Abstract
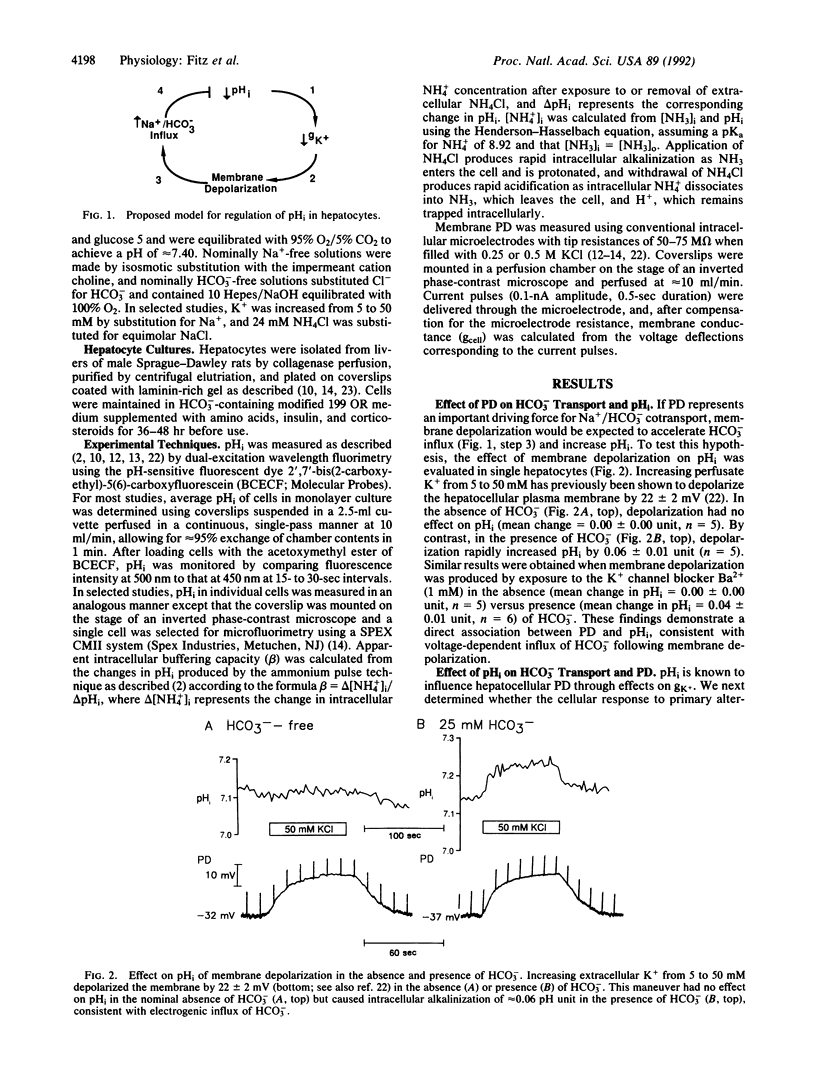
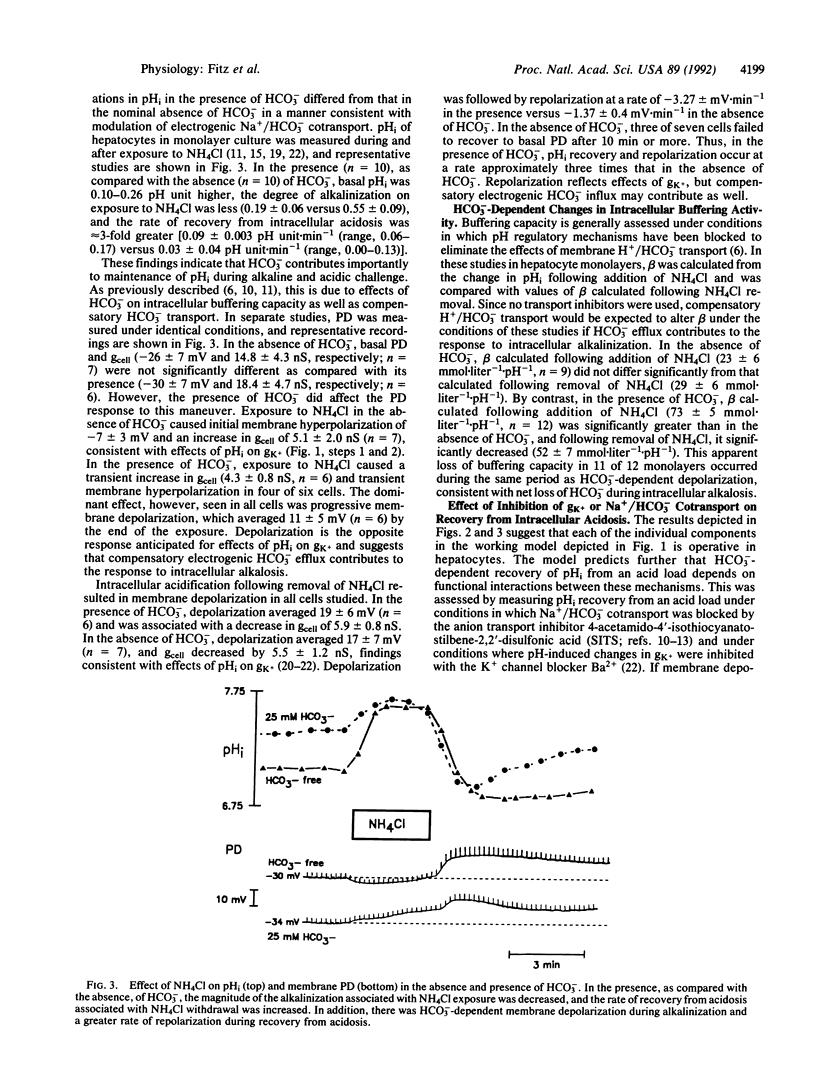
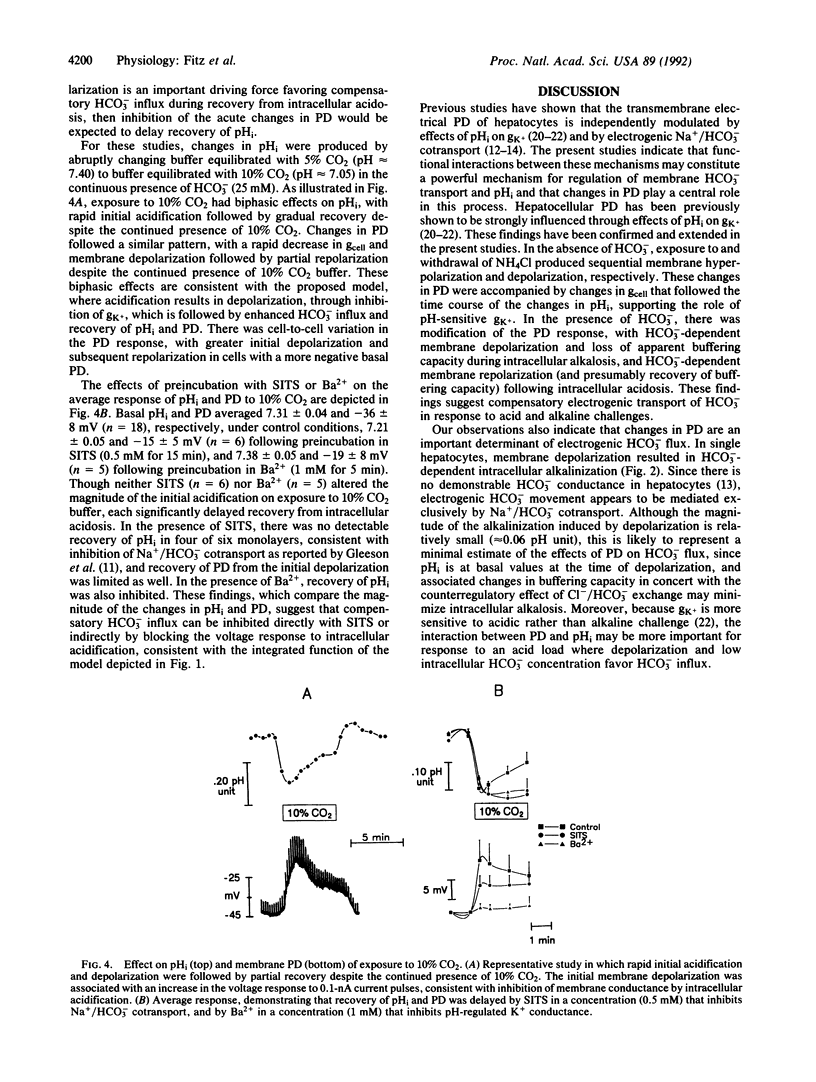
We have examined the hypothesis that a regulatory interplay between pH-regulated plasma membrane K+ conductance (gK+) and electrogenic Na+/HCO3- cotransport contributes importantly to regulation of intracellular pH (pHi) in hepatocytes. In individual cells, membrane depolarization produced by transient exposure to 50 mM K+ caused a reversible increase in pHi in the presence, but not absence, of HCO3-, consistent with voltage-dependent HCO3- influx. In the absence of HCO3-, intracellular alkalinization and acidification produced by NH4Cl exposure and withdrawal produced membrane hyperpolarization and depolarization, respectively, as expected for pHi-induced changes in gK+. By contrast, in the presence of HCO3-, NH4Cl exposure and withdrawal produced a decrease in apparent buffering capacity and changes in membrane potential difference consistent with compensatory regulation of electrogenic Na+/HCO3- cotransport. Moreover, the rate of pHi and potential difference recovery was several-fold greater in the presence as compared with the absence of HCO3-. Finally, continuous exposure to 10% CO2 in the presence of HCO3- produced intracellular acidification, and the rate of pHi recovery from intracellular acidosis was inhibited by Ba2+, which blocks pHi-induced changes in gK+, and by 4-acetamido-4'-isothiocyanatostilbene-2,2'-disulfonic acid, which inhibits Na+/HCO3- cotransport. These findings suggest that in hepatocytes, changes in transmembrane electrical potential difference, mediated by pH-sensitive gK+, play a central role in regulation of pHi through effects on electrogenic Na+/HCO3- cotransport.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpern R. J. Mechanism of basolateral membrane H+/OH-/HCO-3 transport in the rat proximal convoluted tubule. A sodium-coupled electrogenic process. J Gen Physiol. 