Abstract
Huntington disease is a neurological movement disorder involving massive neuronal death in the caudate-putamen region of the brain. Neither preventive nor curative therapy exists for this disease. The implantation of cross-species striatal neural precursor cells into the lesioned striatum of nonhuman primates (baboons) reduced the abnormal movements seen in the disease model. These abnormal movements reappeared after immunological rejection of the implanted striatal cells and were not modified by transplantation with nonstriatal cells. These findings encourage further experimentation toward the use of cell sources other than human fetal cells in a potential clinical application to Huntington disease.
Full text
PDF
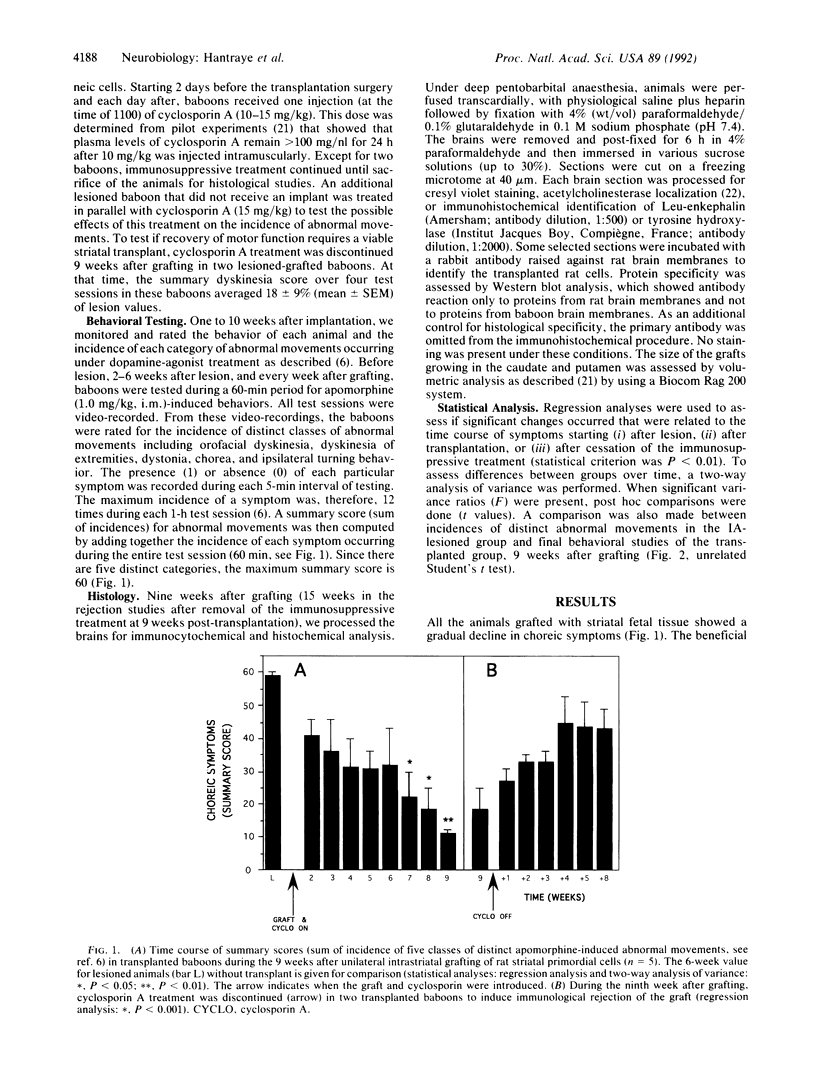
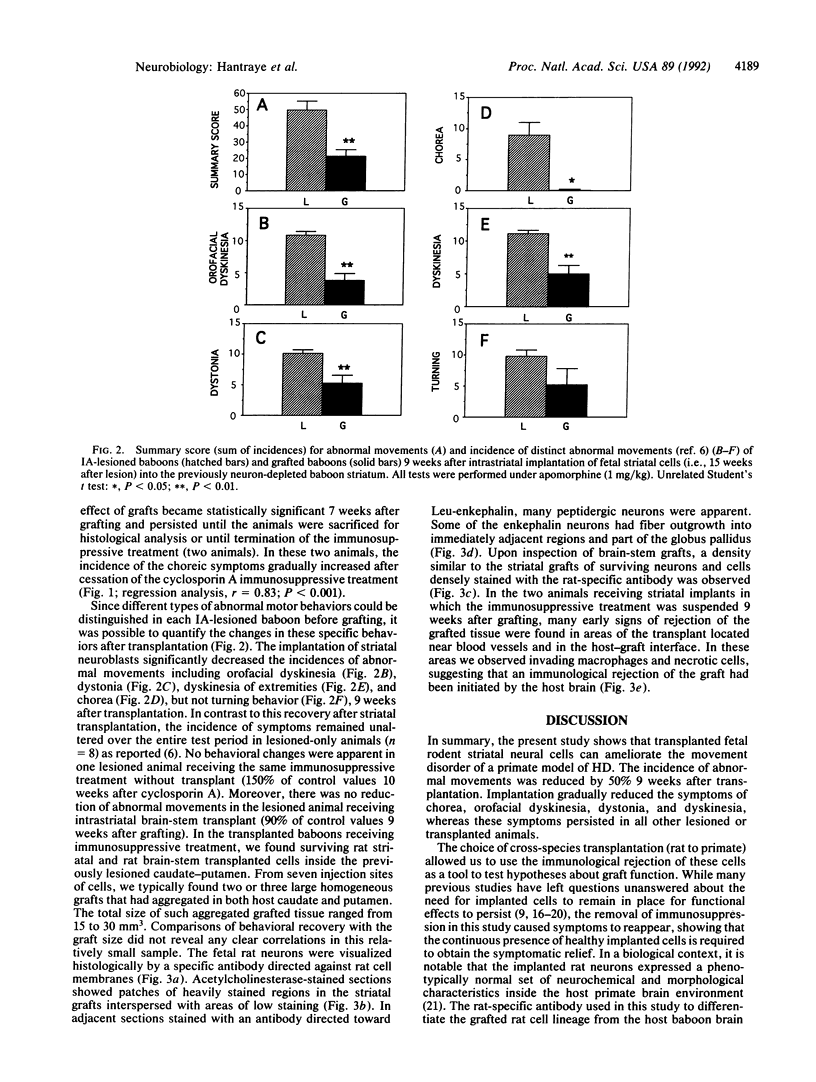


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albin R. L., Reiner A., Anderson K. D., Penney J. B., Young A. B. Striatal and nigral neuron subpopulations in rigid Huntington's disease: implications for the functional anatomy of chorea and rigidity-akinesia. Ann Neurol. 1990 Apr;27(4):357–365. doi: 10.1002/ana.410270403. [DOI] [PubMed] [Google Scholar]
- Albin R. L., Young A. B., Penney J. B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989 Oct;12(10):366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Beal M. F., Kowall N. W., Ellison D. W., Mazurek M. F., Swartz K. J., Martin J. B. Replication of the neurochemical characteristics of Huntington's disease by quinolinic acid. Nature. 1986 May 8;321(6066):168–171. doi: 10.1038/321168a0. [DOI] [PubMed] [Google Scholar]
- Bird E. D. Chemical pathology of Huntington's disease. Annu Rev Pharmacol Toxicol. 1980;20:533–551. doi: 10.1146/annurev.pa.20.040180.002533. [DOI] [PubMed] [Google Scholar]
- Brundin P., Widner H., Nilsson O. G., Strecker R. E., Björklund A. Intracerebral xenografts of dopamine neurons: the role of immunosuppression and the blood-brain barrier. Exp Brain Res. 1989;75(1):195–207. doi: 10.1007/BF00248542. [DOI] [PubMed] [Google Scholar]
- Chevalier G., Deniau J. M. Disinhibition as a basic process in the expression of striatal functions. Trends Neurosci. 1990 Jul;13(7):277–280. doi: 10.1016/0166-2236(90)90109-n. [DOI] [PubMed] [Google Scholar]
- Clarke D. J., Dunnett S. B. Ultrastructural organization within intrastriatal striatal grafts. Prog Brain Res. 1990;82:407–415. doi: 10.1016/s0079-6123(08)62629-1. [DOI] [PubMed] [Google Scholar]
- Crossman A. R. Primate models of dyskinesia: the experimental approach to the study of basal ganglia-related involuntary movement disorders. Neuroscience. 1987 Apr;21(1):1–40. doi: 10.1016/0306-4522(87)90322-8. [DOI] [PubMed] [Google Scholar]
