Abstract
Dysregulation of the Hypothalamic-Pituitary-Adrenal (HPA) axis and disruptions of restorative processes (e.g., sleep) have been implicated as two key mechanisms through which loneliness leads to medical morbidity in adults and late adolescents. Whether loneliness acts through these biological and behavioral intermediaries in children as well remains unexplored. In a sample of 645 children aged 8 to 15 affected by parental HIV/AIDS in rural China, trait and state (i.e., daily) loneliness were measured in a 3-day diary study, wherein participants also provided cortisol samples and sleep measures. Whereas high levels of trait loneliness were found to predict lower morning cortisol levels, longer time in bed, lower sleep quality, and a higher number of night awakenings, daily loneliness was associated with a flatter diurnal cortisol slope and shorter time in bed. Although the association between trait loneliness and daily loneliness with HPA activity remained significant after controlling for psychological constructs that overlap with loneliness (e.g., depression and daily negative affect), some of the associations between loneliness and sleep measures became non-significant after including these additional covariates. These findings provide the first empirical evidence to our knowledge of associations between trait and state loneliness and health-related outcomes among school-aged children and young adolescents.
Keywords: Loneliness, Sleep, Cortisol, Physical Health, Childhood
The “Pit of Despair” and “Well of Loneliness” were among the most common names given by psychologist Harry F. Harlow to the small vertical apparatus he used for his experiments on social isolation in rhesus macaques. The consequences of placing animals in these inescapable metal cages were lethal; in Harlow’s words: “Placed in a free-living situation, most of these animals would be driven off or eliminated before they could have an opportunity to learn to adapt to the group” (Harlow et al., 1965).
In humans, social isolation, the measurable condition of having a withered social network (i.e., few and infrequent social contacts), is associated with broad adult medical morbidity and mortality. For example, receiving low social support has been associated with higher risk of heart disease (Barth et al., 2010), susceptibility to common respiratory illness (Cohen et al., 1997), and mortality (Eng et al., 2002). Similar findings have emerged in people reporting high levels of loneliness (Caspi et al., 2006; Patterson and Veenstra, 2010), the emotional discomfort associated with the perceived discrepancy between desired and available quality and quantity of social contacts. Despite being obviously related, social isolation and loneliness can have non-overlapping effects on health (Steptoe et al., 2013), especially when social isolation does not predict loneliness (e.g., Fees et al., 1999).
Although loneliness is a common experience, stigmatized populations, such those living with HIV, are at greater risk of feeling socially isolated. HIV-infected individuals are often socially rejected and must face recurrent discrimination across a variety of social realms, including work and health care settings (Nyblade et al., 2009). This chronic psychological stressor can have daunting mental and physical consequences not only for HIV-positive individuals, but also for their children, who predominantly depend on them, regardless of their HIV status (Chi et al., 2014). Children affected by parental HIV, in fact, are more often socially excluded by peers and family members, and report recurrent experiences of humiliation and reduced social support (Cluver et al., 2008). Understanding how the multifaceted construct of loneliness influences health outcomes among children affected by parental HIV has important implications for tailoring multilevel (individual, family, community) intervention programs in this population.
The overarching aim of the current study was to investigate the contribution of different aspects of loneliness to two biobehavioral intermediaries—sleep and cortisol secretion—associated with physical health in a group of children affected by parental HIV. From this perspective, a distinction can be made between trait loneliness, the chronic perception—accumulated over time—of inadequate quantity and quality of social relationships, and state (i.e., daily) loneliness, a transient and more circumstantial feeling (De Jong-Gierveld and Raadschelders, 1982). This distinction is particularly meaningful in diary studies, where data from participants are collected as they live through their quotidian life, as it allows the researcher to disentangle the effect of sustained loneliness (i.e., trait loneliness) as well as between people variability in transient experiences of loneliness (i.e., daily loneliness), which provide a more proximal picture of the individual feelings of isolation during the testing days (Doane & Adam, 2010). This approach is also important in light of the fact that daily loneliness may not strongly correlate with trait loneliness, especially during childhood, wherein the degrees of stability and fluidity of one’s social network are more malleable (Cairns et al., 1995).
