Abstract
The in vivo biodistribution and pharmacokinetics of 1329, a novel spectinamide antibiotic with anti-tubercular activity, were studied during intravenous administration of an tritium-labeled compound for nine consecutive, 12-hourly doses to rats. Serial blood samples were collected after the first and the eighth dose, and major organs and tissues were collected 1 h after the ninth dose. Urinary and fecal excretion was monitored throughout the dosing period. Radioactivity in the collected samples was assessed by scintillation counting. During the course of treatment, 86.6% of the administered radioactivity was recovered in urine, feces, organs, and muscle tissue. Urinary excretion was the major route of elimination, with 70% of radioactivity recovered from urine and 12.6% from feces. The time profiles of radioactivity in serum after the first and the eighth dose were identical for the first 2 h post-dose, with similar Cmax (3.39 vs. 3.55 mCi/L) and AUC0−τ (5.08 vs. 5.17 mCi • h/L), indicating no substantial accumulation of 1329 during multiple dosing. Radioactivity in major target organs for pulmonary tuberculosis infection, the lungs and spleen, was 2.79- and 3.06-fold higher than in the blood. Similarly, the intracellular uptake of 1329 into macrophages was sixfold higher than for streptomycin. Overall, these observations suggest biodistribution properties favorable for targeting pulmonary tuberculosis infections.
Electronic supplementary material
The online version of this article (doi:10.1208/s12248-016-9900-7) contains supplementary material, which is available to authorized users.
Keywords: biodistribution, mass balance, pharmacokinetics, spectinamide, tuberculosis
INTRODUCTION
Spectinamides are novel amide derivatives of the antibiotic spectinomycin synthesized by chemical modification on the 3′ position of spectinomycin (Fig. 1). We have previously demonstrated that spectinamides exhibit potent anti-tuberculosis activity in vitro and in several in vivo rodent models of Mycobacterium tuberculosis (MTB) infection that is a product of high affinity for the mycobacterial ribosome and avoidance of efflux by the MTB efflux pump Rv1258c (1). In the current study, we investigated the biodistribution, tissue accumulation, mass balance, and pharmacokinetics of a radiolabeled version of one of our spectinamide lead compounds 1329, designated as 3H-1329, as vital information to identify potential toxicity and safety concerns of novel drug candidates (2,3).
Fig. 1.
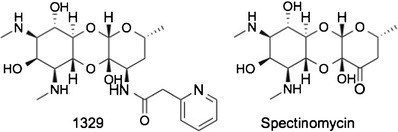
Structure of spectinamide anti-tubercular agents. Spectinamide 1329, developed from the parent antibiotic spectinomycin
MATERIALS AND METHODS
Biodistribution Study of 3H-1329
Radiolabeling of 1329 [3′-Dihydro-3′-deoxy-3′(R)-(pyridin-2yl) acetylamino spectinomycin trihydrobromide] was performed as described in the Online Supplementary Material. Catheterized male Sprague-Dawley rats (n = 6 plus one control; Harlan Bioscience, Indianapolis, IN) weighing 229 to 247 g (average 237 ± 7 g) were housed in individual metabolic cages in a temperature- and air-controlled room and kept on a 12-h light/dark cycle with access to food and water ad libitum. The animals were administered 3H-1329 intravenously (IV) via a femoral vein catheter at a dose of 0.9 mg/kg body weight (0.87 mCi/kg) every 12 h for 4.5 days (a total of nine doses for each animal). The control rat received vehicle (normal saline) only. Serial blood samples (approx. 250 μL) were collected from a jugular vein catheter at 0 (pre-dose), 0.25, 2, 8, and 12 h after the first dose and at 0 (before eighth dose), 0.25, 0.5, 1, 2, 4, 6, and 12 h after the eighth dose. Serum was separated from blood after clotting at room temperature by centrifugation (10,000g for 10 min). Urine and feces specimens were collected at 0 h (pre-dose) and every 12 h after dosing up to the end of the study. One hour after the administration of the ninth dose (on day 5), the animals were euthanized, total blood was collected by cardiac puncture, and all major organs and tissues including the liver, kidneys, spleen, lung, heart, brain, and thigh muscle tissue were collected. All samples were stored at −80°C until analysis. Total volume of blood, urine, total weight of the organs, muscle (4), and feces was noted for the calculation of mass balance.
