
| + CVD risk factors and medications‡; HR (95% Cl) |
1.00 (ref) | 1.61 (1.17, 2.20) | 1.81 (1.38, 2.38) |
| + eGFR§; HR (95% CI) | 1.00 (ref) | 1.60 (1.17, 2.19) | 1.68 (1.27, 2.22) |
| + Intervening MI and HF hospitalization and Competing Risk¶; HR (95% Cl) |
1.00 (ref) | 1.53 (1.11, 2.11) | 1.56 (1.17, 2.11) |
adjusted for age, sex, race, education, income
adjusted for above plus systolic and diastolic blood pressure, diabetes, total cholesterol, HDL, smoking, body mass index, left ventricular hypertrophy, physical activity, baseline coronary heart disease, stroke, peripheral artery disease or aortic aneurysm, digoxin use, antihypertensive medication, ACE inhibitor, ARB, statins
adjusted for above plus eGFR
adjusted for above plus intervening MI and HF hospitalization events as time-dependent covariates and competing risk of non-SCD and non-cardiovascular death
Similarly, the incidence of SCD increased across eGFR categories and ranged from 0.15% per year among individuals with an eGFR ≥ 90 ml/min/1.73m2 to 0.50% per year among individuals with an eGFR < 45 ml/min/1.73m2 (Figure 1B). Nearly 90% of participants had an eGFR ≥ 60 ml/min/1.73m2, and only 4% had an eGFR < 45 ml/min/1.73m2. Similar to the analysis that assessed ACR, adjustment for demographics and cardiovascular conditions resulted in the greatest attenuation in the risk of SCD, and no significant differences were observed between the eGFR ≥ 90 and 60–89 ml/min/1.73m2 groups (Table 3). After the final adjustment, the eGFR < 45 ml/min/1.73m2 retained a significantly higher risk of SCD compared with those with an eGFR ≥ 90 ml/min/1.73m2.
Table 3.
Association of Estimated Glomerular Filtration Rate with Sudden Cardiac Death
| eGFR Range, ml/min/1.73m2 | ||||
|---|---|---|---|---|
| eGFR ≥90 | eGFR = 60– 89 |
eGFR = 45– 59 |
eGFR <45 | |
| Annual Event Rate (#events/#at risk) |
0.15% (93/12,291) |
0.19% (163/12,018) |
0.27% (42/1,984) |
0.50% (37/1,003) |
| Unadjusted; HR (95% Cl) | 1.00 (ref) | 1.75 (1.36, 2.26) |
2.95 (2.05, 4.24) |
5.79 (3.96, 8.48) |
| Adjusted for demographics†; HR (95% CI) |
1.00 (ref) | 1.34 (1.02, 1.76) |
1.86 (1.26, 2.75) |