Alteration in the activity of the Hypothalamic-Pituitary-Adrenal (HPA) axis has emerged as one of the key mechanisms underlying the association between loneliness and health. For example, several cross sectional studies have found small—but consistent—positive associations between subjective feelings of social isolation and salivary cortisol (Cacioppo et al., 2000; Steptoe et al., 2004; Pressman et al., 2005; Edwards et al., 2010) and urinary cortisol (Kiecolt-Glaser et al., 1984; Hawkley et al., 2006). These findings have been confirmed with daily dairy data, wherein daily assessments of loneliness were prospectively associated with larger cortisol responses to awakening in adults and late adolescents (i.e. cortisol awakening response, or CAR, Adam et al., 2006; Doane and Adam, 2010, but see Sladek and Doane, 2015), while trait loneliness was associated with a flatter decline in diurnal cortisol in late adolescence (Doane and Adam, 2010, but see, Sladek and Doane, 2015), for a null association between trait loneliness and cortisol slope in the same age group). Despite the insights provided by those data, critical gaps remain unaddressed in this literature. To date, no work has investigated the link between loneliness and health-related biology in children. Extending the link between the HPA axis and loneliness in children is important for at least two reasons. Recent empirical evidence showed that loneliness at a young age is predictive of health risk factors in young adulthood (Caspi et al., 2006) and dysregulation in cortisol secretion might be a key biological intermediary through which these effects take place.
A second mechanism that has been recognized as crucial in mediating the effect of loneliness on physical health is the dysregulation of restorative processes (e.g., sleep) (Hawkley and Cacioppo, 2010). The intuitive association between sleep and various biological risk factors (for a reviews, see (Irwin, 2015; Mullington et al., 2009) and, consequently, medical morbidity during adulthood (e.g., King et al., 2008) and mortality (Kripke et al., 2002) is well established. Within this framework, loneliness in adulthood has emerged as a reliable antecedent of poor sleep quality (Cacioppo et al., 2002b; Steptoe et al., 2004; Pressman et al., 2005) and disruptions in sleep continuity (Cacioppo et al., 2002a), but not sleep duration (Cacioppo et al., 2002b; Hawkley et al., 2010; Kurina et al., 2011). Studies on adolescents replicated and extended these findings by showing that not only was loneliness positively associated with sleep disturbances (e.g., number of night awakenings) (Mahon, 1994; Harris et al., 2013), but also with difficulty of falling asleep (i.e. sleep onset latency or SOL) (Harris et al., 2013). Overall, this pattern of results demonstrates the importance of measuring different sleep outcomes across subsequent days rather than single assessments at one point in time.
The aim of the current study was to investigate the impact of trait loneliness and daily loneliness on diurnal cortisol and daily reports of sleep in a large sample of children aged 8 to 15 years affected by parental HIV/AIDS in rural China. Loneliness among children in rural China is widespread (Chen et al., 2014) and, as mentioned above, this feeling can be further exacerbated among children whose parents belong to a stigmatized group, such as HIV infected individuals (Chi and Li, 2013). Working with this high-risk sample offers a unique opportunity to investigate the link between loneliness and biobehavioral health mechanisms among youth living in adversity.
Method
Participants
Seven hundred and ninety children aged 6–17 affected by parental HIV participated in a randomized controlled trial of a psychological intervention currently under way; the current investigation used baseline data (i.e., prior to intervention) drawn from this larger study. Of the larger sample of 790 children, 746 fit the inclusion criterion of 8 to 15 years of age, based on the age range for which the self-report measures used in the present analyses were normed. Further, of those 746 children, 645 (86.4%) provided valid saliva samples for cortisol analyses (48.1% female, age, M = 10.67 years, SD = 1.79 years) and were therefore used in the analyses. At the end of the study, each child received either toys or school supplies depending on their age as tokens of appreciation. Appropriate informed consent/assent was obtained before participation and all procedures received approval by the Institutional Review Boards at Wayne State University in the United States and Henan University in China.
Procedure
Children and their caregivers completed confidential survey questionnaires in Chinese. The survey included detailed measures of demographic information and several psychosocial scales. The majority of the child surveys were self-administrated in a small group in which two interviewers were present. The daily diary data collection occurred during the same time as daily saliva collection. From Thursday to Saturday, children were instructed in detail to fill the daily sleep diary immediately after the first saliva sample was collected, and to fill the daily mood diary (including momentary loneliness and other daily emotions). The primary caregiver was allowed to provide assistance during the collection of daily diary measures.
Measures
Trait Loneliness
The Children’s Loneliness Scale (CLS, Asher et al., 1984) was used to assess trait loneliness. An overall index (M = 2.10, SD = .47) is calculated by averaging children’s self-reported scores on 16 items rated on a 4-point Likert scale, ranging from “Always True” to “Not True at All”. Sample items included, “It’s easy for me to make new friends at school”; “I feel alone” (reversed); “I have nobody to talk to” (reversed). The scale also contained 10 (8 in the original scale) additional filler items that are not included in the scoring. The high reliability obtained in our sample (α = .78) matches the strong reliability found in other non-clinical samples (Parkhurst and Asher, 1992), including Chinese children (Chen et al., 2004; Li et al., 2009; Du et al., 2014; Qiao et al., 2014).
