Abstract
Regulator of G-protein signaling (RGS) proteins were originally identified as negative regulators of G-protein-coupled receptor (GPCR) signaling via their GTPase-accelerating protein (GAP) activity. All RGS proteins contain evolutionarily conserved RGS domain; however, they differ in their size and regulatory domains. RGS1 and RGS10 are smaller than other RGS proteins, and their functions involve various inflammatory responses including autoimmune responses in both the periphery and the central nervous system (CNS). Neuroinflammation is the chronic inflammatory response in the CNS. Acute inflammatory response in the CNS is believed to be beneficial by involving the neuroprotective actions of immune cells in the brain, particularly microglia, to limit tissue damage and to aid in neuronal repair. However, chronically elevated levels of cytokines serve to maintain activation of abundant numbers of immune cells potentiating prolonged inflammatory responses and creating an environment of oxidative stress, which further hastens oxidative damage of neurons. In this review, we describe the implications and features of RGS proteins (specifically RGS1 and RGS10) in neuroinflammation and neurodegenerative diseases. We will discuss the experimental and epidemiological evidence on the benefits of anti-inflammatory interventions by targeting RGS1 and/or RGS10 protein function or expression in order to delay or attenuate the progression of neurodegeneration, particularly in multiple sclerosis (MS) and Parkinson’s disease (PD).
KEY WORDS: G-protein-coupled receptor (GPCR), multiple sclerosis, neuroinflammation, Parkinson’s disease, regulator of G-protein signaling
INTRODUCTION
G-protein-coupled receptors (GPCRs) signal through heterotrimeric G-proteins that consist of an α subunit and a βγ heterodimer (1). Regulator of G-protein signaling (RGS) proteins play a role in turning off GPCR signaling. All of the RGS proteins contain a conserved RGS domain that interacts with a Gαi, Gαq/11, or Gα12/13 subunit with variable selectivity, which accelerates the GTPase-activating activity of the Gα subunit (2–4). Since the mid-1990s, more than 30 functional RGS genes have been identified and subdivided into eight subfamilies that are expressed in eukaryotic organisms, from fungi to animals such as mice and humans (3, 5, 6). RGS proteins differ widely in their size and contain a variety of structural domains in addition to the RGS domain and motifs that regulate their activity and determine regulatory binding partners (3, 5; also reviewed in 6–9). Early evidence suggested that RGS proteins acted primarily as negative regulators of G-protein signaling. Today, it is well documented that these proteins act as tightly regulated modulators and multifunctional interactors of G-protein signaling (reviewed in 8). In addition, it has been recently appreciated that the non-RGS regions of RGS proteins can provide non-canonical functions distinct from the inactivation of Gα subunits or even from G-protein signaling entirely (reviewed in 9, 10).
RGS proteins are highly conserved from yeast to mammals and are abundant in the retina, brain, heart, and immune organs (11; see review in 12). Tissue-specific patterns of RGS protein expression in the human peripheral tissues and brain were reported by Larminie and his group (13). They showed that the major RGS10 proteins in human lymphocytes are RGS1, RGS2, RGS10, RGS13, RGS14, RGS16, and RGS18 (13). The RGS proteins may acquire functional diversity in immune cells by a fine-tuned and dynamic regulation of the expression of multiple RGS proteins.
RGS protein profiling in human lymphocytes displays a similar expression profile to rodent lymphocytes except for RGS18 (14), suggesting that observations in rodent lymphocytes may be translated into what would be occurring in human lymphocytes. In this review, we will discuss how RGS proteins, and more precisely RGS1 and RGS10, play important roles in inflammatory and neurodegenerative diseases and act as therapeutic potentials mainly in Parkinson’s disease (PD) and multiple sclerosis (MS).
RGS PROTEIN FAMILY AND MS
MS is a chronic inflammatory disease of the central nervous system (CNS) associated with demyelination that is thought to have an underlying autoimmune etiology. Currently in the USA, around 250,000–300,000 people have been diagnosed with MS and there are 200 new cases diagnosed every week. Although there are varieties of immune-based therapeutic drugs available for the treatment of MS, it is difficult for clinicians to predict which drugs would work best for an individual patient due to a lack of mechanistic information of the disease (15). Also, major MS drugs target broad ranges of immune cells, which significantly affect leukocyte trafficking and function. Therefore, it is important to identify biomarkers and/or cellular regulators specifically modulating function of autoimmune-reactive leukocytes.
