Abstract
One approach in wound dressing development is to incorporate active molecules or drugs in the dressing. In order to reduce the frequency of dressing changes as well as to prolong wound healing efficacy, wound dressings that can sustain the release of the active molecules should be developed. In our previous work, we developed chitosan/sericin (CH/SS) microspheres that released sericin in a controlled rate. However, the difficulty of applying the microspheres that easily diffuse and quickly degrade onto the wound was its limitations. In this study, we aimed to develop wound dressing materials which are easier to apply and to provide extended release of sericin. Different amounts of CH/SS microspheres were embedded into various compositions of polyvinyl alcohol/gelatin (PVA/G) scaffolds and fabricated using freeze-drying and glutaraldehyde crosslinking techniques. The obtained CH/SS microspheres-embedded scaffolds with appropriate design and formulation were introduced as a wound dressing material. Sericin was released from the microspheres and the scaffolds in a sustained manner. Furthermore, an optimized formation of the microspheres-embedded scaffolds (2PVA2G+2CHSS) was shown to possess an effective antimicrobial activity against both gram-positive and gram-negative bacteria. These microspheres-embedded scaffolds were not toxic to L929 mouse fibroblast cells, and they did not irritate the tissue when applied to the wound. Finally, probably by the sustained release of sericin, these microspheres-embedded scaffolds could promote wound healing as well as or slightly better than a clinically used wound dressing (Allevyn®) in a mouse model. The antimicrobial CH/SS microspheres-embedded PVA/G scaffolds with sustained release of sericin would appear to be a promising candidate for wound dressing application.
KEY WORDS: chitosan, microsphere, sericin, sustained release, wound dressing
INTRODUCTION
In wound healing, appropriate formulation design is necessary not only for drug delivery but also for accelerating the healing process and prolonging antimicrobial activity. Recently, various kinds of wound dressing materials have been developed to address the problems of delayed healing, excessive inflammation, and scar formation. The design of wound dressing should take into account the following goals: (1) provides mechanical stability and conformability for convenient applicability, (2) provides a suitable environment such as moisture and pH for cell proliferation and tissue formation, (3) provides high porosity to allow the absorption and penetration of wound exudate and oxygen over the wound, and (4) encompasses non-adhesive property to minimize damage to the wound surface upon removal. Furthermore, to reduce the frequency of dressing changes, the wound dressing should prolong the release and enhance the permeability of the active molecules or drugs (wound healing accelerating component) that have been incorporated (1,2). Further, the wound dressing should be fabricated from biocompatible, biological active, non-toxic, and non-irritation materials. The combination of natural-derived or synthetic polymers is often introduced to produce novel compositions that improve the physical and biological properties of the wound dressing.
In this research, the naturally derived gelatin (G) and synthetic polyvinyl alcohol (PVA) were selected to fabricate the wound dressing scaffold. Gelatin is obtained from a naturally abundant collagen by hydrolysis. It serves as an important extracellular matrix component which provides inherent biocompatibility, biodegradability, non-immunogenicity, and promoting cell attachment and proliferation. In addition, gelatin contains natural components such as amino acids which can promote cellular activities, foster hemostasis, and facilitate adhesion and proliferation of skin cells during wound healing (3). Although gelatin scaffolds have been clinically used as a wound dressing, they possess insufficient flexibility and are rapidly degraded by bacteria during the application period. The properties of gelatin can be improved by cross-linking and/or blending with other polymers (4). Polyvinyl alcohol is one of the most widely used synthetic polymers due to its biocompatibility, biodegradability, and high thermal stability while inducing minimal inflammation. Polyvinyl alcohol scaffolds have been applied in various biomedical applications such as wound dressing, implant materials, and artificial organs (5,6). However, PVA scaffolds lack the inherent bioactive properties. Herein, we introduce the blending of gelatin and PVA to form a scaffold with enhanced physical and biological properties. Gelatin provides the biological activities to the scaffold while PVA would improve the mechanical properties and stability of the scaffold. In this work, the blending compositions of PVA and gelatin were optimized to investigate the properties as wound dressing materials.
As a potential wound healing accelerating component, sericin (SS), a glue-like protein that envelops the fibroin fibers of Bombyx mori silkworm, has become of interest in wound dressing applications due to its non-toxicity, non-immunogenicity, antioxidant action, moisture regulating ability, UV resistance, anti-bacterial, and anti-cancer properties (7–9). Sericin is also found to promote the activities of keratinocytes and primary cultured human fibroblasts as well as to enhance epithelialization and collagen formation (10–14). We have previously reported the accelerated wound healing rate in both animal and clinical trials by the application of various kinds of wound dressing releasing sericin, e.g., sericin/PVA scaffold and sericin-incorporated silk fibroin/gelatin scaffold (15–18).
In our recent study, the new formulation of sericin-releasing material, the ionic-crosslinked chitosan/sericin (CH/SS) microspheres, was developed (19). The CH/SS microspheres could encapsulate sericin at the high percentage and release sericin in a controlled rate while it shows no toxicity to L929 mouse fibroblast cells. Chitosan is also known for its antimicrobial activity (20,21) which will be another benefit of these microspheres. However, the application of microspheres to the wound bed was complicated because the microspheres can be diffused easily, resulting in the non-uniform dispersion over the wound surface. This study introduces the embedding of these microspheres into the PVA/G matrix scaffold for the easier applicability as a wound dressing material and for extending the sericin release rate. The amount of microspheres incorporated in the scaffold was varied to obtain different profiles of sericin release. The morphology, water absorption ability, in vitro degradation rate, in vitro release of sericin, in vitro cytotoxicity, attachment, and proliferation of L929 mouse fibroblast cells on the scaffolds were evaluated. Furthermore, the antimicrobial activity of the scaffolds against gram-positive and gram-negative bacteria was tested. Finally, the scaffold was applied to the full-thickness wound of Wistar rats to evaluate the wound healing in terms of inflammatory response (tissue irritation), the production of collagen, epithelialization, and the reduction of the wound area. We hypothesized that our new design of CH/SS-embedded PVA/G scaffold would show improved stability and prolong the release of sericin. If confirmed, the combination of the four chosen components (PVA, gelatin, sericin, and chitosan) in which each component exerts its contributing functions would become a novel formulation for wound dressing.