1985 Nov;86(5):613–636. doi: 10.1085/jgp.86.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear C. E., Davison J. S., Shaffer E. A. Intracellular pH influences the resting membrane potential of isolated rat hepatocytes. Biochim Biophys Acta. 1988 Oct 6;944(2):113–120. doi: 10.1016/0005-2736(88)90424-5. [DOI] [PubMed] [Google Scholar]
- Benedetti A., Strazzabosco M., Corasanti J. G., Haddad P., Graf J., Boyer J. L. Cl(-)-HCO3- exchanger in isolated rat hepatocytes: role in regulation of intracellular pH. Am J Physiol. 1991 Sep;261(3 Pt 1):G512–G522. doi: 10.1152/ajpgi.1991.261.3.G512. [DOI] [PubMed] [Google Scholar]
- Bissell D. M., Arenson D. M., Maher J. J., Roll F. J. Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver. J Clin Invest. 1987 Mar;79(3):801–812. doi: 10.1172/JCI112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F. Intracellular pH regulation in epithelial cells. Annu Rev Physiol. 1986;48:377–388. doi: 10.1146/annurev.ph.48.030186.002113. [DOI] [PubMed] [Google Scholar]
- Fitz J. G., Lidofsky S. D., Weisiger R. A., Xie M. H., Cochran M., Grotmol T., Scharschmidt B. F. HCO3(-)-coupled Na+ influx is a major determinant of Na+ turnover and Na+/K+ pump activity in rat hepatocytes. J Membr Biol. 1991 May;122(1):1–10. doi: 10.1007/BF01872734. [DOI] [PubMed] [Google Scholar]
- Fitz J. G., Lidofsky S. D., Xie M. H., Cochran M., Scharschmidt B. F. Plasma membrane H(+)-HCO3- transport in rat hepatocytes: a principal role for Na(+)-coupled HCO3- transport. Am J Physiol. 1991 Nov;261(5 Pt 1):G803–G809. doi: 10.1152/ajpgi.1991.261.5.G803. [DOI] [PubMed] [Google Scholar]
- Fitz J. G., Persico M., Scharschmidt B. F. Electrophysiological evidence for Na+-coupled bicarbonate transport in cultured rat hepatocytes. Am J Physiol. 1989 Mar;256(3 Pt 1):G491–G500. doi: 10.1152/ajpgi.1989.256.3.G491. [DOI] [PubMed] [Google Scholar]