Although the etiology of MS has not been identified, increasing evidence indicates that disease onset involves the combined influence of environmental factors and genetic susceptibility (16). GPCR signaling plays an important role in various aspects of MS pathogenesis including : antigen presentation, cytokine/chemokine production, and T-cell differentiation, proliferation, and invasion (see review in (17)). RGS family proteins that are important modulators of GPCR signaling pathways are recently implicated in the development of MS and other autoimmune diseases. Multiple points of genetic evidences have shown the following: (1) single nucleotide polymorphisms (SNPs) of RGS1, RGS7, RGS9, and RGS14 are reported to be of high correlation with the diagnosis of MS, Crohn’s disease, and ulcerative colitis (18–22), and (2) the messenger RNA (mRNA) level of RGS10 and RGS1 is higher in peripheral blood mononuclear cells (PBMCs) from patients with MS according to the Gene Expression Omnibus (GEO) profile database (23, 24). However, the role of RGS proteins in the context of the onset or progression of autoimmune diseases is yet to be explored.
RGS1 AND MS
RGS1 is a novel MS susceptibility locus as recently identified by the International Multiple Sclerosis Genetics Consortium (IMSGC) (18). A search of the GEO profile database (25) revealed that levels of RGS1 gene expression are higher in MS patients (Fig. 1) and are induced in response to interferon (IFN)-γ therapy in early treatment on day 1 (NCBI GEO database, accession number GDS2419). Tran et al. reported that IFN-γ induced RGS1 mRNA and protein expression in PBMC from human MS patients as early as 4 h after treatment (26). However, the role of RGS1 in the onset or progression of MS has never been explored.
Fig. 1.
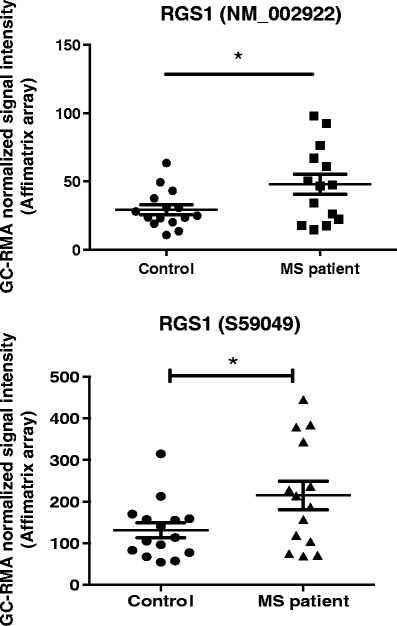
RGS1 mRNA expression level is higher in peripheral blood mononuclear cells (PBMCs) of MS patients. Microarray experiments identify genes and pathways involved in MS pathogenesis (data accessible at the NCBI GEO database, accession number GSE21942). PBMCs were isolated from the whole blood from 12 MS patients and 15 controls, and total RNA was extracted (22). . GC-RMA refers Guanine Cytosine Robust Multi-Array Analysis. *p<0.05, Student's t test.
RGS1 is expressed in lymphocytes, dendritic cells, monocytes (27), and microglia (28). In B lymphocytes, RGS1 impairs Gαi signaling responses (29) and its silencing enhances responsiveness to chemokines such as chemokine (C-X-C motif) ligand (CXCL)12 and CXCL13 and impairs desensitization (30). RGS1 overexpression inhibits T cell migration in response to chemokines that control lymphoid homing, while depletion of RGS1 selectively enhances such chemotaxis. These chemokines include chemokine (C-C motif) ligand 19 (CCL19) and CXCL12 in gut T cells (31). CCL19 is mediated by chemokine (C-C motif) receptor 7 (CCR7), and CXCL12 is mediated by chemokine (C-X-C motif) receptor 4 (CXCR4) (31) which both promote T cell egress from tissues to lymph nodes. The high level of RGS1 in human T cells in the gut especially in inflammatory bowel disease (IBD) suggests that RGS1 may contribute to the colitogenic potential of T cells via limiting gut T cell response to chemokines (31).