MATERIALS AND METHODS
Materials
Fresh bivoltine white-shell cocoons of B. mori produced in a controlled environment were kindly supplied by Chul Thai Silk Co., Ltd. (Petchaboon province, Thailand). SS was extracted using a high temperature and pressure degumming method, as described previously (13). The molecular weight of sericin obtained ranged from 25 to 150 kDa. Low molecular weight chitosan (CH, molecular weight ~ 5000 Da) was purchased from Shenzhen Naturactive Inc., China (CAS number 9012-76-4). A gelatin (G) sample prepared by acidic treatment of porcine skin collagen (isoelectric point (IEP) = 9.0) was kindly supplied by Nitta Gelatin Inc., Osaka, Japan. Polyvinyl alcohol (PVA, MW 77,000-82,000) was purchased from Ajax Finechem (New South Wales, Australia). Sodium tripolyphosphate (TPP), glutaraldehyde (GA), and other chemicals were purchased from Sigma-Aldrich Co. Ltd. (USA) and used without further purification.
Fabrication of CH/SS Microspheres
The CH/SS microspheres were fabricated according to the method reported previously (19). Briefly, chitosan solution was mixed with sericin solution at a CH/SS blending ratio of 80/20 to obtain the final solution concentration of 6 wt%. The CH/SS mixture was stirred at room temperature for 1 h. Then, 40 mL of TPP solution (1 wt%, pH 6.5) was slowly dropped into the CH/SS mixture and stirred at room temperature for 1 h. The CH/SS microspheres were collected by centrifugation at 3486 g for 5 min and washed repeatedly with deionized (DI) water prior to freeze-drying. The dried CH/SS microspheres were obtained.
Fabrication of CH/SS Microspheres-Embedded PVA/G Scaffolds
Polyvinyl alcohol and gelatin solutions were separately prepared in DI water. The PVA solution was mixed with gelatin solution at different PVA/G blending compositions of 0.5/3.5, 1/3, 1.5/2.5, and 2/2 to obtain the final concentration of 4 wt%. Then, the CH/SS microspheres (2 and 4 wt%) were blended with the PVA/G mixture solution and rigorously stirred at 40°C for 1 h. Glutaraldehyde solution (0.2 wt%) was added into the above mixture and mixed for 5 min under darkness. The mixture solution was immediately cast into mold, incubated at 4°C overnight to obtain the solidified gel. The gel was washed repeatedly with glycine solution and water to remove the residual aldehyde groups prior to the freezing at −40°C and lyophilization for 3 days. The various compositions of PVA/G scaffolds incorporated different amounts of CH/SS microspheres were obtained, as summarized in Table I.
Table I.
Formulations of CH/SS Microspheres-Embedded PVA/G Scaffolds
| Nomenclature | Final concentration of PVA/G mixture | PVA ratio | Gelatin ratio | Final concentration of CH/SS microspheres |
|---|---|---|---|---|
| 0.5PVA3.5G+2CH/SS | 4 wt% | 0.5 | 3.5 | 2 wt% |
| 1PVA3G+2CH/SS | 1.0 | 3.0 | 2 wt% | |
| 1.5PVA2.5G+2CH/SS | 1.5 | 2.5 | 2 wt% | |
| 2PVA2G+2CH/SS | 2.0 | 2.0 | 2 wt% | |
| 0.5PVA3.5G+4CH/SS | 0.5 | 3.5 | 4 wt% | |
| 1PVA3G+4CH/SS | 1.0 | 3.0 | 4 wt% | |
| 1.5PVA2.5G+4CH/SS | 1.5 | 2.5 | 4 wt% | |
| 2PVA2G+4CH/SS | 2.0 | 2.0 | 4 wt% |
Morphological Observation
Cross-sectioned morphology and the distribution of microspheres on the scaffolds were observed on a scanning electron microscope (SEM, JSM 5400, JEOL) at an accelerating voltage of 12–15 kV after sputter-coating with gold.
Water Absorption Test
The lyophilized scaffolds were weighed and then immersed in phosphate-buffered saline solution (PBS, pH 7.4) at 37°C for 1, 2, 4, 6, and 24 h. The water-absorbed scaffolds were carefully wiped with lint-free paper and weighed. The percentage of water absorption of the scaffolds was calculated from the following equation:
where W 0 and W t represent weights of the dry- and water-absorbed scaffold, respectively. The experiment was performed in triplicate (n = 3).
In Vitro Enzymatic Degradation Test
The lyophilized scaffolds were weighed and then subjected to degradation in PBS solution containing collagenase (1 Unit/mL) at 37°C for 1, 3, 5, 7, and 14 days. At each time point, the remained scaffold was collected, washed with DI water, freeze dried, and weighed. The percentage of weight remaining was calculated from the following equation:
where W 0 and W t represent the original and remaining weights of scaffold, respectively. The experiment was performed in triplicate (n = 3).
In Vitro Release Test of Sericin
The CH/SS microspheres-embedded PVA/G scaffolds were placed in 5-mL PBS solution containing collagenase (1 Unit/mL) at 37°C with continuous stirring at 100 rpm in a closed-container. The PBS solution (100 μL) was collected at different time points and replaced with the same volume of fresh PBS. The amount of sericin released into the solution was measured using a BCA protein assay kit (Pierce, Rockford, IL). The absorbance was measured at 562 nm and the amount of sericin released was determined from a standard curve prepared from different concentrations of sericin. The experiment was performed in triplicate (n = 3).
In Vitro Cytotoxic Test
The cytotoxicity of CH/SS microspheres-embedded PVA/G scaffolds was evaluated with L929 mouse fibroblast cells using an indirect method according to ISO 10993-Part 5 (1992). Scaffolds were incubated in Dulbecco’s modified eagle powder medium (DMEM) without fetal bovine serum at 37°C/5% CO2. After 24 h of incubation, the hydrogel’s extract solution was obtained. L929 mouse fibroblast cells (20,000 cells/well) were seeded into 48-well plate and incubated at 37°C/5% CO2 for 24 h to allow for cell attachment. Then the medium was replaced with the hydrogel’s extract solution and incubated for further 24, 48, and 72 h. At each time period, the number of cells was measured using MTT assay (22). The total amount of soluble type I collagen was assayed using the Sircol collagen assay kit (Biocolor, UK). Cells cultured with DMEM and 20 ppm Zn were used as negative and positive controls, respectively.