- Crossman A. R., Sambrook M. A., Jackson A. Experimental hemichorea/hemiballismus in the monkey. Studies on the intracerebral site of action in a drug-induced dyskinesia. Brain. 1984 Jun;107(Pt 2):579–596. doi: 10.1093/brain/107.2.579. [DOI] [PubMed] [Google Scholar]
- Deckel A. W., Robinson R. G., Coyle J. T., Sanberg P. R. Reversal of long-term locomotor abnormalities in the kainic acid model of Huntington's disease by day 18 fetal striatal implants. Eur J Pharmacol. 1983 Sep 30;93(3-4):287–288. doi: 10.1016/0014-2999(83)90150-4. [DOI] [PubMed] [Google Scholar]
- Dunnett S. B., Björklund A. Mechanisms of function of neural grafts in the adult mammalian brain. J Exp Biol. 1987 Sep;132:265–289. doi: 10.1242/jeb.132.1.265. [DOI] [PubMed] [Google Scholar]
- Dunnett S. B., Isacson O., Sirinathsinghji D. J., Clarke D. J., Björklund A. Striatal grafts in rats with unilateral neostriatal lesions--III. Recovery from dopamine-dependent motor asymmetry and deficits in skilled paw reaching. Neuroscience. 1988 Mar;24(3):813–820. doi: 10.1016/0306-4522(88)90069-3. [DOI] [PubMed] [Google Scholar]
- Ellison D. W., Beal M. F., Mazurek M. F., Malloy J. R., Bird E. D., Martin J. B. Amino acid neurotransmitter abnormalities in Huntington's disease and the quinolinic acid animal model of Huntington's disease. Brain. 1987 Dec;110(Pt 6):1657–1673. doi: 10.1093/brain/110.6.1657. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M., Liu F. C., Dunnett S. B. Intrastriatal grafts derived from fetal striatal primordia. I. Phenotypy and modular organization. J Neurosci. 1989 Sep;9(9):3250–3271. doi: 10.1523/JNEUROSCI.09-09-03250.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantraye P., Riche D., Maziere M., Isacson O. A primate model of Huntington's disease: behavioral and anatomical studies of unilateral excitotoxic lesions of the caudate-putamen in the baboon. Exp Neurol. 1990 May;108(2):91–104. doi: 10.1016/0014-4886(90)90014-j. [DOI] [PubMed] [Google Scholar]
- Isacson O., Brundin P., Gage F. H., Björklund A. Neural grafting in a rat model of Huntington's disease: progressive neurochemical changes after neostriatal ibotenate lesions and striatal tissue grafting. Neuroscience. 1985 Dec;16(4):799–817. doi: 10.1016/0306-4522(85)90095-8. [DOI] [PubMed] [Google Scholar]
- Isacson O., Brundin P., Kelly P. A., Gage F. H., Björklund A. Functional neuronal replacement by grafted striatal neurones in the ibotenic acid-lesioned rat striatum. Nature. 1984 Oct 4;311(5985):458–460. doi: 10.1038/311458a0. [DOI] [PubMed] [Google Scholar]