Analysis of Biologic Samples for 3H-1329
Tritium activity (3H) in the specimens (in terms of disintegrations per minute (DPM)) was determined with a daily calibrated Tri-carb 2800 TR liquid scintillation analyzer (PerkinElmer, Waltham, MA) after mixing samples with liquid scintillation cocktail as described in the Online Supplemental Material. The energy window was set from 0 to 20 keV. In order to reduce the luminescence originating from the samples, a 10-min pre-count delay was used. Samples were counted in the luminescence correction mode with three replicates and a 1-min counting time. The average counting efficiency for 3H is 65.45%.
Macrophage Uptake Assay
Macrophages are a common reservoir for MTB and thus effective anti-mycobacterials need to penetrate well into the intracellular space of macrophages (5). A macrophage uptake assay for 1329 was performed using the murine macrophage cell line J774A.1 (ATCC, Manassas, VA). The cells were cultured in Dulbecco’s Modified Eagle’s medium (DMEM; Gibco, USA) with 10% fetal bovine serum (Sigma-Aldrich, USA) at 37°C, 5% CO2 and allowed to grow until 60–80% confluence in a six-well plate (each plate representing an individual time point and concentration). After exchanging the media for DMEM containing a final 1329 concentration of either 25 or 100 μg/mL, plates were incubated and the media containing test compound was aspirated at different time point (0.5, 1, 4, 8, and 24 h). The cells were washed with ice-cold Dulbecco’s phosphate-buffered saline, suspended in 1 mL water and sonicated for 2 min. The cell suspension was centrifuged for 5 min at 10,000 rpm and the supernatant collected was used to determine the drug uptake using an LC-MS/MS assay as previously described (1). The drug uptake was expressed in terms of μg/mg of protein, as determined with the Pierce BCA assay kit (Thermo Fisher Scientific, Waltham, MA).
Data Analysis
The biodistribution of 3H-1329 in blood, serum, organs, muscle, urine, and feces was calculated in terms of 3H activity (μCi or mCi of 3H) from the analyzed DPM using standard conversion provided by PerkinElmer (2.22 × 106 DPM equal to 1 μCi of 3H) and is expressed as μCi of 3H-1329 activity per gram or milliliter of sample or as percent radioactivity recovered relative to the total amount of 3H-1329 activity administered.
Serum 3H-1329 activity versus time profiles were analyzed by standard non-compartmental pharmacokinetic analysis using Phoenix WinNonlin 6.2 (Certara, Mountain View, CA) (6). Statistical differences were considered significant when p < 0.05 by one-way ANOVA followed by Tukey’s multiple range test.
RESULTS AND DISCUSSION
Mass Balance
During the course of nine doses of 0.9 mg/kg 3H-1329 administered intravenously every 12 h, a total of 86.6% of the administered radioactivity was recovered in urine, feces, blood, muscle, and organs (Table I). Mass balance analysis exhibited that 1 h after the ninth dose approximately 70% of the administered 3H-1329 activity had been recovered in urine and 12.6% in feces, while all the major organs, muscle tissue and whole blood contained only 4.0% of the radioactivity. Thus, in the present study, 86.6% of the administered radioactivity was recovered in urine, feces, organs, and tissues, which is in good agreement with recoveries in other mass balance studies (7–9). The amount of radioactivity excreted in urine after each dosing interval is nearly identical, ranging from 119.3 to 138.9 μCi (Fig. 2), suggesting that there is no substantial accumulation of drug in the body during multiple dosing.
Table I.