Antimicrobial Efficacy Test
The antimicrobial efficacy of CH/SS microspheres-embedded PVA/G scaffolds was evaluated by the disc diffusion method (CLSI M2-A9). To enhance the immediate antimicrobial activity, all scaffolds were immersed in 1 wt% chitosan solution in 20 vol% glycerin for 4 h and left to dry for 12 h (23). Six strains of bacteria including Bacillus subtilis (ATCC 6633, gram-positive), Staphylococcus aureus (ATCC 25923, gram-positive), methicillin-resistant S. aureus (MRSA, gram-positive), Escherichia coli (ATCC 25922, gram-negative), Acinetobacter baumannii (ATCC 19606, gram-negative), and Pseudomonas aeruginosa (ATCC 27853, gram-negative) were selected for the test. All bacterial strains were cultured on an agar plate at 37°C for 24 h. The inoculum was prepared by selecting 3–5 isolated colonies of bacteria into 5 mL of Tryptone Soya Broth (TSB) and followed by incubation at 37°C for 4–6 h. The content of bacteria was determined by a UV/VIS spectrometer (Lambda 25, Perkin Elmer, Waltham, MA, USA) at 625 nm. Then, one swab was applied on the surface of the agar plate. The gamma-irradiated scaffolds (1 × 1 × 0.1 cm3) was placed on the agar plate and incubated at 37°C for 24 h. After incubation, the size of the inhibition zone was measured. Acticoat® (highly conformable nanocrystalline silver antimicrobial barrier dressing, Smith & Nephew Healthcare Ltd., Hull, United Kingdom) and gauze pads were used as control materials of the test.
In Vivo Study of Full-Thickness Wound Model
Animals
The animals used in this study were approved by the Mahidol University Animal Care and Use Committee (MU-ACUC), Bangkok, Thailand. All experimental procedures were carried out in compliance with the Guide for the Care and Use of Laboratory Animals, 1996 and implemented by the National Laboratory Animal Center of Mahidol University, Bangkok Thailand. Eight-week-old male Wistar rats (weight 250 ± 5 g) purchased from National Laboratory Animal Centre, Mahidol University, NakhonPathom, Thailand were used for the experiment. The rats were fed with a standard diet and housed individually under controlled temperature (22–23°C).
Full-Thickness Wound Model
The rats were anesthetized and injected with 10% w/v povidone iodine. The full-thickness wounds (1.5 × 1.5 cm) were created on both left and right sides of their back (two wounds/rat). The gamma-irradiated CH/SS microspheres-embedded PVA/G scaffolds and the commercial wound dressing Allevyn® (Hydrophilic adhesive polyurethane foam dressing, Smith & Nephew Healthcare Ltd., Hull, United Kingdom) as a control dressing were randomly applied to the left- and right-side wounds. The wounds were wrapped with medical tape (3 M Corporate Headquarters, Minnesota, USA) to keep the scaffolds in place and to secure the dressings. Carprofen (5 mg/kg body weight) was subcutaneously injected into all rats once daily for 5 days post-operatively to reduce pain.
Histological Staining
At 3, 7, 14, and 21 days post-operatively, the rats were sacrificed and the wound bed tissue was collected for histological examination. The tissue was fixed in 10 vol% neutral buffered formalin at room temperature for 48 h. The fixed tissues were dehydrated with standard tissue processing. Each tissue sample was embedded in a paraffin block and 5-μm sections were prepared. At least three sections were randomly taken from each tissue sample. The tissue sections were then mounted on glass slides for Hematoxylin-Eosin (H&E) staining.
Evaluation of Tissue Irritation
The number of inflammatory cells (polymorphonuclear neutrophils (PMN), lymphocyte, macrophage, and mast cells), vascularization, and fatty infiltration were evaluated from the H&E images of each sample. The semi-quantitative analysis was performed by a blinded investigator according to ISO 10993–6: Biological evaluation of medical devices.
Evaluation of Epithelialization
The analysis was performed on H&E images of 640 × 480 pixel resolution that acquired a light microscope (BX51, Olympus®), a stereoscope (Stemi 2000-C, ZEISS®), and a digital camera (Moticam 1000, Moticam®) running under an imaging analysis program (ImageJ, NIH). Each set of measurement was standardized by calibrated scale of the image analysis program. The distance between both sides of epithelial tips and the length of epithelial tongue were measured.
Measurement of Wound Size Reduction
Size of wounds was measured immediately after operation and at 3, 7, 14, and 21 days post-operatively using a stereomicroscope (1024 × 768 pixels). The percentage of wound size reduction was calculated (n = 6).
Evaluation of Collagen Formation
Histomorphometric study was performed to measure collagen formation in each period of post wounding. The tissue sections were stained with Masson’s trichrome. From each specimen, ten random fields (at 400×) were examined and color images of 640 × 480 pixel resolution were acquired with a light microscope (BX51, Olympus®) and digital camera (DP20, Olympus®). The area fraction of collagen was semi-quantitatively measured from the acquired images using by ImageJ program, NIH. Briefly, color images were transformed to gray scale and the collagen bundle was located as an interested area via threshold mode. Thus, the area fraction of positive reaction of collagen was automatically determined by the program as the percentage/image.
Statistical Analysis
All quantitative data were shown as mean ± SD. For in vitro characterization, the statistical significance was determined by paired and unpaired Student’s t tests along with ANOVA. For in vivo study, all treatment groups were compared by ANOVA and the differences between groups at different time points were compared by post hoc t test. A value of p < 0.05 was considered to be significant.
RESULTS
Morphology of the Scaffolds
Figure 1 presents the cross-sectional structure of CH/SS microspheres-embedded PVA/G scaffolds. Various formulations of PVA/G scaffolds had different structure, however, the interconnected pore was observed on all scaffolds. The CH/SS microspheres were homogeneously distributed throughout the surface of each scaffold.
Fig. 1.