- Gleeson D., Smith N. D., Boyer J. L. Bicarbonate-dependent and -independent intracellular pH regulatory mechanisms in rat hepatocytes. Evidence for Na+-HCO3- cotransport. J Clin Invest. 1989 Jul;84(1):312–321. doi: 10.1172/JCI114156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf J., Henderson R. M., Krumpholz B., Boyer J. L. Cell membrane and transepithelial voltages and resistances in isolated rat hepatocyte couplets. J Membr Biol. 1987;95(3):241–254. doi: 10.1007/BF01869486. [DOI] [PubMed] [Google Scholar]
- Henderson R. M., Graf J., Boyer J. L. Na-H exchange regulates intracellular pH in isolated rat hepatocyte couplets. Am J Physiol. 1987 Jan;252(1 Pt 1):G109–G113. doi: 10.1152/ajpgi.1987.252.1.G109. [DOI] [PubMed] [Google Scholar]
- Henderson R. M., Krumpholz B., Boyer J. L., Graf J. Effect of intracellular pH on potassium conductance in liver. Pflugers Arch. 1988 Aug;412(3):334–335. doi: 10.1007/BF00582518. [DOI] [PubMed] [Google Scholar]
- Hughes B. A., Adorante J. S., Miller S. S., Lin H. Apical electrogenic NaHCO3 cotransport. A mechanism for HCO3 absorption across the retinal pigment epithelium. J Gen Physiol. 1989 Jul;94(1):125–150. doi: 10.1085/jgp.94.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagura T., Deutsch C. J., Taylor J., Erecińska M., Wilson D. F. Dependence of gluconeogenesis, urea synthesis, and energy metabolism of hepatocytes on intracellular pH. J Biol Chem. 1984 Jan 10;259(1):237–243. [PubMed] [Google Scholar]
- Mason M. J., Smith J. D., Garcia-Soto J. J., Grinstein S. Internal pH-sensitive site couples Cl-(-)HCO3- exchange to Na+-H+ antiport in lymphocytes. Am J Physiol. 1989 Feb;256(2 Pt 1):C428–C433. doi: 10.1152/ajpcell.1989.256.2.C428. [DOI] [PubMed] [Google Scholar]