Tissue Distribution and Recovery
| Sample/organ | Relative amount of radioactivity [μCi/g tissue] | Total amount of radioactivity [μCi] | % of radioactivity recovered |
|---|---|---|---|
| Administration | |||
| Total amount administered | 1432 (3.44) | ||
| Recovery | |||
| Blooda | 0.240 (12.2) | 3.47 | 0.242 (26.8) |
| Liver | 1.19 (13.7) | 11.4 | 0.796 (5.99) |
| Kidney | 7.92 (8.98) | 16.4 | 1.15 (10.9) |
| Lung | 0.635 (9.35) | 0.805 | 0.056 (12.1) |
| Spleen | 0.688 (11.5) | 0.658 | 0.046 (13.8) |
| Heart | 0.224 (9.32) | 0.219 | 0.015 (24.9) |
| Brain | 0.104 (18.1) | 0.160 | 0.011 (39.6) |
| Muscle | 0.228 (21.8) | 24.7 | 1.72 (34.9) |
| Urinea | 17.9 (21.2) | 1003 | 70.0 (2.99) |
| Fecesb | 5.91 (39.6) | 181 | 12.6 (29.8) |
| Total amount recovered | 1242 | 86.6 (5.09) | |
Recovery of radioactivity following IV administration of 3H-1329 (0.9 mg/kg, BID for 4.5 days) in rats. All values are expressed as mean (%CV)
aIn μCi/mL
bIn μCi/g feces
Fig. 2.
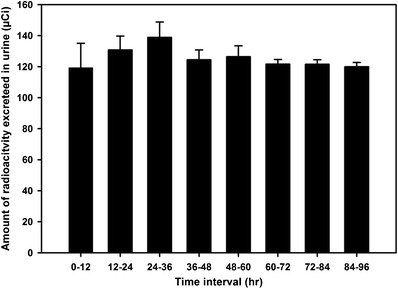
Urinary excretion. Amount of radioactivity excreted in urine after each dosing interval (0.9 mg/kg 3H-1329, BID for 4.5 days). Values are expressed as mean ± SD
Previous pharmacokinetic studies with cold 1329 have shown that 1329 is stable towards hepatic microsomal phase I metabolism, is only bound to 29% to serum proteins, and is excreted to 46% in unchanged form in the urine (1,10). The role of renal excretion as major route of elimination is further supported by the mass balance data.
Tissue Distribution
One hour after the ninth dose, the distribution of radioactivity to major organs (liver, kidney, brain, lung, heart, and spleen) and muscle was analyzed to evaluate the tissue distribution, penetration, and accumulation of 3H-1329 and its labeled metabolites. Relative and absolute radioactivity for each organ and tissue are presented in Table I. The fold difference in amount of radioactivity per milligram or milliliter of tissue relative to whole blood was used as a measure of tissue penetration (Fig. 3). 3H-1329 activity in the lungs and spleen was 2.79- and 3.06-fold higher compared to whole blood, suggesting good tissue penetration of 3H-1329 into those key organs with the highest bacterial burden in pulmonary tuberculosis as most common form of MTB infection. In comparison, penetration of 3H-1329 was less than onefold to other organs such as the heart and brain.
Fig. 3.
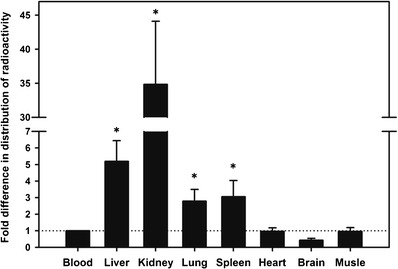
Tissue penetration of 3H-1329. Fold difference in tissue distribution of radioactivity compared to amount in whole blood (after normalizing the distributions to μCi/mL or μCi/mg of blood or tissue). Values are expressed as mean ± SD. The horizontal dotted line indicates the reference value
Pharmacokinetic Profile of Radioactivity in Serum
A comparison of the time profiles of radioactivity after administration of the first dose (day 1) and eighth dose (day 5) exhibited similar values for the average [%CV] systemic exposure measures maximum concentration C max (3.39 [7.4] vs. 3.55 [28.9] mCi/L) and area-under-the-curve during one dosing interval AUC0-τ (5.08 [6.8] vs. 5.17 [20.8] mCi · h/L), suggesting an effective half-life of 2.05 h and the absence of any substantial drug accumulation in serum (11,12). While the concentration-time profiles are superimposable for the first 2 h after the first and eighth dose, respectively, concentrations of the radiosignal were elevated by approximately 50% beyond 2 h post-dose (Fig. 4). A potential formation of circulating metabolites of 3H-1329 as cause for this elevation would be in line with the renal excretion data. Alternatively, this could be explained by the in vivo formation of tritiated water which has been reported to follow a half-life of 85–98 h in rats (7). Tritium exchange and the potential formation of tritiated water in vivo is recognized as a potential limitation for the use of tritiated compounds for drug disposition studies (7), although our in vitro data did not show any tritium exchange for 3H-1329.