Daily Sleep Outcomes
At the beginning of each day, participants were asked to report the time they woke up, the time they went to bed the night before, how many minutes it took them to fall asleep (i.e., SOL) (M = 11.88, SD = 11.56) and the number of night awakenings (M = 1.20, SD = .98). Total time in bed (M = 10.11 SD = 1.25) was calculated in hours by subtracting the time they woke up from the time they went to bed. Participants were also asked to rate, on a scale from 1 (very bad) to 4 (very good), their sleep quality (M = 3.41, SD = .66).
Daily Loneliness and Negative Affect
At the end of each day, participants were asked to report, on a scale from 1 (not at all) to 3 (most of the day), how much they felt each of the following affective states: lonely, sad, upset, fear, angry, and worried. Responses on the “lonely” item were used for our measure of daily loneliness (across days M = 1.25, SD = .42), while an average of the other moods were used for our measure of general negative affect (across days M = 1.37, SD = .37) (for a similar approach, see Doane and Adam, 2010).
Depression
A short version of the Center for Epidemiologic Studies Depression Scale for children (Fendrich et al., 1990) was used to assess depression. Children were asked to report on a 4-point Likert scale (1 = not at all, 4 = a lot) how they felt or acted in the previous week. Examples of the 10 items include, “I was bothered by things that usually don’t bother me,” and “I felt like I was too tired to do things this past week” (α = .62). Scores on this scale were obtained by summing the responses and were computed only for participants who answered all 10 items. Higher scores indicated higher depression symptoms (M = 20.17, SD = 4.29).
Perceived Health Status
We also controlled for perceived health status, which was self-reported by each youth and by their caregiver on a 5-point Likert-type scale ranging from 1 (very poor) to 5 (very good). This scale was constructed by calculating the mean of the two items and was computed for cases that had valid values on both items. Higher scores indicate higher perceived health status (M = 4.1, SD = .75).
Parental death
One-hundred and twelve children lost at least one of their parents, while 495 had both parents still alive. Data for this variable were not available for 38 children.
Other Stressful Life Events
Fifteen items were used to assess children’s experience of a number of stressful life events. Children reported whether these events occurred during the past six months (M = 2.47, SD = 2.12). Sample items included being in a traffic accident, being a witness to involuntary violence, hospitalization, natural disaster, severe sickness or death of friends, relocation of the family, and death of family members.
Additional Covariates
Other covariates included age (M = 10.67, SD = 1.79), gender (48.1% female), family socioeconomic status (SES), which was derived by adding together caregiver education and family income after having z scored them (M = .01, SD = 1.53), daily wake up time, and day of the week (weekday = 0, weekend = 1). At the momentary level (i.e., at collection time of each saliva sample), youths reported whether they smoked or practiced any sport. Missing cases for these two variables were replaced by the mode. Variables listed in this section are standard covariates in diurnal cortisol studies (Adam et al., 2006).
Cortisol
Participants self-collected saliva samples at four time points each day for three days: immediately upon waking (prior to any eating, drinking, or exercise), thirty minutes later to assess cortisol awakening response (CAR), one hour before dinnertime, and then at bedtime. Prior to saliva collection, the investigators showed children the correct procedure to collect saliva samples using Salivettes (Sarstedt, Rommelsdorft, Germany) and emphasized the importance of compliance with the time of collection. Salivettes were stored at room temperature before being returned to the researchers, who refrigerated them until the day of the assay at Huaihe Hospital. Prior work has shown that Salivette storage at room temperature for as long as two weeks (much longer than in this study) does not adversely affect cortisol concentration (Garde and Hansen, 2005). Participants provided a total of 11.17 out of 12 samples on average (SD = 1.60), with 93% of all possible saliva samples collected. Altogether, 61.3% of participants did not miss any samples, with 90.4% providing between 10 and 12 samples, and 96% of participants providing at least 8 of the 12 possible saliva samples across the 3 days. Cortisol levels were determined via chemiluminescent immunoassay (Access Cortisol kit YZB/USA 2802, Beckman Coulter, Inc, Fullerton, CA). Cortisol values were natural log-transformed to correct for positive skew in the cortisol distribution (Adam and Kumari, 2009). In order to ensure that all transformed scores were positive, a constant of 1 was added before the transformation.