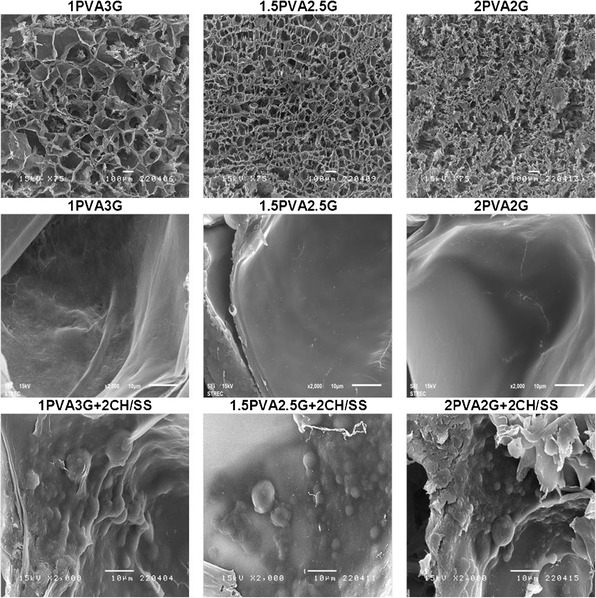
Cross-sectioned and surface structure of the CH/SS microspheres-embedded PVA/G scaffolds prepared from different compositions
Water Absorption Ability of the Scaffolds
The water absorption ability of various CH/SS microspheres-embedded PVA/G scaffolds is shown in Fig. 2a. The water absorption percentage of all scaffolds increased rapidly within the first 4 h and gradually rose thereafter. The percentage seemed to be increased with the increasing ratio of PVA. For the 1.5PVA2.5G and 2PVA2G scaffolds, 800–1000% equilibrium water absorption percentage was reached. On the other hand, the amount of CH/SS microspheres incorporated did not significantly affect the water absorption ability of scaffolds.
Fig. 2.
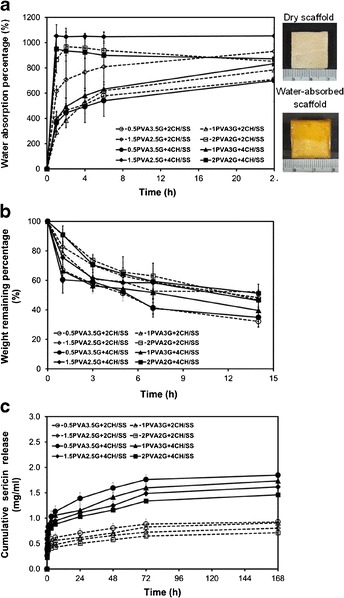
a Water absorption percentage, b Weight remaining percentage, and c Cumulative sericin release of the CH/SS microspheres-embedded PVA/G scaffolds prepared from different compositions
Degradation Rate of the Scaffolds
The degradation profiles of various CH/SS microspheres-embedded PVA/G scaffolds are shown in Fig. 2b. All scaffolds gradually degraded within the study period. The scaffolds with the high ratio of gelatin tended to have the lowest remaining weight (~40%) after 14 h of incubation period. However, no significant difference was detected in the degradation rate among the various scaffolds.
In Vitro Release Profiles of Sericin
Figure 2c shows the profiles of sericin released from various CH/SS microspheres-embedded PVA/G scaffolds. Sericin gradually released from all scaffolds and its release plateaued after 72 h. It seemed that the release of sericin depended mainly on the amount of CH/SS microspheres incorporated. The scaffolds incorporated with 4 wt% microspheres released sericin at the significantly higher extent than those incorporated with 2 wt% microspheres. Among various scaffolds incorporated the same amount of microspheres, it appeared that the scaffolds with higher gelatin content released more amount of sericin. However, the difference was not significant.
In Vitro Cytotoxicity of the Scaffolds
Figure 3a presents the percentage of L929 cell viability when cultured in various scaffolds’ medium extracts for 1 day. The cells cultured in all scaffolds’ extracts showed around 100% viability, as that cultured in DMEM. On the other hand, the viability of cells cultured in medium containing 20 ppm Zn was only 40%. Attachment and proliferation of L929 cells cultured in various scaffolds’ medium extracts for 1, 2, and 3 days are shown in Fig. 3b. At 1 day, the number of attached cells was not significantly different for all scaffolds’ extracts. At 2 and 3 days later, the cells, except those cultured in medium containing 20 ppm Zn, could grow continuously. Interestingly, the number of proliferated cells cultured in the medium extracts of scaffold containing higher PVA composition (1.5PVA2.5G and 2PVA2G) tended to be higher than that of cells cultured in control DMEM and the medium extracts of scaffold containing lower PVA composition (0.5PVA3.5G), irrespective of the amount of microspheres incorporated. Furthermore, the release of sericin from scaffolds, particularly 1.5PVA2.5G + 2CH/SS, 1.5PVA2.5G + 4CH/SS, and 2PVA2G + 4CH/SS scaffolds, subsequently promoted the proliferation of cells. Figure 3c shows the concentration of collagen produced by L929 cells cultured in various scaffolds’ medium extracts for 1, 2, and 3 days. The highest collagen concentration was found for the cells cultured in 2PVA2G + 4CH/SS scaffold, as can be seen obviously at 2 and 3 days of the culture. Therefore, only the 2PVA2G + 4CH/SS scaffold was selected for further evaluations.
Fig. 3.
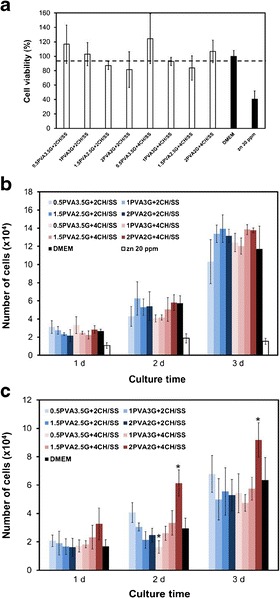
a Viability percentage of L929 mouse fibroblast cells cultured with the extract solutions of the hydrogels for 1 day. b The number of attached and proliferated L929 mouse fibroblast cells cultured with extract solutions of the hydrogels for 1, 2, and 3 days. c The concentration of collagen produced by L929 mouse fibroblast cells cultured with the extract solutions of the hydrogels for 1, 2, and 3 days. The cells cultured with DMEM and Zn were served as negative and positive control, respectively
Antimicrobial Efficiency of the Scaffolds
Table II presents the clear zone of gram-positive and gram-negative bacteria when cultured in the presence of CH/SS microspheres-embedded PVA/gelatin scaffolds (2PVA2G + 4CH/SS). It can be seen that the clear zone distances of all gram-positive bacteria cultured in the presence of microspheres-embedded scaffold (14–15 mm) were significantly higher than those of the Acticoat® (11–12.67 mm). On the other hand, all gram-negative bacteria cultured with the microspheres-embedded scaffold had the lower clear zone distance, comparing to those of Acticoat®. The microbial clear zone was not observed in case of gauze pads.