Fig. 4.
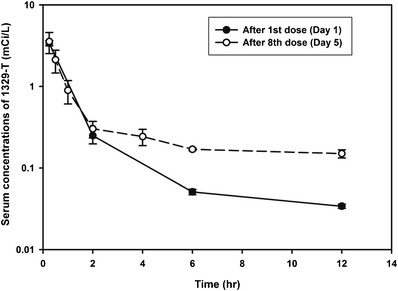
Comparison of single and multiple dose pharmacokinetics. Average (±SD) serum concentration-time profiles of radioactivity after the first dose and eighth dose during multiple dosing with 0.9 mg/kg 3H-1329 given intravenously every 12 h
Macrophage Uptake
Intracellular uptake into macrophages is a prerequisite for antibiotic activity against MTB residing in infected macrophages (5). MTB continues to replicate after it gets ingested by macrophages (13). Hence, it is essential to determine the penetration of anti-tuberculosis agents into the intracellular space of macrophages. 1329 showed a significantly higher penetration into macrophages as compared to spectinomycin, the parent compound of 1329, and streptomycin, an aminoglycoside antibiotic that is used as second-line agent to treat tuberculosis. As shown in Fig. 5, 1329 has a sixfold higher uptake than streptomycin and a 2.2-fold higher uptake than spectinomycin, which is in line with the good tissue penetration on 1329 observed in the mass balance study.
Fig. 5.
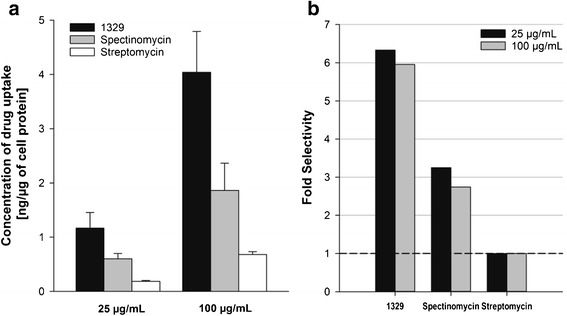
Macrophage cellular uptake. a Average (±SEM) concentration of drug uptake in macrophages after 24 h of incubation at drug concentrations of 25 and 100 μg/mL (N = 6). b Fold difference in cellular uptake of 1329 and spectinomycin as compared to streptomycin at both concentration levels after 24 h of incubation. The horizontal dashed line specifies the reference line
CONCLUSION
Spectinamide 1329 has exhibited properties favorable for targeting pulmonary MTB infection in these initial biodistribution studies. Further, more definitive drug disposition and biodistribution studies with isotopes such as 14C will need to corroborate and expand the observations of this report.
ELECTRONIC SUPPLEMENTARY MATERIAL
Below is the link to the electronic supplementary material.
(PDF 459 kb)
Acknowledgments
This research was supported by grant R01AI090810 by the National Institute of Allergy and Infectious Diseases and grant S10OD016226, Office of the Director, National Institutes of Health and the American Lebanese Syrian Associated Charities (ALSAC). We thank Tom Mohaupt, Jennings Payne, Kellen Thuo, and Warner Turner (Radiation Safety Division, St. Jude Children’s Research Hospital, Memphis, TN) for their help in determining the total and specific activity of the radiolabeled compound. We thank Dr. Feng Yin and Dr. James T. Dalton (GTx Inc., Memphis, TN) for the opportunity to use their sample oxidation equipment.