Statistical Analyses
The incidence of missing data was 8.67% at the daily level (sleep analyses) and 5.4% at the person levels (sleep analyses and cortisol analyses). In order to curtail the bias associated with pairwise or listwise deletion of missing data (Collins et al., 2001), we used the expectation maximization (EM) algorithm to impute missing data. Estimates obtained with this approach are less biased than estimates obtained with ad hoc methods (e.g., listwise deletion of missing data) (Schafer and Graham, 2002; Peng et al., 2006, see also Harris and colleagues’ work on loneliness for a similar approach; Harris et al., 2013). Because this algorithm does not allow value replacement for categorical data, mode imputation was used to replace missing cases for parental death (n = 38).
Inferential Statistics
Cortisol analyses
Hierarchical Linear Modeling (HLM) was employed in order to properly account for the strong diurnal rhythm of cortisol. HLM allows for the simultaneous estimation of more than one cortisol parameter (e.g., wakeup values, CAR, and slope) along with the prediction of individual differences in diurnal cortisol profiles. Based on previous diurnal cortisol research (Adam and Kumari, 2009), Time Since Waking, Time Since Waking-squared, and CAR (dummy coded 0 and 1) were modeled at Level 1 in order to estimate each participants’ diurnal cortisol profile. In our sample CAR observations that deviated by 10 min or more from the requested 30-min interval were dropped from the analyses. At Level 2 (person-level), we tested the effect of trait and daily loneliness (i.e., average of loneliness across the three testing days). Analyses were conducted without controlling for covariates (Model 1), controlling only for demographic covariates (age, gender, family SES, average wakeup time, sport, smoke, parental death, stressful events, and health status) (Model 2), and then controlling for both demographics and psychological covariates (depression and daily negative affect) (Model 3). Cortisol intercept, slope (effect of time), and CAR were all allowed to vary randomly at Level-2, while Time Since Waking-squared was considered as a fixed effect with no Level-2 predictors. Age was centered such that a score of zero would represent the youngest children in the sample (i.e., age 8), while stressful life events was left uncentered (0 = no stressful life events). All other continuous person-level variables were grand-mean centered. All significance tests were two-tailed (α = .05) with robust standard errors.
Sleep outcomes analyses
Given the nested nature of our sleep data (i.e. days within people), hierarchical linear modeling (HLM) was used for data analyses. For each sleep outcome (subjective sleep quality, SOL, number of awakenings, total time in bed), analyses were conducted without controlling for covariates (Model 1), controlling only for demographics covariates (age, gender, family SES, weekend, parental death, stressful events, and health status) (Model 2), and then controlling for both demographics and psychological covariates (depression and daily negative affect) (Model 3). The intercept of each model was allowed to vary randomly at the day level (e.g., treated as random effects). All significance tests were two- tailed (α = .05) with robust standard errors.
Results
Cortisol analyses
Interestingly, trait loneliness was only weakly associated with daily loneliness (r = .119, p = .002) and daily negative affect (r = .113, p = .004), but strongly associated with depression (r = .497, p < .001). Daily loneliness was associated strongly with daily negative affect (r = .629, p < .001) and moderately with depression (r = .212, p < .001). As reported in Table 1, Model 1, trait loneliness was associated with lower morning cortisol (t = −2.678, p = .008), while average daily loneliness was associated with a flatter cortisol slope (t = 2.384, p = .017), suggesting that those individual who experienced more daily loneliness across the three testing days had a less steep diurnal decline in cortisol (Figure 1). No effects were found for CAR, which overall was not statistically significant in this sample. These effects remained significant once we controlled for demographic covariates (Model 2, Table 1) and demographics and psychosocial covariates (Model 3, Table 1).
Table 1.