Table II.
Clear Zone of Gram-Positive and Gram-Negative Bacteria When Culture in the Presence of 2PVA2G+2CHSS Microspheres
| Clear zone (mm) | ||||
|---|---|---|---|---|
| Scaffold + 1% CH | Acticoat | Guaze pads | ||
| Gram-positive bacteria | MRSA | 14.00 ± 1.00 | 11.00 ± 0.0 | – |
| S. aureus | 15.00 ± 1.41 | 12.67 ± 0.58 | – | |
| B. subtilis | 15.00 ± 1.41 | 12.33 ± 2.31 | – | |
| Gram-negative bacteria | P. aeruginosa | 12.00 ± 0.0 | 18.67 ± 0.58 | – |
| A. baumannii | 12.67 ± 0.58 | 19.00 ± 1.0 | – | |
| E. coli | 12.00 ± 0.0 | 15.33 ± 0.58 | – | |
Healing in Full-Thickness Wound
Tissue irritation scores in terms of the number of inflammatory cells (PMN, lymphocyte, macrophage, and mast cells), vascularization, and fatty infiltration of the wound tissue treated with microspheres-embedded scaffold and Allevyn® are shown in Fig. 4. At 3 days of treatment, a higher extent of macrophages and fat infiltration was found in the wound treated with microspheres-embedded scaffold than that of the Allevyn®. At 7 days, the numbers of PMN and mast cells in the wound treated with microspheres-embedded scaffold were significantly higher than those of Allevyn®. At 14 days, the treatment of microspheres-embedded scaffold recruited the significantly higher number of lymphocytes and macrophage to the wound than the treatment of Allevyn®. However, at 21 days, the extents of all above inflammatory cells and fat infiltration in the wounds treated with either microspheres-embedded scaffold or Allevyn® were not different. No significant difference in vascularization of both wound treatments was observed along the treatment period.
Fig. 4.
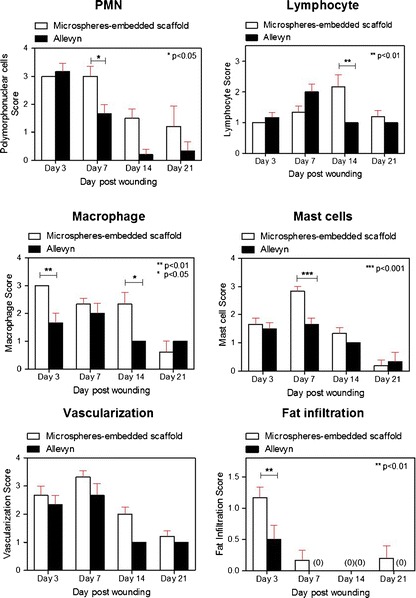
The irritation score of polymorphonuclear neutrophils (PMN), lymphocyte, macrophage, mast cells, vascularization, and fatty infiltration in the wound tissues treated with 2PVA2G + 2CHSS microspheres-embedded scaffold or Allevyn® at 3, 7, 14, and 21 days posttreatment
Figures 5 and 6 show the epithelialization of the wounds treated with microspheres-embedded scaffold and Allevyn®. The distance between epithelial tips of both wounds was not significantly different along the treatment period (Fig. 5). The epidermal widths (Fig. 6b) and epithelial tongue distances (Fig. 6c) of both wounds were not significantly different. However, we found that the epithelial tongue distances of wound treated with microsphere-embedded scaffold at 14 days posttreatment were significant higher compared to the control group.
Fig. 5.
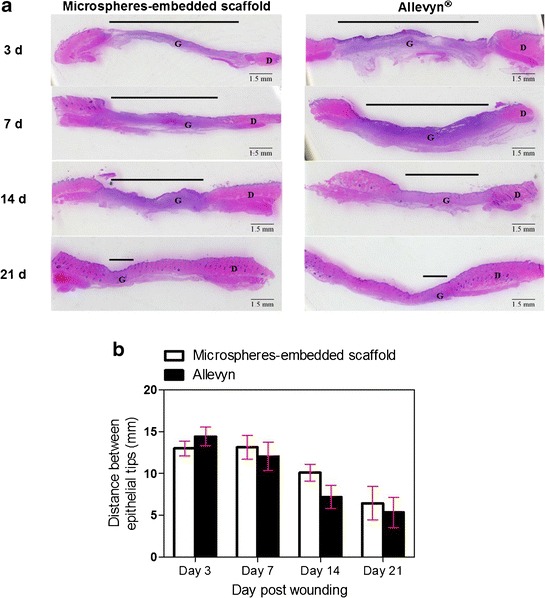
a H&E-stained images indicating the distance between epithelial tips in the wound tissues treated with 2PVA2G + 2CHSS microspheres-embedded scaffold or Allevyn® at 3, 7, 14, and 21 days posttreatment (G granulation tissue, D dermis). b Quantitative distance between epithelial tips in the wound tissues treated with 2PVA2G + 2CHSS microspheres-embedded scaffold or Allevyn® at 3, 7, 14, and 21 days posttreatment
Fig. 6.
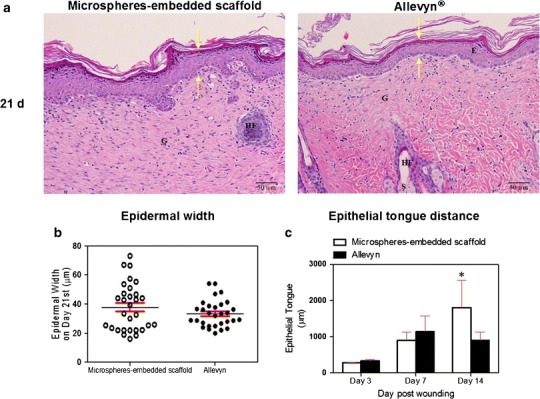
a H&E-stained images indicating the distance of epidermal width in the wound tissues treated with 2PVA2G + 2CHSS microspheres-embedded scaffold or Allevyn® at 21 days posttreatment (Arrow indicates the distance of epidermal width, E epidermis, G granulation tissue, HF hair follicle, S sebaceous gland). b Quantitative distance of epidermal width in the wound tissues treated with microspheres-embedded scaffold or Allevyn® at 21 days posttreatment. c Quantitative epithelial tongue distance in the wound tissues treated with 2PVA2G + 2CHSS microspheres-embedded scaffold or Allevyn® at 3, 7, and 14 days posttreatment
The reduction of wound size after treatment with microspheres-embedded scaffold and Allevyn® was shown in Fig. 7. Interestingly, the wound treated with microspheres-embedded scaffold showed significantly reduced size than that of the Allevyn® at 3, 7, and 14 days. The complete healing (100% wound size reduction) of the wound treated with microspheres-embedded scaffold was achieved at 14 days while that of the Allevyn® was achieved at 21 days. Figure 8 shows the collagen formation in the wounds. The data indicated that the amount of collagen found in wound treated with microspheres-embedded scaffold at 3, 14, and 21 days posttreatment was significantly higher than that of the wound treated with Allevyn®.