Compliance with Ethical Standards
All animal experiments were conducted in accordance with the Animal Welfare Act and the Public Health Service Policy on Humane Care and Use of Laboratory Animals. The study protocol was approved by the Institutional Animal Care and Use Committee and the Radiation Safety Committee of the University of Tennessee Health Science Center.
Conflict of Interest
J.L., R.E.L., and B.M. disclose intellectual property rights ownership associated with the spectinamide series.
References
- 1.Lee RE, Hurdle JG, Liu J, Bruhn DF, Matt T, Scherman M, et al. Spectinamides: a new class of semisynthetic antituberculosis agents that overcome native drug efflux. Nat Med. 2014;20(2):152–8. doi: 10.1038/nm.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meibohm B, Derendorf H. Pharmacokinetic/pharmacodynamic studies in drug product development. J Pharm Sci. 2002;91(1):18–31. doi: 10.1002/jps.1167. [DOI] [PubMed] [Google Scholar]
- 3.Mdluli K, Kaneko T, Upton A. The tuberculosis drug discovery and development pipeline and emerging drug targets. Cold Spring Harb Perspect Med. 2015;5(6). doi: 10.1101/cshperspect.a021154. [DOI] [PMC free article] [PubMed]
- 4.Usynin IF, Khar’kovsky AV, Balitskaya NI, Panin LE. Gadolinium chloride-induced Kupffer cell blockade increases uptake of oxidized low-density lipoproteins by rat heart and aorta. Biochemistry (Mosc) 1999;64(6):620–4. [PubMed] [Google Scholar]
- 5.Pieters J. Mycobacterium tuberculosis and the macrophage: maintaining a balance. Cell Host Microbe. 2008;3(6):399–407. doi: 10.1016/j.chom.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Muir KT, Gomeni RO. Non-compartmental analysis. In: Bonate PL, Howard DR, editors. Pharmacokinetics in drug development: clinical study design and analysis. Arlington: AAPS Press; 2004. pp. 235–58. [Google Scholar]
- 7.Shaffer CL, Gunduz M, Thornburgh BA, Fate GD. Using a tritiated compound to elucidate its preclinical metabolic and excretory pathways in vivo: exploring tritium exchange risk. Drug Metab Dispos: Biol Fate Chem. 2006;34(9):1615–23. doi: 10.1124/dmd.106.010934. [DOI] [PubMed] [Google Scholar]
- 8.Yang S, Zhang Q, Chen J, Han D, Zhao D, Chen X. Pharmacokinetics and disposition study of calf thymus DNA in rats by applying 3H-labeling method. J Pharm Biomed Anal. 2012;64-65:35–9. doi: 10.1016/j.jpba.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Roffey SJ, Obach RS, Gedge JI, Smith DA. What is the objective of the mass balance study? A retrospective analysis of data in animal and human excretion studies employing radiolabeled drugs. Drug Metab Rev. 2007;39(1):17–43. doi: 10.1080/03602530600952172. [DOI] [PubMed] [Google Scholar]
- 10.Madhura DB, Trivedi A, Rathi C, Liu J, Lee RE, Meibohm B. Pharmacokinetic evaluation of novel spectinamides. AAPS J. 2012;14(S2):W5350. [Google Scholar]
- 11.Wagner JG. Drug accumulation. J Clin Pharmacol. 1967;7(2):84–8. doi: 10.1002/j.1552-4604.1967.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 12.Boxenbaum H, Battle M. Effective half-life in clinical pharmacology. J Clin Pharmacol. 1995;35(8):763–6. doi: 10.1002/j.1552-4604.1995.tb04117.x. [DOI] [PubMed] [Google Scholar]
- 13.Knechel NA. Tuberculosis: pathophysiology, clinical features, and diagnosis. Crit Care Nurse. 2009;29(2):34–43. doi: 10.4037/ccn2009968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 459 kb)