HLM Models of Diurnal Cortisol Parameters
| Fixed effect (independent variable) | Model 1
|
Model 2
|
Model 3
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | P | Estimate | SE | P | Estimate | SE | P | |
| Morning cortisol, π0 | |||||||||
| Average Morning Cortisol (Intercept), β00 | 0.6620 | 0.0064 | <.001 | 0.6571 | 0.0164 | <.001 | 0.6569 | 0.0162 | <.001 |
| Trait Loneliness, β01 | −0.0368 | 0.0137 | 0.008 | −0.0329 | 0.0140 | 0.019 | −0.0320 | 0.0158 | 0.043 |
| Daily Loneliness, β02 | −0.0236 | 0.0152 | 0.120 | −0.0234 | 0.0154 | 0.128 | −0.0217 | 0.0197 | 0.272 |
| Female, β03 | – | – | – | −0.0071 | 0.0131 | 0.590 | −0.0072 | 0.0132 | 0.585 |
| Parental death, β04 | – | – | – | −0.0137 | 0.0174 | 0.430 | −0.0137 | 0.0174 | 0.431 |
| Health status, β05 | – | – | – | −0.0017 | 0.0085 | 0.843 | −0.0018 | 0.0086 | 0.837 |
| Life events, β06 | – | – | – | −0.0032 | 0.0027 | 0.241 | −0.0031 | 0.0027 | 0.254 |
| Family SES β07 | – | – | – | 0.0016 | 0.0041 | 0.693 | 0.0016 | 0.0041 | 0.698 |
| Age, β08 | – | – | – | 0.0057 | 0.0037 | 0.130 | 0.0057 | 0.0037 | 0.127 |
| Wakeup time, β09 | – | – | – | −0.0003 | 0.0105 | 0.978 | −0.0003 | 0.0105 | 0.975 |
| Daily Negative Affect, β010 | – | – | – | – | – | – | −0.0029 | 0.0247 | 0.906 |
| Depression, β011 | – | – | – | – | – | – | −0.0002 | 0.0017 | 0.907 |
| Cortisol Awakening Response, π1 | |||||||||
| Average CAR, β10 | 0.0023 | 0.0069 | 0.740 | −0.0059 | 0.0155 | 0.705 | −0.0056 | 0.0154 | 0.716 |
| Trait Loneliness, β11 | −0.0057 | 0.0129 | 0.658 | −0.0029 | 0.0132 | 0.826 | −0.0037 | 0.0149 | 0.801 |
| Daily Loneliness, β12 | −0.0029 | 0.0161 | 0.858 | −0.0083 | 0.0162 | 0.609 | −0.0093 | 0.0199 | 0.638 |
| Female, β13 | – | – | – | −0.0311 | 0.0136 | 0.022 | −0.0310 | 0.0135 | 0.022 |
| Parental death, β14 | – | – | – | 0.0178 | 0.0179 | 0.320 | 0.0178 | 0.0179 | 0.320 |
| Health status, β15 | – | – | – | 0.0077 | 0.0099 | 0.434 | 0.0078 | 0.0099 | 0.432 |
| Life events, β16 | – | – | – | −0.0001 | 0.0034 | 0.979 | −0.0002 | 0.0035 | 0.957 |
| Family SES β17 | – | – | – | −0.0044 | 0.0042 | 0.296 | −0.0044 | 0.0042 | 0.296 |
| Age, β18 | – | – | – | 0.0067 | 0.0037 | 0.071 | 0.0067 | 0.0037 | 0.070 |
| Wakeup time, β19 | – | – | – | −0.0048 | 0.0129 | 0.710 | −0.0047 | 0.0129 | 0.714 |
| Daily Negative Affect, β1110 | – | – | – | – | – | – | 0.0017 | 0.0255 | 0.949 |
| Depression, β1111 | – | – | – | – | – | – | 0.0002 | 0.0018 | 0.911 |
| Time Since Waking, π2 | |||||||||
| Average Linear Slope, β20 | −0.0403 | 0.0020 | <.001 | −0.0389 | 0.0022 | <.001 | −0.0389 | 0.0022 | <.001 |
| Trait Loneliness, β21 | 0.0017 | 0.0012 | 0.162 | 0.0013 | 0.0012 | 0.288 | 0.0011 | 0.0013 | 0.402 |
| Daily Loneliness, β22 | 0.0034 | 0.0014 | 0.017 | 0.0033 | 0.0014 | 0.020 | 0.0038 | 0.0019 | 0.041 |
| Female, β23 | – | – | – | −0.0008 | 0.0011 | 0.489 | −0.0008 | 0.0011 | 0.506 |
| Parental death, β24 | – | – | – | 0.0020 | 0.0016 | 0.204 | 0.0020 | 0.0016 | 0.204 |
| Health status, β25 | – | – | – | −0.0001 | 0.0007 | 0.890 | −0.0001 | 0.0007 | 0.867 |
| Life events, β26 | – | – | – | 0.0002 | 0.0003 | 0.526 | 0.0002 | 0.0003 | 0.537 |
| Family SES β27 | – | – | – | −0.0002 | 0.0004 | 0.702 | −0.0002 | 0.0004 | 0.675 |
| Age, β28 | – | – | – | −0.0006 | 0.0003 | 0.055 | −0.0006 | 0.0003 | 0.054 |
| Wakeup time, β29 | – | – | – | −0.0014 | 0.0009 | 0.145 | −0.0014 | 0.0009 | 0.144 |
| Daily Negative Affect, β210 | – | – | – | – | – | – | −0.0010 | 0.0023 | 0.658 |
| Depression, β211 | – | – | – | – | – | – | 0.0000 | 0.0002 | 0.742 |
| Time Since Waking2, π3 | |||||||||
| Average Curvature, β30 | 0.0011 | 0.0001 | <.001 | 0.0011 | 0.0001 | <.001 | 0.0011 | 0.0001 | <.001 |
| Smoke, π4 | |||||||||
| Intercept, β40 | – | – | – | 0.1558 | 0.0442 | <.001 | 0.1566 | 0.0444 | <.001 |
| Exercise, π5 | |||||||||
| Intercept, β50 | – | – | – | 0.0227 | 0.0086 | 0.008 | 0.0229 | 0.0086 | 0.008 |
Note. Intercepts indicate average cortisol values at wakeup; average slopes of time since waking indicate change in cortisol per 1-hour change in time; average slopes of time since waking2 indicate change in cortisol per 1-hour change in time2. CAR = Cortisol Awakening Response. SES: Socioeconomic Status.