Fig. 7.
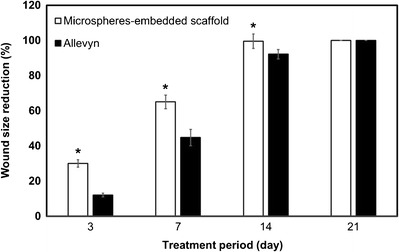
Percentage of wound size reduction after the treatment with 2PVA2G + 2CHSS microspheres-embedded scaffold or Allevyn® for 3, 7, 14, and 21 days posttreatment
Fig. 8.
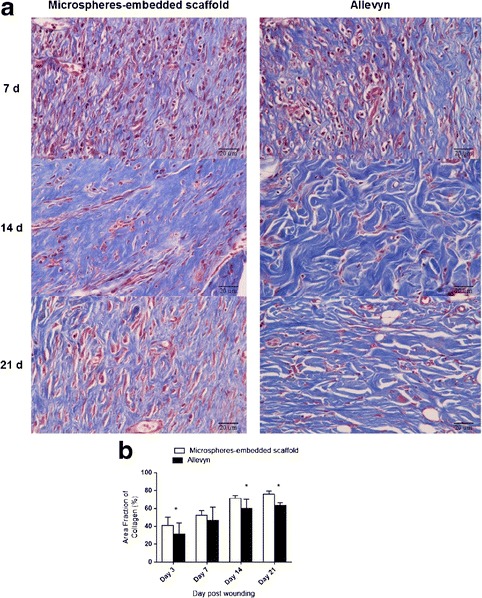
a Masson’s trichrome-stained images indicating collagen formation in the wound tissues treated with 2PVA2G + 2CHSS microspheres-embedded scaffold or Allevyn® at 7, 14, and 21 days posttreatment. b Quantitative area fraction of collagen formed in the wound tissues treated with 2PVA2G + 2CHSS microspheres-embedded scaffold or Allevyn® at 3, 7, 14, and 21 days posttreatment
DISCUSSION
Accelerated wound healing by sustained release sericin formulations of wound dressing materials has been confirmed previously (15–18). In our recent work, the non-toxic chitosan/sericin (CH/SS) microspheres that could release sericin in a sustained manner were developed (19). Unfortunately, the difficulty of applying the microspheres that easily diffuse and quickly degrade onto the wound site limited its use. In this study, we incorporated these CH/SS microspheres into a scaffolding material for the convenient use as a wound dressing and for the extended sustained release of sericin. The microspheres prepared from CH/SS at 80/20 were selected for this study based on the data of the previous work (19). It was shown that the microspheres prepared from CH/SS at 80/20 had homogeneously size distribution, slowest degradation rate, and released sericin in a sustained manner. In term of scaffolds, the compositions of PVA/G were optimized for suitable physical and biological properties for encapsulation of CH/SS microspheres and wound dressing application. The amount of microspheres incorporated was also varied in order to alter the release profiles of sericin. Herein, the sericin could be controlled released from the CH/SS microspheres that embedded in the PVA/G scaffolds. It was supposed that the microspheres were diffused from scaffolds along the scaffolds’ degradation, then the sericin was released out.
By using freeze-drying and glutaraldehyde crosslinking techniques, the scaffolds with interconnected porous structure could be formed (Fig. 1) although the arrangement of pores seemed to depend on the composition of PVA/G. The composition of the scaffolds would influence the penetration and distribution of water molecules in the hydrogel network, contributing to the different size and shape of ice crystal (porogen) formed during the freezing process (24). Furthermore, it was showed that the immediate freezing of polymer matrix solution could form the homogeneously distributed microspheres throughout the surface of all scaffolds. We therefore named these scaffolds as “microspheres-embedded scaffolds”.
All compositions of the microspheres-embedded scaffolds showed high equilibrium water absorption ability up to 600–1000% (Fig. 2a) which was appropriate for the application because high exudate wound needs the dressing to possess a high absorption capability (1). Surprisingly, the water absorption ability of scaffolds seemed to increase with the increasing ratio of PVA. As it is known that the glutaraldehyde can crosslink amine groups of gelatin molecules, it is likely that the gelatin molecules were highly crosslinked and its hydrophilicity was reduced (25). Nevertheless, the scaffolds with higher gelatin content degraded at the faster rate than the scaffolds with higher PVA content (Fig. 2b). This was because collagenase enzyme specifically degraded collagen and collagen derivatives like gelatin (26). Generally, the water absorption ability and degradation rate of the scaffolds could affect the release profiles of molecule incorporated (27). In this case, the scaffolds with higher gelatin content released the higher amount of sericin within the study period (Fig. 2c). This meant that the release of sericin was mainly controlled by the degradation rather than the swelling of scaffolds.
Next, the in vitro non-cytotoxicity and antimicrobial activity of the scaffolds were examined. The results confirmed that, under the study conditions, all scaffolds were not toxic to L929 mouse fibroblast cells (Fig. 3a). Furthermore, the sustained release of sericin from 2PVA2G + 4CH/SS scaffold subsequently promoted cell proliferation and collagen production (Fig. 3b, c). Then, the 2PVA2G + 4CH/SS scaffold was selected for further evaluations. This scaffold was immersed in 1 wt% chitosan solution to enhance its bust release in order to allow the sufficient chitosan amount to produce a strong adjacent antibacterial effect immediately (23,28). This scaffold was found to be effective in antimicrobial activity against the gram-positive bacteria rather than the gram-negative bacteria. Furthermore, the antimicrobial activity of the scaffolds was comparable to that of Acticoat® which is a clinically available antimicrobial wound dressing. The antimicrobial activity of this scaffold was possibly due to both the chitosan and sericin components (29–31). Therefore, our microspheres-embedded scaffolds would act as an antimicrobial wound dressing which could reduce the risk of wound infection during treatment.