3.36 (2.23, 5.06) |
| + CVD risk factors and medications‡; HR (95% Cl) |
1.00 (ref) | 1.29 (0.97, 1.71) |
1.52 (1.02, 2.28) |
2.22 (1.44, 3.43) |
| + ACR§; HR (95% CI) | 1.00 (ref) | 1.27 (0.96, 1.69) |
1.42 (0.95, 2.13) |
1.92 (1.23, 2.99) |
| + Intervening MI and HF hospitalization and Competing Risk¶; HR (95% Cl) |
1.00 (ref) | 1.26 (0.97, 1.68) |
1.35 (0.90, 2.03) |
1.66 (1.06, 2.58) |
adjusted for age, sex, race, education, income
adjusted for above plus systolic and diastolic blood pressure, diabetes, total cholesterol, HDL, smoking, body mass index, left ventricular hypertrophy, physical activity, baseline coronary heart disease, stroke, peripheral artery disease or aortic aneurysm, digoxin use, antihypertensive medication, ACE inhibitor, ARB, statins
adjusted for above plus ACR
adjusted for above plus intervening MI and HF hospitalization events as time-dependent covariates and competing risk of non-SCD and non-cardiovascular death
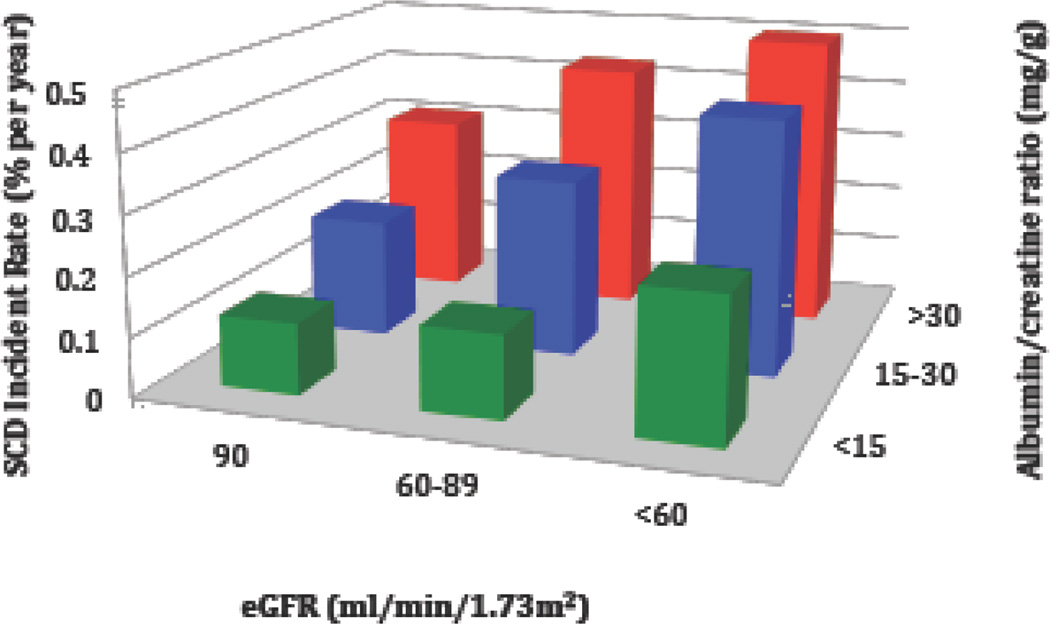
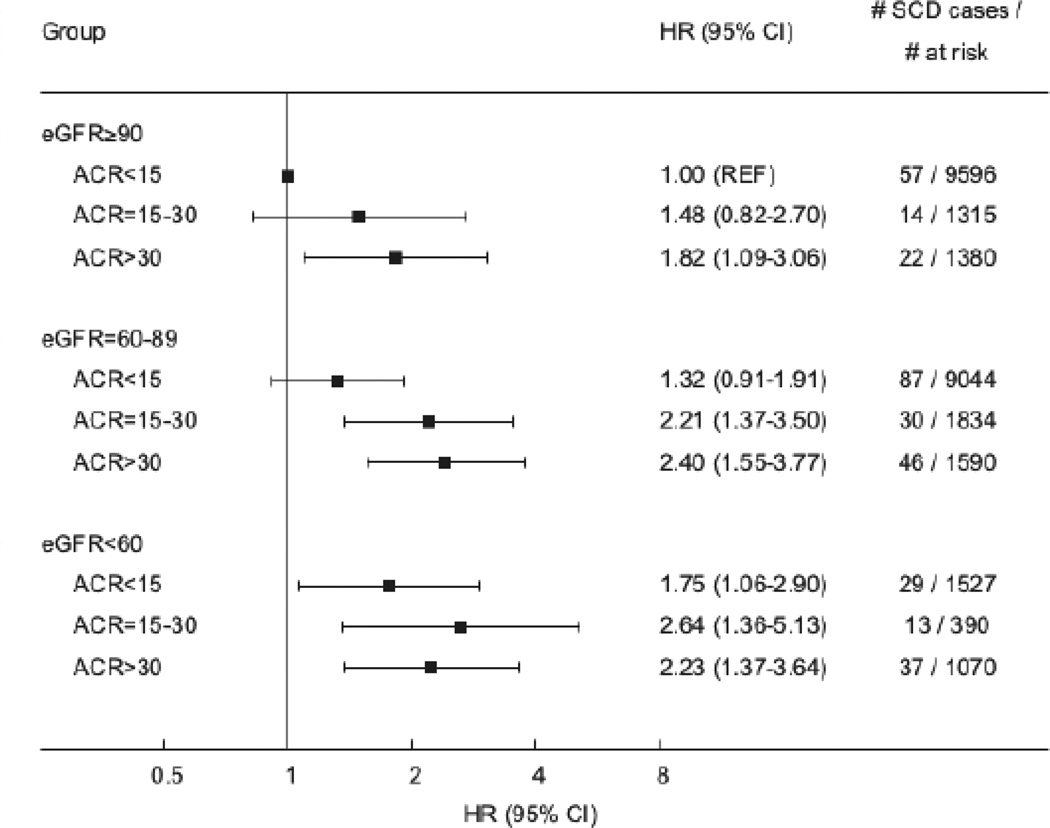
In the analysis that combined ACR and eGFR categories as the primary predictor variable, we detected a progressive increase in SCD incidence as both measures of kidney health declined (Figure 2). The age adjusted incidence rate of SCD ranged from 0.12% per year among those with no abnormalities in eGFR and ACR (eGFR ≥ 90 ml/min/1.73m2 and ACR < 15 mg/g) to 0.52% per year among those with the most advanced kidney disease (eGFR < 60 ml/min/1.73m2 and ACR ≥ 30 mg/g). In further analysis, albuminuria consistently identified individuals with an eGFR ≥ 60 ml/min/1.73m2 that were at significantly increased risk of SCD (Figure 3). In particular, among the eGFR ≥ 90 ml/min/1.73m2 group, an ACR > 30 mg/g was associated with a near two-fold higher SCD risk compared with individuals with eGFR ≥ 90 ml/min/1.73m2 and ACR < 15 mg/g. Further, among participants with an eGFR 60–89 ml/min/1.73m2, an ACR > 15 mg/g was present in 25% of participants and associated with a greater than two-fold SCD risk after multivariable adjustment. The Online Supplemental Results and Supplemental Tables 2 and 3 demonstrate associations between ACR, eGFR, and SCD in those without any baseline history of CHD.