It has been shown that chemokine receptor signaling plays an important role in disease progression in mouse experimental autoimmune encephalitis (EAE), the most commonly used murine model of MS. The key chemokines that are overproduced at the site of active MS lesions are CXCL10, CCL3, CCL4, CCL2, CCL8, and CCL5 (32) all of which are important for lymphocyte recruitment to the CNS. It will be particularly interesting to explore the role of the microglial response to chemokines that become elevated in MS. Microglia are the myeloid-derived brain-resident macrophages of the CNS that are responsible for immune surveillance and have been proposed to have regulatory and effector functions in disease progression during MS (33). Microglia activation has been shown to correlate with demyelination and occurs in early pre-demyelinating lesions (34–36). Ablation of microglia suppresses onset and severity of EAE (37–39). However, it has been difficult to determine the role of microglia in the onset and progression of MS in part because they are also likely to play a beneficial role in the termination of the inflammatory response (40). Microglia express various chemokine receptors including those present in active human MS lesions such as CCR1, CCR2, CCR3, CCR5, and CCR8 (41). Chemokines regulate leukocyte trafficking during inflammatory responses. In addition to their role in immune cell trafficking, chemokines are known to have other functional roles including induction of phagocytosis, modulation of cell adhesion, cytokine activation, cell activation, and apoptosis (42). It has been shown that chemokine receptor signaling plays an important role in mouse EAE progression. For example, it has been shown that blockade or genetic deletion of CCR2 (which is involved in monocyte and T cell trafficking) prevents severe disease and can lead to faster remission in the mouse EAE model (43). Many of the RGS proteins have been shown to desensitize (limit the signal of) a variety of chemotactic receptors that are G-protein-coupled receptors. Given that RGS1 is the most abundant RGS protein in microglia, it may also play a role in microglia chemotaxis which is yet to be addressed.
RGS10 AND MS
RGS10 is a 20-kDa protein that selectively accelerates the GTPase activity of Gαi3, Gαq, and Gαz (44). RGS10 belongs to the R12 subfamily and is highly expressed in the brain, thymus, and lymph nodes (3, 44–46). Burgon et al. demonstrated that RGS10 can be phosphorylated at Ser168 by the cAMP-dependent protein kinase A (PKA), which is required for its nuclear translocation (47), implicating a non-canonical role of RGS10. RGS10 is also one of the most highly expressed RGS proteins in mouse lymphocytes (14). Garcia-Bernal et al. reported that RGS10 modulates CXCL12-induced T cell adhesion through integrin α4β1 (48). They showed that RGS10 could function as an inhibitor of CXCL12-induced T cell adhesion, which is mediated by α4β1 and αLβ2 (48). Integrin α4 is known as a critical molecule for the homing of lymphocytes to the CNS (49). Currently, a humanized antibody that blocks integrin α4, natalizumab, has been approved for treatment of MS (50). These suggest a potential role of RGS10 in lymphocyte adhesion and/or migration. Interestingly, the mRNA level of RGS10 is higher in PBMC from patients with MS (NCBI GEO database, accession number GDS2419) (23), Crohn’s disease, and ulcerative colitis (NCBI GEO database, accession number GDS1615) (24).
The pathogenesis of MS is far more complicated than it had been predicted from early studies of the EAE model system. However, it is now well documented from studies of human MS patients as well as animal models that autoimmune T cells mediate the initial stages of MS lesions similar to the EAE model (51–53). From our arrays of experiments, we showed that RGS10-null mice immunized with myelin oligodendrocyte glycoprotein peptide fragment 35–55 (MOG35–55) displayed significantly milder clinical EAE in the acute phase and remained mild all throughout the progressive phases of the disease (54). Moreover, they displayed significantly fewer incidences of EAE with a delayed onset and a lower mean maximum score (54). These data suggest the role for RGS10 in augmenting instead of attenuating autoimmune response through autoreactive Th1 response. In-depth investigations are needed to establish how RGS10 mediates lymphocyte effector function to augment autoimmune responses. If RGS10 plays a role in specific subsets of autoreactive lymphocytes during EAE, this may enable the targeting of RGS10 in disease-modifying strategies.