The in vivo safety of our wound dressing scaffold was confirmed by tissue irritation test. Excessive inflammation was not found on the wounds treated with either the microspheres-embedded scaffold or the clinically used wound dressing, Allevyn® (Fig. 4). Although there were more infiltration of inflammatory cells in the microspheres-embedded scaffold-treated wound than those treated with Allevyn® within the first 2 weeks of treatment, the inflammation reaction of both wounds was almost the same after 3 weeks of treatment. Helbig et al. reported that inflammatory cells are involved in the replacement of the necrotic zones and seem to be crucial for wound healing (32). The higher number of macrophages found in wounds treated with microspheres-embedded scaffold may indicate the stronger signal from cells for the repairing process. In term of wound healing, epithelialization, wound size reduction, and collagen formation were considered. The epithelialization is the migration and growth of keratinocytes on the neodermis to form a structural and mechanical stable basement membrane to cover the wound surface. We found here that the wound treated with microspheres-embedded scaffold showed the distance of epithelial tip, epithelial tongue, and epidermal width similar to those of the Allevyn®-treated wound (Figs. 5 and 6). Interestingly, the wound treated with our microspheres-embedded scaffold showed the significantly lower wound size than the Allevyn®-treated wound along the treatment period. This might be due to the more contraction as a result of the higher inflammation response and higher collagen content of wound treated with microspheres-embedded scaffold (Fig. 8) (33). This confirmed that sericin has high potential to induce the formation of collagen, as reported previously (12–14). All the results from this study demonstrated that the PVA/G scaffolds embedding CH/SS microspheres could be applied as wound dressing material with appropriate physical and biological properties to support wound healing. The accelerated wound healing would mainly be a result of sustained release of sericin while the antimicrobial activity of the wound dressing might come from the function of either sericin or chitosan.
CONCLUSION
The previously developed CH/SS microspheres were embedded into various compositions of PVA/G scaffolds. All microspheres-embedded scaffolds showed an interconnected pore structure while the microspheres were distributed homogeneously throughout the scaffolds’ surface. The scaffolds showed high water absorption ability and biodegraded gradually. The extent of water absorption and degradation rate of the scaffolds slightly depended on the composition of PVA/G. Sericin could be released in a sustained manner from the microspheres and scaffolds, and the release rate of sericin seemed to be governed by degradation rather than by the swelling of the scaffolds. We also showed that the microspheres-embedded scaffolds were not toxic to fibroblast cells and they did not irritate the tissue when applied to the wound. Lastly, we confirmed that the microspheres-embedded scaffolds showed antimicrobial activity against both gram-positive and gram-negative bacteria by the activity of either chitosan or sericin, and these formulations also promoted wound healing probably by the sustained release of sericin.
ACKNOWLEDGMENTS
This research was supported by Chula Research Scholar from The Ratchadaphiseksomphot Endowment Fund, Chulalongkorn University (Contract number GCURS_58_07_33_01).
REFERENCES
- 1.Hasatsri S, Yamdech R, Chanvorachote P, Aramwit P. Physical and biological assessments of the innovative bilayered wound dressing made of silk and gelatin for clinical applications. J Biomater Appl. 2015;29:1304–13. doi: 10.1177/0885328214559138. [DOI] [PubMed] [Google Scholar]
- 2.Aramwit P, Ratanavaraporn J, Siritientong T. Improvement of physical and wound adhesion properties of silk sericin and polyvinyl alcohol dressing using glycerin. Adv Skin Wound Care. 2015;28:358Y67. doi: 10.1097/01.ASW.0000467304.77196.b9. [DOI] [PubMed] [Google Scholar]
- 3.Saarai A, Kasparkova V, Sedlacek AT, Saha P. On the development and characterization of crosslinked sodium alginate/gelatin hydrogels. J Mech Behav Biomed Mater. 2013;18:152–66. doi: 10.1016/j.jmbbm.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Kuijpers AJ, Engbers GHM, Krijgsveld J, Zaat SAJ, Dankert J, Feijen J. Crosslinkingand characterization of gelatin matrices for biomedical applications. J Biomater Sci Polym Ed. 2000;11:225–43. doi: 10.1163/156856200743670. [DOI] [PubMed] [Google Scholar]
- 5.Juang JH, Bonner WS, Ogawa YJ, Vacanti P, Weir GC. Outcome of subcutaneous islet transplantation improved by polymer device. Transplantation. 1996;61:1557–61. doi: 10.1097/00007890-199606150-00001. [DOI] [PubMed] [Google Scholar]
- 6.Chen DH, Leu JC, Huang TC. Transport and hydrolysis of urea in a reactor–separator combining an anion exchangemembrane and immobilized urease. J Chem Technol Biotechnol. 1994;61:351–7. doi: 10.1002/jctb.280610411. [DOI] [PubMed] [Google Scholar]
- 7.Yang M, Shuai Y, Zhou G, Mandal N, Zhu L, Mao C. Tuning molecular weights of Bombyx mori (B. mori) silk sericin to modify its assembly structures and materials formation. ACS Appl Mater Interfaces. 2014;6:13782–9. doi: 10.1021/am503214g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang YQ. Applications of natural silk protein sericin in biomaterials. Biotechnol Adv. 2002;20:91–100. doi: 10.1016/S0734-9750(02)00003-4. [DOI] [PubMed] [Google Scholar]
- 9.Zhaorigetu S, Yanaka N, Sasaki M, Watanabe H, Kato N. Inhibitory effects of silk protein, sericin on UVB-induced acute damage and tumor promotion by reducing oxidative stress in the skin of hairless mouse. J Photochem Photobiol B. 2003;71:11–7. doi: 10.1016/S1011-1344(03)00092-7. [DOI] [PubMed] [Google Scholar]