- Meier P. J., Knickelbein R., Moseley R. H., Dobbins J. W., Boyer J. L. Evidence for carrier-mediated chloride/bicarbonate exchange in canalicular rat liver plasma membrane vesicles. J Clin Invest. 1985 Apr;75(4):1256–1263. doi: 10.1172/JCI111824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugharbil A., Knickelbein R. G., Aronson P. S., Dobbins J. W. Rabbit ileal brush-border membrane Cl-HCO3 exchanger is activated by an internal pH-sensitive modifier site. Am J Physiol. 1990 Oct;259(4 Pt 1):G666–G670. doi: 10.1152/ajpgi.1990.259.4.G666. [DOI] [PubMed] [Google Scholar]
- Renner E. L., Lake J. R., Persico M., Scharschmidt B. F. Na+-H+ exchange activity in rat hepatocytes: role in regulation of intracellular pH. Am J Physiol. 1989 Jan;256(1 Pt 1):G44–G52. doi: 10.1152/ajpgi.1989.256.1.G44. [DOI] [PubMed] [Google Scholar]
- Renner E. L., Lake J. R., Scharschmidt B. F., Zimmerli B., Meier P. J. Rat hepatocytes exhibit basolateral Na+/HCO3- cotransport. J Clin Invest. 1989 Apr;83(4):1225–1235. doi: 10.1172/JCI114005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani M., Grassi S. M., Aronson P. S. Stoichiometry of Na+-HCO-3 cotransport in basolateral membrane vesicles isolated from rabbit renal cortex. J Clin Invest. 1987 Apr;79(4):1276–1280. doi: 10.1172/JCI112948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltoff S. P., Cantley L. C. Mitogens and ion fluxes. Annu Rev Physiol. 1988;50:207–223. doi: 10.1146/annurev.ph.50.030188.001231. [DOI] [PubMed] [Google Scholar]
- Stewart D. J. Sodium-proton exchanger in isolated hepatocytes exhibits a set point. Am J Physiol. 1988 Sep;255(3 Pt 1):G346–G351. doi: 10.1152/ajpgi.1988.255.3.G346. [DOI] [PubMed] [Google Scholar]
- Wang W. H., Wang Y., Silbernagl S., Oberleithner H. Fused cells of frog proximal tubule: II. Voltage-dependent intracellular pH. J Membr Biol. 1988 Mar;101(3):259–265. doi: 10.1007/BF01872840. [DOI] [PubMed] [Google Scholar]