RGS10 IN NEUROINFLAMMATION AND PARKINSON’S DISEASE
In brain-resident microglia, RGS10 has been shown to play a critical role in inflammatory microglial activation via negative regulation of NF-kB signaling. In dopaminergic neuroblastoma cells, RGS10 modulates sensitivity to inflammation-induced cell survival by interaction with the PKA/CREB pathway (55–57). Chronic peripheral administration of lipopolysaccharide (LPS) in RGS10-null mice results in chronic microgliosis and loss of dopaminergic (DA) neurons (57). RGS10-null mice displayed increased microglial burden in the CNS and dysregulated inflammation-related gene expression in microglia as well as nigral DA neuron degeneration with repeated systemic administration of low-dose LPS injection. Moreover, RGS10-null microglia produced significantly higher levels of proinflammatory cytokines including TNF, IL-1β, and IL-6 than wild-type (WT) microglia upon LPS treatment. Another study showed that RGS10-null mice exhibited impaired osteoclast differentiation due to the absence of RGS10-dependent calcium current oscillations and the loss of nuclear factor of activated T cell c1 (NFATc1) expression (58). This suggests that RGS10 may regulate intracellular calcium oscillations in microglia. The primary function of RGS proteins is believed to be the regulation of heterotrimeric G-protein signaling at the plasma membrane. However, our findings as well as those of others (47, 59, 60) reveal that RGS proteins translocate to the nucleus and are found at high abundance at other intracellular sites. Given that the cellular distribution of RGS10 is both nuclear and cytoplasmic (59), it would be interesting to study the possible function of RGS10 in regulating microglial stress responses through mechanisms that include changes in gene transcription and cellular calcium regulation in addition to its GTPase-accelerating protein (GAP) activity at the plasma membrane. A better understanding of the normal function of RGS10 in the CNS along with proof-of-concept studies to demonstrate that manipulation of RGS10 levels and/or its activity in the ventral midbrain has positive effects on DA neuron survival without exerting unwanted effects on other cell types may reveal its potential as a therapeutic target for blocking or delaying the progressive loss of nigrostriatal DA neurons in PD.
In conclusion, it is possible that an increase in the RGS activity, especially RGS10, would be beneficial in PD-associated neuroinflammation but detrimental in the treatment of MS. However, identifying novel modulators and their mechanism will open up the possibility for the development of new mechanism-based therapeutic interventions for the treatment of neurodegenerative diseases.
REFERENCES
- 1.Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296(5573):1636–9. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 2.Berman DM, Wilkie TM, Gilman AG. GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein alpha subunits. Cell. 1996;86(3):445–52. doi: 10.1016/S0092-8674(00)80117-8. [DOI] [PubMed] [Google Scholar]
- 3.Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- 4.Siderovski DP, Diverse-Pierluissi M, De Vries L. The GoLoco motif: a Galphai/o binding motif and potential guanine-nucleotide exchange factor. Trends Biochem Sci. 1999;24(9):340–1. doi: 10.1016/S0968-0004(99)01441-3. [DOI] [PubMed] [Google Scholar]
- 5.Zheng B, De Vries L, Gist Farquhar M. Divergence of RGS proteins: evidence for the existence of six mammalian RGS subfamilies. Trends Biochem Sci. 1999;24(11):411–4. doi: 10.1016/S0968-0004(99)01474-7. [DOI] [PubMed] [Google Scholar]
- 6.Willars GB. Mammalian RGS, proteins: multifunctional regulators of cellular signalling. Semin Cell Dev Biol. 2006;17(3):363–76. doi: 10.1016/j.semcdb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Abramow-Newerly M, Roy AA, Nunn C, Chidiac P. RGS proteins have a signalling complex: interactions between RGS proteins and GPCRs, effectors, and auxiliary proteins. Cell Signal. 2006;18(5):579–91. doi: 10.1016/j.cellsig.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Hollinger S, Hepler JR. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev. 2002;54(3):527–59. doi: 10.1124/pr.54.3.527. [DOI] [PubMed] [Google Scholar]