- 10.Dash R, Mandal M, Ghosh SK, Kundu SC. Silk sericin protein of tropical tasar silkworm inhibits UVB-induced apoptosis in human skin keratinocytes. Mol Cell Biochem. 2008;311:111–9. doi: 10.1007/s11010-008-9702-z. [DOI] [PubMed] [Google Scholar]
- 11.Tsubouchi K, Igarashi Y, Takasu Y, Yamada H. Sericin enhances attachment of cultured human skin fibroblasts. Biosci Biotechnol Biochem. 2005;69:403–5. doi: 10.1271/bbb.69.403. [DOI] [PubMed] [Google Scholar]
- 12.Aramwit P, Kanokpanont S, De-Eknamkul W, Kamei K, Srichana T. The effect of sericin with variable amino-acid content from different silk strains on the production of collagen and nitric oxide. J Biomater Sci Polym Ed. 2009;20:1295–306. doi: 10.1163/156856209X453006. [DOI] [PubMed] [Google Scholar]
- 13.Aramwit P, Kanokpanont S, Nakpheng T, Srichana T. The effect of sericin from various extraction methods on cell viability and collagen production. Int J Mol Sci. 2010;11:2200–11. doi: 10.3390/ijms11052200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aramwit P, Sangcakul A. The effects of sericin cream on wound healing in rats. Biosci Biotechnol Biochem. 2007;712473–7. [DOI] [PubMed]
- 15.Aramwit P, Siritienthong T, Srichana T, Ratanavaraporn J. Accelerated healing of full-thickness wounds by genipin-crosslinked silk sericin/PVA scaffolds. Cells Tissues Organs. 2013;197:224–38. doi: 10.1159/000345600. [DOI] [PubMed] [Google Scholar]
- 16.Siritienthong T, Angspatt A, Ratanavaraporn J, Aramwit P. Clinical potential of a silk sericin-releasing bioactive wound dressing for the treatment of split-thickness skin graft donor sites. Pharm Res. 2014;31:104–16. doi: 10.1007/s11095-013-1136-y. [DOI] [PubMed] [Google Scholar]
- 17.Siritienthong T, Ratanavaraporn J, Aramwit P. Development of ethyl alcohol-precipitated silk sericin/polyvinyl alcohol scaffolds for accelerated healing of full-thickness wounds. Int J Pharm. 2012;439:175–86. doi: 10.1016/j.ijpharm.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 18.Siritienthong T, Ratanavaraporn J, Srichana T, Aramwit P. Preliminary characterization of genipin-cross-linked silk sericin/poly(vinyl alcohol) films as two-dimensional wound dressings for the healing of superficial wounds. Biomed Res Int. 2013;Article ID 904314. [DOI] [PMC free article] [PubMed]
- 19.Aramwit P, Ekasit S, Yamdech R. The development of non-toxic ionic-crosslinked chitosan-based microspheres as carriers for the controlled release of silk sericin. Biomed Microdevices. 2015;17:9991. doi: 10.1007/s10544-015-9991-4. [DOI] [PubMed] [Google Scholar]
- 20.Rabea EI, Badawy ME, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003;4:1457–65. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- 21.Goy RC, Britto D, Assis OBG. A review of the antimicrobial activity of chitosan. Polímeros: Ciência e Tecnologia. 2009;19:241–7. doi: 10.1590/S0104-14282009000300013. [DOI] [Google Scholar]
- 22.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–6. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 23.Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release. 2001;73:121–36. doi: 10.1016/S0168-3659(01)00248-6. [DOI] [PubMed] [Google Scholar]
- 24.Kang HW, Tabata Y, Ikada Y. Fabrication of porous gelatin scaffolds for tissue engineering. Biomaterials. 1999;20:1339–44. doi: 10.1016/S0142-9612(99)00036-8. [DOI] [PubMed] [Google Scholar]
- 25.Vartiainen J, Harlin A. Crosslinking as an efficient tool for decreasing moisture sensitivity of biobased nanocomposite films. Mater Sci Appl. 2011;2:346–54. [Google Scholar]
- 26.Komsa-Penkova RS, Rashap RK, Yomtova VM. Advantages of orange-labelled collagen and gelatine as substrates for rapid collagenase activity measurement. J Biochem Biophys Methods. 1997;34:237–49. doi: 10.1016/S0165-022X(97)00016-X. [DOI] [PubMed] [Google Scholar]
- 27.Ratanavaraporn J, Furuya H, Kohara H, Tabata Y. Synergistic effects of the dual release of stromal cell-derived factor-1 and bonemorphogenetic protein-2 from hydrogels on bone regeneration. Biomaterials. 2011;32:2797–811. doi: 10.1016/j.biomaterials.2010.12.052. [DOI] [PubMed] [Google Scholar]
- 28.Lischer S, Körner E, Balazs DJ, Shen D, Wick P, Grieder K, et al. Antibacterial burst-release from minimal Ag-containing plasma polymer coatings. J R Soc Interface. 2011;8:1019–30. doi: 10.1098/rsif.2010.0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ong SY, Wu J, Moochhala SM, Tan MH, Lu J. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials. 2008;29:4323–32. doi: 10.1016/j.biomaterials.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 30.Fouda MMG, Wittke R, Knittel D, Schollmeyer E. Use of chitosan/polyamine biopolymers based cotton as a model system to prepare antimicrobial wound dressing. Int J Diabetes Mellit. 2009;1:61–4. doi: 10.1016/j.ijdm.2009.05.005. [DOI] [Google Scholar]
- 31.Wittayasuporn M, Rengpipat S, Palaga T, Asawanonda P, Anumansirikul N, Wanichwecharungruang S. Chitosan derivative nanocarrier: safety evaluation, antibacterial property and ascorbylpalmitate encapsulation. J Microencapsul. 2010;27:218–25. doi: 10.3109/02652040903067836. [DOI] [PubMed] [Google Scholar]
- 32.Helbig D, Bodendorf MO, Grunewald S, Kendler M, Simon JC, Paasch U. Immunohistochemical investigation of wound healing in response to fractional photothermolysis. J Biomed Opt. 2009;14:064044. doi: 10.1117/1.3275479. [DOI] [PubMed] [Google Scholar]
- 33.Tranquillo RT, Murray JD. Continuum model of fibroblast-driven wound contraction: inflammation-mediation. J Theor Biol. 1992;158:135–72. doi: 10.1016/S0022-5193(05)80715-5. [DOI] [PubMed] [Google Scholar]