Figure 2.
SCD incident rates across estimated glomerular filtration rate (eGFR) and urinary albumin-to-creatinine ratio (ACR) categories
Figure 3.
Hazard ratios (HR) and 95% confidence intervals (CI) of sudden cardiac death (SCD) according to eGFR and ACR. Cox proportional hazard model adjusted for age, sex, race, education, income, systolic and diastolic blood pressure, diabetes, total cholesterol, HDL cholesterol, smoking, body mass index, left ventricular hypertrophy, physical activity, baseline coronary heart disease, stroke, peripheral artery disease or aortic aneurism, use of digoxin, antihypertensive medication, ACE inhibitor, ARB, statins, intervening MI and HF hospitalization events as time dependent covariates and competing risk of non-SCD and non-cardiovascular death.
In stratified analysis, an ACR > 15 mg/g continued to identify nearly 25% of the REGARDS population without any baseline history of CHD at a higher risk of SCD compared with individuals with an ACR < 15 mg/g (Online Supplemental Table 2). Similarly, an eGFR < 45 ml/min/1.73m2 identified less than 3% of the subgroup without baseline CHD that was at a significantly higher risk of SCD (Online Supplemental Table 3). No significant interactions were detected (Online Supplemental Results).
DISCUSSION
Our findings from REGARDS, a large population-based study from across the United States, demonstrate that albuminuria is not only present in more than 25% of participants, but even at low levels, is independently associated with SCD after accounting for eGFR. An ACR between 15–30 mg/g, which is below the cutoff for defining microalbuminuria, was associated with a 50% higher risk of SCD compared to the referent group of < 15 mg/g. When combined with eGFR, this level of albuminuria was associated with 2.2 – 2.6 fold elevations in SCD risk among those with an eGFR < 90 ml/min/1.73m2. Greater degrees of albuminuria (ACR > 30 mg/g), which were present in 17% of the population, were also associated with an elevation in SCD risk among individuals with preserved eGFR (≥ 90 ml/min/1.73m2).