- 9.Kach J, Sethakorn N, Dulin NO. A finer tuning of G-protein signaling through regulated control of RGS proteins. Am J Physiol Heart Circ Physiol. 2012;303(1):H19–35. doi: 10.1152/ajpheart.00764.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sethakorn N, Yau DM, Dulin NO. Non-canonical functions of RGS proteins. Cell Signal. 2010;22(9):1274–81. doi: 10.1016/j.cellsig.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dohlman HG, Thorner J. RGS proteins and signaling by heterotrimeric G proteins. J Biol Chem. 1997;272(7):3871–4. doi: 10.1074/jbc.272.7.3871. [DOI] [PubMed] [Google Scholar]
- 12.Koelle MR. A new family of G-protein regulators—the RGS proteins. Curr Opin Cell Biol. 1997;9(2):143–7. doi: 10.1016/S0955-0674(97)80055-5. [DOI] [PubMed] [Google Scholar]
- 13.Larminie C, Murdock P, Walhin JP, Duckworth M, Blumer KJ, Scheideler MA, et al. Selective expression of regulators of G-protein signaling (RGS) in the human central nervous system. Brain Res Mol Brain Res. 2004;122(1):24–34. doi: 10.1016/j.molbrainres.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Moratz C, Harrison K, Kehrl JH. Regulation of chemokine-induced lymphocyte migration by RGS proteins. Methods Enzymol. 2004;389:15–32. doi: 10.1016/S0076-6879(04)89002-5. [DOI] [PubMed] [Google Scholar]
- 15.Steinman L, Merrill JT, McInnes IB, Peakman M. Optimization of current and future therapy for autoimmune diseases. Nat Med. 2012;18(1):59–65. doi: 10.1038/nm.2625. [DOI] [PubMed] [Google Scholar]
- 16.Handel AE, Handunnetthi L, Giovannoni G, Ebers GC, Ramagopalan SV. Genetic and environmental factors and the distribution of multiple sclerosis in Europe. Eur J Neurol. 2010;17(9):1210–4. doi: 10.1111/j.1468-1331.2010.03003.x. [DOI] [PubMed] [Google Scholar]
- 17.Du C, Xie X. G protein-coupled receptors as therapeutic targets for multiple sclerosis. Cell Res. 2012;22(7):1108–28. [DOI] [PMC free article] [PubMed]
- 18.International Multiple Sclerosis Genetics Consortium (IMSGC) IL12A, MPHOSPH9/CDK2AP1 and RGS1 are novel multiple sclerosis susceptibility loci. Genes Immun. 2010;11(5):397–405. doi: 10.1038/gene.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gourraud PA. When is the absence of evidence, evidence of absence? Use of equivalence-based analyses in genetic epidemiology and a conclusion for the KIF1B rs10492972*C allelic association in multiple sclerosis. Genet Epidemiol. 2011;35(6):568–71. doi: 10.1002/gepi.20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunt KA, Zhernakova A, Turner G, Heap GA, Franke L, Bruinenberg M, et al. Newly identified genetic risk variants for celiac disease related to the immune response. Nat Genet. 2008;40(4):395–402. doi: 10.1038/ng.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smyth DJ, Plagnol V, Walker NM, Cooper JD, Downes K, Yang JH, et al. Shared and distinct genetic variants in type 1 diabetes and celiac disease. N Engl J Med. 2008;359(26):2767–77. doi: 10.1056/NEJMoa0807917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson BA, Wang J, Taylor EM, Caillier SJ, Herbert J, Khan OA, et al. Multiple sclerosis susceptibility alleles in African Americans. Genes Immun. 2010;11(4):343–50. doi: 10.1038/gene.2009.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kemppinen AK, Kaprio J, Palotie A, Saarela J. Systematic review of genome-wide expression studies in multiple sclerosis. BMJ Open. 2011;1(1) doi: 10.1136/bmjopen-2011-000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burczynski ME, Peterson RL, Twine NC, Zuberek KA, Brodeur BJ, Casciotti L, et al. Molecular classification of Crohn’s disease and ulcerative colitis patients using transcriptional profiles in peripheral blood mononuclear cells. J Mol Diagn. 2006;8(1):51–61. doi: 10.2353/jmoldx.2006.050079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh MK, Scott TF, LaFramboise WA, Hu FZ, Post JC, Ehrlich GD. Gene expression changes in peripheral blood mononuclear cells from multiple sclerosis patients undergoing beta-interferon therapy. J Neurol Sci. 2007;258(1–2):52–9. doi: 10.1016/j.jns.