Figure 1.
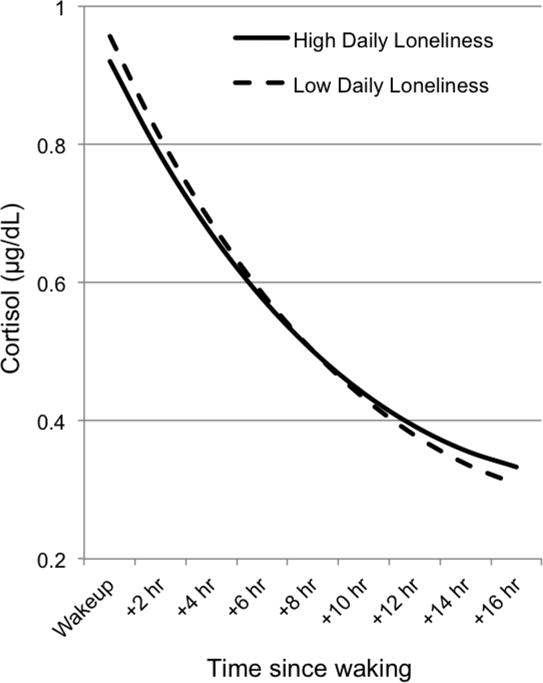
Associations between daily loneliness and diurnal cortisol. Cortisol levels (μg/dL) is graphed as a function of time since waking, separately for children that reported low (1 SD below the mean) and high (1 SD above the mean) daily loneliness.
Sleep outcomes analyses
As shown in Table 2, Model 1 and Model 2, high trait loneliness was associated with low sleep quality (t = −2.612, p = .009; t = −2.451, p = .014, after controlling for demographic covariates) and high self-reported number of night awakenings (t = 3.897, p < .001; t = 3.113, p = .002, after controlling for demographic covariates). Similarly, average daily loneliness was associated with higher number of night awakenings (t = 2.007, p = .045; t = 2.195, p = .028, after controlling for demographic covariates). Average daily loneliness was also associated with lower sleep quality (t = −2.042, p = .042), but this relationship disappeared after controlling for demographic covariates (t = −1.468, p = .143). Trait loneliness was associated with more time in bed (t = 3.747, p < .001; t = 2.524, p = .012, after controlling for demographic covariates), while the opposite was true for average daily loneliness (t = −2.596, p = .010; t = −2.084, p = .038, after controlling for demographic covariates). None of the measures of loneliness was predictive of SOL1.
Table 2.