Previous studies have demonstrated that moderate reductions in eGFR are an independent risk factor for SCD.8, 15 We used a recently derived estimate of GFR, which provides a more sensitive measure of early declines in kidney function.20, 21 Although our adjusted findings demonstrated a gradual increase in SCD risk across 4 categories of eGFR, the risk of SCD was not significant and independent until the eGFR was less than 45 ml/min/1.73m2. This category represents less than 5% of the total REGARDS population and limits the impact of using eGFR only in identifying participants at an intermediate risk for SCD. Instead, the incorporation of both kidney filtration and injury markers was required to understand SCD risk in the population with CKD.
This study represents one of the first to assess comprehensively SCD risk across a range of ACR values in a general population-based study. Albuminuria identified SCD risk attributable to CKD among persons who would have been considered to have normal kidneys using eGFR alone. An ACR > 15 mg/g, which was present in more than 20% of individuals with an eGFR ≥ 90 ml/min/1.73m2 and 25% of those with an eGFR between 60 and 90 ml/min/1.73m2, was associated with an approximate doubling of risk for SCD. Since individuals with an eGFR ≥ 60 ml/min/1.73m2 are not considered high risk for adverse events, the addition of albuminuria could be an important step in further identifying persons with kidney injury that are at an intermediate risk for SCD. In addition, prior studies have demonstrated independent associations between albuminuria at levels below the threshold for microalbuminuria (ACR = 30 mg/g) and cardiovascular disease and all-cause mortality.16–18 As a result, our statistical modeling approach evaluated competing risks of mortality and intervening, non-fatal cardiovascular events. The findings from our analysis suggest that population-based interventions aimed at preventing fatal arrhythmias and the progression of kidney injury may be more effective among those with mild kidney disease as compared with more advanced CKD patients who have a high competing risk of non-SCD.9, 28 Strategies for the primary prevention of SCD at more advanced stages of CKD or ESRD have proven challenging especially since placement of the implantable cardioverter defibrillator (ICD) appears to have reduced efficacy.9, 28,29
Our findings provide greater clarity around population-based SCD and potential cardiorenal pathways that may be implicated in arrhythmic outcomes. As indicated previously, the majority of SCD events occur in individuals without a history of clinical cardiovascular disease and a normal left ventricular ejection fraction.11–14, 30 Of the SCD cases from the Oregon Sudden Unexpected Death Study that had an echocardiographic assessment of left ventricular function prior to the cardiac arrest, only 20% would have been eligible for ICD implantation for the primary prevention of SCD.30 The majority of SCD cases in the population are likely to have subclinical abnormalities in cardiac remodeling. Albuminuria is a well-recognized marker of systemic microvascular injury and has been associated with subclinical myocardial dysfunction in individuals with normal left ventricular ejection fraction (LVEF).31 In nearly 1900 individuals from the HyperGEN Study, all of whom had normal left ventricular function, even mild degrees of albuminuria were associated with abnormalities in cardiac mechanics including longitudinal strain,31 which is a sensitive marker for the health of the myocardium. Albuminuria may signify early declines in cardiac function and may help to explain our observations of an ACR-SCD link among individuals without a history of CHD. This group likely represents a healthier population than the overall cohort, and very few would be expected to have severe left ventricular dysfunction. The pathways linking albuminuria to early declines in cardiac function and increased cardiovascular disease risk remain speculative; however, albuminuria likely represents a sensitive marker of early dysregulation in the cardiorenal axis.
Our study has several strengths. It is one of the first to systematically assess SCD risk across the spectrum of kidney health in a large, broadly representative, modern prospective cohort study. By adjusting for intervening cardiovascular events and competing risk of mortality, we were able to distinguish the association of kidney disease with SCD risk from its well-known associations with other cardiovascular and non-cardiovascular outcomes. Several limitations of our study should be considered. Our analysis did not account for left ventricular function. Although a depressed LVEF is a strong risk factor for SCD, multiple studies have demonstrated that the majority of SCD in the community occur in individuals with normal LVEF. Given the design of this national cohort where in-home visits were performed, it was not feasible to obtain measures of LVEF using echocardiograms in this population. In addition, there was only a single measurement of ACR and eGFR, which may have led to exposure misclassification for some study participants. Further, in the combined ACR/eGFR evaluation, some categories had relatively few SCD events, which reduced the power to detect more precise estimates.