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 26.Tran T, Paz P, Velichko S, Cifrese J, Belur P, Yamaguchi KD, et al. Interferonβ-1b induces the expression of RGS1 a negative regulator of G-protein signaling. Int J Cell Biol. 2010;2010:529376. doi: 10.1155/2010/529376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bansal G, Druey KM, Xie Z. R4 RGS proteins: regulation of G-protein signaling and beyond. Pharmacol Ther. 2007;116(3):473–95. doi: 10.1016/j.pharmthera.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atwood BK, Lopez J, Wager-Miller J, Mackie K, Straiker A. Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genomics. 2011;12:14. doi: 10.1186/1471-2164-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moratz C, Kang VH, Druey KM, Shi CS, Scheschonka A, Murphy PM, et al. Regulator of G protein signaling 1 (RGS1) markedly impairs Gi alpha signaling responses of B lymphocytes. J Immunol. 2000;164(4):1829–38. doi: 10.4049/jimmunol.164.4.1829. [DOI] [PubMed] [Google Scholar]
- 30.Han JI, Huang NN, Kim DU, Kehrl JH. RGS1 and RGS13 mRNA silencing in a human B lymphoma line enhances responsiveness to chemoattractants and impairs desensitization. J Leukoc Biol. 2006;79(6):1357–68. doi: 10.1189/jlb.1105693. [DOI] [PubMed] [Google Scholar]
- 31.Gibbons DL, Abeler-Dorner L, Raine T, Hwang IY, Jandke A, Wencker M, et al. Cutting edge: regulator of G protein signaling-1 selectively regulates gut T cell trafficking and colitic potential. J Immunol. 2011;187(5):2067–71. doi: 10.4049/jimmunol.1100833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005;48(1):16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 33.Sanders P, De Keyser J. Janus faces of microglia in multiple sclerosis. Brain Res Rev. 2007;54(2):274–85. doi: 10.1016/j.brainresrev.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Marik C, Felts PA, Bauer J, Lassmann H, Smith KJ. Lesion genesis in a subset of patients with multiple sclerosis: a role for innate immunity? Brain. 2007;130(Pt 11):2800–15. doi: 10.1093/brain/awm236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol. 2004;55(4):458–68. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- 36.Vos CM, Geurts JJ, Montagne L, van Haastert ES, Bo L, van der Valk P, et al. Blood-brain barrier alterations in both focal and diffuse abnormalities on postmortem MRI in multiple sclerosis. Neurobiol Dis. 2005;20(3):953–60. doi: 10.1016/j.nbd.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 37.Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hovelmeyer N, et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med. 2005;11(2):146–52. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- 38.Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11(3):328–34. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 39.McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11(3):335–9. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 40.Chan A, Seguin R, Magnus T, Papadimitriou C, Toyka KV, Antel JP, et al. Phagocytosis of apoptotic inflammatory cells by microglia and its therapeutic implications: termination of CNS autoimmune inflammation and modulation by interferon-beta. Glia. 2003;43(3):231–42. doi: 10.1002/glia.10258. [DOI] [PubMed] [Google Scholar]
- 41.Balashov KE, Rottman JB, Weiner HL, Hancock WW. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci U S A. 1999;96(12):6873–8. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matejuk A, Dwyer J, Ito A, Bruender Z, Vandenbark AA, Offner H. Effects of cytokine deficiency on chemokine expression in CNS of mice with EAE. J Neurosci Res. 2002;67(5):680–8. doi: 10.1002/jnr.10156. [DOI] [PubMed] [Google Scholar]
- 43.Gaupp S, Pitt D, Kuziel WA, Cannella B, Raine CS. Experimental autoimmune encephalomyelitis (EAE) in CCR2(−/−) mice: susceptibility in multiple strains. Am J Pathol. 2003;162(1):139–50. doi: 10.1016/S0002-9440(10)63805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunt TW, Fields TA, Casey PJ, Peralta EG. RGS10 is a selective activator of G alpha i GTPase activity. Nature. 1996;383(6596):175–7. doi: 10.1038/383175a0. [DOI] [PubMed] [Google Scholar]