HLM Models of Sleep Outcomes
| Fixed effect (independent variable) | Model 1
|
Model 2
|
Model 3
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | P | Estimate | SE | P | Estimate | SE | P | |
| Time in bed, π0 | |||||||||
| Average Time in Bed (Intercept), β00 | 10.1238 | 0.0360 | <.001 | 10.4030 | 0.0831 | <.001 | 10.3925 | 0.0835 | <.001 |
| Trait Loneliness, β01 | 0.3089 | 0.0824 | <.001 | 0.1938 | 0.0768 | 0.012 | 0.2229 | 0.0889 | 0.012 |
| Daily Loneliness, β02 | −0.2385 | 0.0919 | 0.010 | −0.1838 | 0.0882 | 0.038 | −0.0955 | 0.1149 | 0.406 |
| Female, β03 | – | – | – | −0.0404 | 0.0694 | 0.560 | −0.0436 | 0.0698 | 0.532 |
| Parental death, β04 | – | – | – | −0.0541 | 0.1021 | 0.596 | −0.0520 | 0.1020 | 0.611 |
| Health status, β05 | – | – | – | −0.0513 | 0.0446 | 0.251 | −0.0541 | 0.0444 | 0.223 |
| Life events, β06 | – | – | – | 0.0087 | 0.0176 | 0.620 | 0.0125 | 0.0181 | 0.491 |
| Family SES β07 | – | – | – | −0.0154 | 0.0260 | 0.554 | −0.0170 | 0.0258 | 0.511 |
| Age, β08 | – | – | – | −0.1571 | 0.0199 | <.001 | −0.1563 | 0.0198 | <.001 |
| Daily Negative Affect, β09 | – | – | – | – | – | – | −0.1494 | 0.1400 | 0.286 |
| Depression, β010 | – | – | – | – | – | – | −0.0064 | 0.0099 | 0.519 |
| Weekend, β10 | – | – | – | 0.4448 | 0.0464 | <.001 | 0.4448 | 0.0464 | <.001 |
|
| |||||||||
| SOL, π0 | |||||||||
| Average SOL (Intercept), β00 | 11.8929 | 0.3973 | <.001 | 10.9313 | 0.8736 | <.001 | 10.9747 | 0.9043 | <.001 |
| Trait Loneliness, β01 | −0.1615 | 0.8299 | 0.846 | 0.0275 | 0.8449 | 0.974 | 0.3680 | 1.0800 | 0.733 |
| Daily Loneliness, β02 | 0.0311 | 0.8780 | 0.972 | −0.2731 | 0.9086 | 0.764 | −1.3752 | 1.5227 | 0.367 |
| Female, β03 | – | – | – | −0.4118 | 0.7982 | 0.606 | −0.4579 | 0.7923 | 0.564 |
| Parental death, β04 | – | – | – | 0.7329 | 1.3129 | 0.577 | 0.7337 | 1.3189 | 0.578 |
| Health status, β05 | – | – | – | −0.3930 | 0.4780 | 0.411 | −0.3516 | 0.4813 | 0.465 |
| Life events, β06 | – | – | – | 0.1828 | 0.2099 | 0.384 | 0.1785 | 0.2072 | 0.389 |
| Family SES β07 | – | – | – | 0.5107 | 0.4392 | 0.245 | 0.5434 | 0.4437 | 0.221 |
| Age, β08 | – | – | – | 0.2510 | 0.2266 | 0.269 | 0.2469 | 0.2283 | 0.280 |
| Daily Negative Affect, β09 | – | – | – | – | – | – | 2.3238 | 1.8952 | 0.221 |
| Depression, β010 | – | – | – | – | – | – | −0.0916 | 0.1323 | 0.489 |
| Weekend (Intercept), β10 | – | – | – | −0.2702 | 0.2356 | 0.252 | −0.2702 | 0.2356 | 0.252 |
|
| |||||||||
| Sleep quality, π0 | |||||||||
| Average Sleep quality (Intercept), β00 | 3.411 | 0.018 | <.001 | 3.5094 | 0.0458 | <.001 | 3.5038 | 0.0460 | <.001 |
| Trait Loneliness, β01 | −0.102 | 0.039 | 0.009 | −0.0981 | 0.0400 | 0.014 | −0.0719 | 0.0461 | 0.119 |
| Daily Loneliness, β02 | −0.096 | 0.047 | 0.042 | −0.0681 | 0.0464 | 0.143 | −0.0375 | 0.0597 | 0.530 |
| Female, β03 | – | – | – | 0.0137 | 0.0357 | 0.700 | 0.0106 | 0.0357 | 0.766 |
| Parental death, β04 | – | – | – | 0.0538 | 0.0514 | 0.295 | 0.0552 | 0.0512 | 0.281 |
| Health status, β05 | – | – | – | 0.0993 | 0.0245 | <.001 | 0.0984 | 0.0244 | <.001 |
| Life events, β06 | – | – | – | −0.0211 | 0.0093 | 0.024 | −0.0188 | 0.0094 | 0.047 |
| Family SES β07 | – | – | – | 0.0092 | 0.0121 | 0.447 | 0.0090 | 0.0120 | 0.457 |
| Age, β08 | – | – | – | −0.0322 | 0.0102 | 0.002 | −0.0317 | 0.0102 | 0.002 |
| Daily Negative Affect, β09 | – | – | – | – | – | – | −0.0414 | 0.0691 | 0.549 |
| Depression, β010 | – | – | – | – | – | – | −0.0061 | 0.0050 | 0.224 |
| Weekend, β10 | – | – | – | 0.0721 | 0.0255 | 0.005 | 0.0721 | 0.0255 | 0.005 |
|
| |||||||||
| Night awakenings, π0 | |||||||||
| Average Night awakenings (Intercept), β00 | 1.194 | 0.028 | <.001 | 1.2894 | 0.0702 | <.001 | 1.2910 | 0.0703 | <.001 |
| Trait Loneliness, β01 | 0.234 | 0.060 | <.001 | 0.1900 | 0.0610 | 0.002 | 0.1904 | 0.0697 | 0.006 |