- Isacson O., Dunnett S. B., Björklund A. Graft-induced behavioral recovery in an animal model of Huntington disease. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2728–2732. doi: 10.1073/pnas.83.8.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacson O., Riche D., Hantraye P., Sofroniew M. V., Maziere M. A primate model of Huntington's disease: cross-species implantation of striatal precursor cells to the excitotoxically lesioned baboon caudate-putamen. Exp Brain Res. 1989;75(1):213–220. doi: 10.1007/BF00248544. [DOI] [PubMed] [Google Scholar]
- KOELLE G. B. The histochemical localization of cholinesterases in the central nervous system of the rat. J Comp Neurol. 1954 Feb;100(1):211–235. doi: 10.1002/cne.901000108. [DOI] [PubMed] [Google Scholar]
- Kanazawa I., Tanaka Y., Cho F. 'Choreic' movement induced by unilateral kainate lesion of the striatum and L-DOPA administration in monkey. Neurosci Lett. 1986 Nov 11;71(2):241–246. doi: 10.1016/0304-3940(86)90566-5. [DOI] [PubMed] [Google Scholar]
- Kelly P. A., Graham D. I., McCulloch J. Specific alterations in local cerebral glucose utilization following striatal lesions. Brain Res. 1982 Feb 4;233(1):157–172. doi: 10.1016/0006-8993(82)90937-4. [DOI] [PubMed] [Google Scholar]
- Kesslak J. P., Nieto-Sampedro M., Globus J., Cotman C. W. Transplants of purified astrocytes promote behavioral recovery after frontal cortex ablation. Exp Neurol. 1986 May;92(2):377–390. doi: 10.1016/0014-4886(86)90089-0. [DOI] [PubMed] [Google Scholar]
- Labbe R., Firl A., Jr, Mufson E. J., Stein D. G. Fetal brain transplant: reduction of cognitive deficits in rats with frontal cortex lesions. Science. 1983 Jul 29;221(4609):470–472. doi: 10.1126/science.6683427. [DOI] [PubMed] [Google Scholar]
- Martin J. B. Huntington's disease: new approaches to an old problem. The Robert Wartenberg lecture. Neurology. 1984 Aug;34(8):1059–1072. doi: 10.1212/wnl.34.8.1059. [DOI] [PubMed] [Google Scholar]
- Norman A. B., Lehman M. N., Sanberg P. R. Functional effects of fetal striatal transplants. Brain Res Bull. 1989 Jan;22(1):163–172. doi: 10.1016/0361-9230(89)90141-x. [DOI] [PubMed] [Google Scholar]
- Olney J. W. Excitatory amino acids and neuropsychiatric disorders. Biol Psychiatry. 1989 Sep;26(5):505–525. doi: 10.1016/0006-3223(89)90072-3. [DOI] [PubMed] [Google Scholar]
- Reiner A., Albin R. L., Anderson K. D., D'Amato C. J., Penney J. B., Young A. B. Differential loss of striatal projection neurons in Huntington disease. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5733–5737. doi: 10.1073/pnas.85.15.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanberg P. R., Coyle J. T. Scientific approaches to Huntington's disease. CRC Crit Rev Clin Neurobiol. 1984;1(1):1–44. [PubMed] [Google Scholar]