CONCLUSIONS
In summary, albuminuria identifies individuals from the general population at an increased risk of SCD even below the thresholds of CKD. Albuminuria also identifies nearly 25% of people with an eGFR ≥ 60 ml/min/1.73m2 that have increased risk of SCD, based on their underlying kidney disease. Therapies aimed at preventing the progression of kidney disease may impact SCD risk.
Supplementary Material
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org
Dr. Deo was supported by grant K23DK089118 from the National Institutes of Health. This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The REGARDS-MI study was supported by NIH grants R01 HL080477 and K24 HL111154.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
REFERENCES
- 1.Fishman GI, Chugh SS, Dimarco JP, et al. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122:2335–2348. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herzog CA, Li S, Weinhandl ED, Strief JW, Collins AJ, Gilbertson DT. Survival of dialysis patients after cardiac arrest and the impact of implantable cardioverter defibrillators. Kidney Int. 2005;68:818–825. doi: 10.1111/j.1523-1755.2005.00462.x. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS. Controlling the epidemic of cardiovascular disease in chronic renal disease: where do we start? Am J Kid Dis. 1998;32:S5–S13. doi: 10.1053/ajkd.1998.v32.pm9820463. [DOI] [PubMed] [Google Scholar]
- 4.Parekh RS, Plantinga LC, Kao WH, Meoni LA, Jaar BG, Fink NE, Powe NR, Coresh J, Klag MJ. The association of sudden cardiac death with inflammation and other traditional risk factors. Kidney Int. 2008;74:1335–1342. doi: 10.1038/ki.2008.449. [DOI] [PubMed] [Google Scholar]
- 5.Wang AY, Lam CW, Chan IH, Wang M, Lui SF, Sanderson JE. Sudden cardiac death in end-stage renal disease patients: a 5-year prospective analysis. Hypertension. 2010;56:210–216. doi: 10.1161/HYPERTENSIONAHA.110.151167. [DOI] [PubMed] [Google Scholar]
- 6.Whitman IR, Feldman HI, Deo R. CKD and sudden cardiac death: epidemiology, mechanisms, and therapeutic approaches. J Am Soc Nephrol. 2012;23:1929–1939. doi: 10.1681/ASN.2012010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 8.Deo R, Lin F, Vittinghoff E, Tseng ZH, Hulley SB, Shlipak MG. Kidney dysfunction and sudden cardiac death among women with coronary heart disease. Hypertension. 2008;51:1578–1582. doi: 10.1161/HYPERTENSIONAHA.107.103804. [DOI] [PubMed] [Google Scholar]
- 9.Goldenberg I, Moss AJ, McNitt S, Zareba W, Andrews ML, Hall WJ, Greenberg H, Case RB. Relations among renal function, risk of sudden cardiac death, and benefit of the implanted cardiac defibrillator in patients with ischemic left ventricular dysfunction. Am J Cardiol. 2006;98:485–490. doi: 10.1016/j.amjcard.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 10.Pun PH, Smarz TR, Honeycutt EF, Shaw LK, Al-Khatib SM, Middleton JP. Chronic kidney disease is associated with increased risk of sudden cardiac death among patients with coronary artery disease. Kidney Int. 2009;76:652–658. doi: 10.1038/ki.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albert CM, Chae CU, Grodstein F, Rose LM, Rexrode KM, Ruskin JN, Stampfer MJ, Manson JE. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107:2096–2101. doi: 10.1161/01.CIR.0000065223.21530.11. [DOI] [PubMed] [Google Scholar]
- 12.Chugh SS, Uy-Evanado A, Teodorescu C, Reinier K, Mariani R, Gunson K, Jui J. Women have a lower prevalence of structural heart disease as a precursor to sudden cardiac arrest: The Ore-SUDS (Oregon Sudden Unexpected Death Study) J Am Coll Cardiol. 2009;54:2006–2011. doi: 10.1016/j.jacc.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deo R, Albert CM. Epidemiology and genetics of sudden cardiac death. Circulation. 2012;125:620–637. doi: 10.1161/CIRCULATIONAHA.111.023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J, Chugh SS. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47:1161–1166. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 15.Deo R, Sotoodehnia N, Katz R, Sarnak MJ, Fried LF, Chonchol M, Kestenbaum B, Psaty BM, Siscovick DS, Shlipak MG. Cystatin C and sudden cardiac death risk in the elderly. Circ Cardiovasc Qual Outcomes. 2010;3:159–164. doi: 10.1161/CIRCOUTCOMES.109.875369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miettinen H, Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Proteinuria predicts stroke and other atherosclerotic vascular disease events in nondiabetic and non-insulin-dependent diabetic subjects. Stroke. 1996;27:2033–2039. doi: 10.1161/01.str.27.11.2033. [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez OM, Khodneva YA, Muntner P, Rizk DV, McClellan WM, Cushman M, Warnock DG, Safford MM. Association between urinary albumin excretion and coronary heart disease in black vs white adults. JAMA. 2013;310:706–714. doi: 10.1001/jama.2013.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrassy KM. Comments on 'KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease'. Kidney Int. 2013;84:622–623. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 20.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS. Estimating glomerular filtration rate from serum creatinine and cystatin C. New Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 22.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 23.Peralta CA, Shlipak MG, Judd S, Cushman M, McClellan W, Zakai NA, Safford MM, Zhang X, Muntner P, Warnock D. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011;305:1545–1552. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redmond N, Richman J, Gamboa CM, Albert MA, Sims M, Durant RW, Glasser SP, Safford MM. Perceived stress is associated with incident coronary heart disease and all-cause mortality in low- but not high-income participants in the Reasons for Geographic And Racial Differences in Stroke study. J Am Heart Assoc. 2013;2:e000447. doi: 10.1161/JAHA.113.000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pullicino PM, McClure LA, Wadley VG, Ahmed A, Howard VJ, Howard G, Safford MM. Blood pressure and stroke in heart failure in the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Stroke. 2009;40:3706–3710. doi: 10.1161/STROKEAHA.109.561670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howard VJ, Woolson RF, Egan BM, Nicholas JS, Adams RJ, Howard G, Lackland DT. Prevalence of hypertension by duration and age at exposure to the stroke belt. J Am Soc Hypertens. 2010;4:32–41. doi: 10.1016/j.jash.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 28.Charytan DM, Patrick AR, Liu J, Setoguchi S, Herzog CA, Brookhart MA, Winkelmayer WC. Trends in the use and outcomes of implantable cardioverter-defibrillators in patients undergoing dialysis in the United States. Am J Kidney Dis. 2011;58:409–417. doi: 10.1053/j.ajkd.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 29.Hess PL, Hellkamp AS, Peterson ED, Sanders GD, Al-Khalidi HR, Curtis LH, Hammill BG, Pun PH, Curtis JP, Anstrom KJ, Hammill SC, Al-Khatib SM. Survival after primary prevention implantable cardioverter-defibrillator placement among patients with chronic kidney disease. Circ Arrhythm Electrophysiol. 2014;7:793–799. doi: 10.1161/CIRCEP.114.001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narayanan K, Reinier K, Uy-Evanado A, Teodorescu C, Chugh H, Marijon E, Gunson K, Jui J, Chugh SS. Frequency and determinants of implantable cardioverter defibrillator deployment among primary prevention candidates with subsequent sudden cardiac arrest in the community. Circulation. 2013;128:1733–1738. doi: 10.1161/CIRCULATIONAHA.113.002539. [DOI] [PubMed] [Google Scholar]
- 31.Katz DH, Selvaraj S, Aguilar FG, et al. Association of low-grade albuminuria with adverse cardiac mechanics: findings from the hypertension genetic epidemiology network (HyperGEN) study. Circulation. 2014;129:42–50. doi: 10.1161/CIRCULATIONAHA.113.003429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.