- 45.Gold SJ, Ni YG, Dohlman HG, Nestler EJ. Regulators of G-protein signaling (RGS) proteins: region-specific expression of nine subtypes in rat brain. J Neurosci. 1997;17(20):8024–37. doi: 10.1523/JNEUROSCI.17-20-08024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sierra DA, Gilbert DJ, Householder D, Grishin NV, Yu K, Ukidwe P, et al. Evolution of the regulators of G-protein signaling multigene family in mouse and human. Genomics. 2002;79(2):177–85. doi: 10.1006/geno.2002.6693. [DOI] [PubMed] [Google Scholar]
- 47.Burgon PG, Lee WL, Nixon AB, Peralta EG, Casey PJ. Phosphorylation and nuclear translocation of a regulator of G protein signaling (RGS10) J Biol Chem. 2001;276(35):32828–34. doi: 10.1074/jbc.M100960200. [DOI] [PubMed] [Google Scholar]
- 48.Garcia-Bernal D, Dios-Esponera A, Sotillo-Mallo E, Garcia-Verdugo R, Arellano-Sanchez N, Teixido J. RGS10 restricts upregulation by chemokines of T cell adhesion mediated by α4β1 and αLβ2 integrins. J Immunol. 2011;187(3):1264–72. doi: 10.4049/jimmunol.1002960. [DOI] [PubMed] [Google Scholar]
- 49.Yednock TA, Butcher EC, Stoolman LM, Rosen SD. Receptors involved in lymphocyte homing: relationship between a carbohydrate-binding receptor and the MEL-14 antigen. J Cell Biol. 1987;104(3):725–31. doi: 10.1083/jcb.104.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 51.Hartung HP, Gonsette R, Konig N, Kwiecinski H, Guseo A, Morrissey SP, et al. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360(9350):2018–25. doi: 10.1016/S0140-6736(02)12023-X. [DOI] [PubMed] [Google Scholar]
- 52.McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007;8(9):913–9. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 53.Coles AJ, Wing MG, Molyneux P, Paolillo A, Davie CM, Hale G, et al. Monoclonal antibody treatment exposes three mechanisms underlying the clinical course of multiple sclerosis. Ann Neurol. 1999;46(3):296–304. doi: 10.1002/1531-8249(199909)46:3<296::AID-ANA4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 54.Lee JK, Kannarkat GT, Chung J, Lee HJ, Graham KL, Tansey MG. RGS10 deficiency ameliorates the severity of disease in experimental autoimmune encephalomyelitis. J Neuroinflammation. 2016;13(1):24. [DOI] [PMC free article] [PubMed]
- 55.Lee JK, Chung J, Druey KM, Tansey MG. RGS10 exerts a neuroprotective role through the PKA/c-AMP response-element (CREB) pathway in dopaminergic neuron-like cells. J Neurochem. 2012;122(2):333–43. doi: 10.1111/j.1471-4159.2012.07780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee JK, Chung J, McAlpine FE, Tansey MG. Regulator of G-protein signaling-10 negatively regulates NF-{kappa}B in microglia and neuroprotects dopaminergic neurons in hemiparkinsonian rats. J Neurosci. 2011;31(33):11879–88. doi: 10.1523/JNEUROSCI.1002-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee JK, McCoy MK, Harms AS, Ruhn KA, Gold SJ, Tansey MG. Regulator of G-protein signaling 10 promotes dopaminergic neuron survival via regulation of the microglial inflammatory response. J Neurosci. 2008;28(34):8517–28. doi: 10.1523/JNEUROSCI.1806-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang S, Li YP. RGS10-null mutation impairs osteoclast differentiation resulting from the loss of [Ca2+]i oscillation regulation. Genes Dev. 2007;21(14):1803–16. doi: 10.1101/gad.1544107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chatterjee TK, Fisher RA. Cytoplasmic, nuclear, and Golgi localization of RGS proteins. Evidence for N-terminal and RGS domain sequences as intracellular targeting motifs. J Biol Chem. 2000;275(31):24013–21. doi: 10.1074/jbc.M002082200. [DOI] [PubMed] [Google Scholar]
- 60.Waugh JL, Lou AC, Eisch AJ, Monteggia LM, Muly EC, Gold SJ. Regional, cellular, and subcellular localization of RGS10 in rodent brain. J Comp Neurol. 2005;481(3):299–313. doi: 10.1002/cne.20372. [DOI] [PubMed] [Google Scholar]