- Sanberg P. R., Giòrdano M., Henault M. A., Nash D. R., Ragozzino M. E., Hagenmeyer-Houser S. H. Intraparenchymal striatal transplants required for maintenance of behavioral recovery in an animal model of Huntington's disease. J Neural Transplant. 1989;1(1):23–31. doi: 10.1155/NP.1989.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanberg P. R., Henault M. A., Deckel A. W. Locomotor hyperactivity: effects of multiple striatal transplants in an animal model of Huntington's disease. Pharmacol Biochem Behav. 1986 Jul;25(1):297–300. doi: 10.1016/0091-3057(86)90269-8. [DOI] [PubMed] [Google Scholar]
- Schwarcz R., Hökfelt T., Fuxe K., Jonsson G., Goldstein M., Terenius L. Ibotenic acid-induced neuronal degeneration: a morphological and neurochemical study. Exp Brain Res. 1979 Oct;37(2):199–216. doi: 10.1007/BF00237708. [DOI] [PubMed] [Google Scholar]
- Sirinathsinghji D. J., Dunnett S. B., Isacson O., Clarke D. J., Kendrick K., Björklund A. Striatal grafts in rats with unilateral neostriatal lesions--II. In vivo monitoring of GABA release in globus pallidus and substantia nigra. Neuroscience. 1988 Mar;24(3):803–811. doi: 10.1016/0306-4522(88)90068-1. [DOI] [PubMed] [Google Scholar]
- Vonsattel J. P., Myers R. H., Stevens T. J., Ferrante R. J., Bird E. D., Richardson E. P., Jr Neuropathological classification of Huntington's disease. J Neuropathol Exp Neurol. 1985 Nov;44(6):559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Wictorin K., Brundin P., Gustavii B., Lindvall O., Björklund A. Reformation of long axon pathways in adult rat central nervous system by human forebrain neuroblasts. Nature. 1990 Oct 11;347(6293):556–558. doi: 10.1038/347556a0. [DOI] [PubMed] [Google Scholar]
- Wictorin K., Isacson O., Fischer W., Nothias F., Peschanski M., Björklund A. Connectivity of striatal grafts implanted into the ibotenic acid-lesioned striatum--I. Subcortical afferents. Neuroscience. 1988 Nov;27(2):547–562. doi: 10.1016/0306-4522(88)90288-6. [DOI] [PubMed] [Google Scholar]
- Wictorin K., Simerly R. B., Isacson O., Swanson L. W., Björklund A. Connectivity of striatal grafts implanted into the ibotenic acid-lesioned striatum--III. Efferent projecting graft neurons and their relation to host afferents within the grafts. Neuroscience. 1989;30(2):313–330. doi: 10.1016/0306-4522(89)90256-x. [DOI] [PubMed] [Google Scholar]
- Widner H., Brundin P. Immunological aspects of grafting in the mammalian central nervous system. A review and speculative synthesis. Brain Res. 1988 Nov;472(3):287–324. doi: 10.1016/0165-0173(88)90010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]