| Daily Loneliness, β02 | 0.153 | 0.076 | 0.045 | 0.1664 | 0.0758 | 0.028 | 0.1456 | 0.0877 | 0.097 |
| Female, β03 | – | – | – | 0.0237 | 0.0559 | 0.671 | 0.0236 | 0.0562 | 0.675 |
| Parental death, β04 | – | – | – | 0.0676 | 0.0798 | 0.397 | 0.0674 | 0.0797 | 0.398 |
| Health status, β05 | – | – | – | −0.0286 | 0.0386 | 0.459 | −0.0279 | 0.0385 | 0.469 |
| Life events, β06 | – | – | – | 0.0050 | 0.0138 | 0.715 | 0.0046 | 0.0142 | 0.746 |
| Family SES β07 | – | – | – | −0.0307 | 0.0196 | 0.118 | −0.0302 | 0.0196 | 0.124 |
| Age, β08 | – | – | – | −0.0441 | 0.0165 | 0.008 | −0.0442 | 0.0164 | 0.007 |
| Daily Negative Affect, β09 | – | – | – | – | – | – | 0.0400 | 0.1022 | 0.696 |
| Depression, β010 | – | – | – | – | – | – | −0.0003 | 0.0079 | 0.975 |
| Weekend (Intercept), β10 | – | – | – | −0.0380 | 0.0343 | 0.268 | −0.0380 | 0.0343 | 0.268 |
Note: SES: Socioeconomic Status. SOL: Sleep Onset Latency.
Notably, all these effects, except for the negative association between trait loneliness and number of awakenings and the positive association between trait loneliness and time in bed, disappeared when we controlled for demographic and psychosocial covariates (Table 2, Model 3), possibly because of the strong overlap between loneliness and depression and daily negative affect.
Discussion
Two main results emerged from our analyses of cortisol. First, high levels of trait loneliness—but not daily loneliness—were found to predict lower morning cortisol levels. Second, between-person variation in daily loneliness was associated with variation in diurnal cortisol profiles, such that those people reporting high loneliness across the study days experienced a flatter diurnal slope. These results remained significant after controlling for demographic covariates as well as psychological constructs that overlap with loneliness, namely depression and daily negative affect.
The paucity of studies that have looked at the relationship between loneliness and diurnal cortisol across days thwarts any attempt to derive a coherent picture of the most robust findings within this literature. For example, Doane and Adam (Doane and Adam, 2010) found that chronic loneliness was associated with a flatter diurnal cortisol slope in sample of 108 mostly female older adolescents, while the same association was not found by Sladek and Doane in a smaller, but demographically comparable, sample (Sladek and Doane, 2015). In our study, although trait loneliness was not associated with a flattened cortisol circadian rhythm, we found that children reporting high levels of daily loneliness—averaged across the three testing days—had a flatter cortisol slope. These results mirror work by Pressman and colleagues (Pressman et al., 2005), who found that high daily loneliness was associated with high evening cortisol, and complement work done among young adults by Stetler and colleagues (Stetler et al., 2004), who showed that days with more social activities were associated with a more normative (i.e., steeper) cortisol daily decline compared with days where social contact was less present.
Blunted cortisol levels at awakening were observed among children high in trait loneliness. Previous research in youth has shown that low morning cortisol—a result of the overnight down-regulation of an overactive HPA axis—is often observed in tandem with severe neglect (Dozier et al., 2006; Bruce et al., 2009) and maladaptive coping (Slatcher et al., 2015). Because these psychological manifestations are also recurrent among chronically lonely individuals (Cacioppo et al., 2000; Cornman et al., 2003), the association between low morning cortisol at awakening and loneliness found in our study can be read in light of this literature.
Lastly, inconsistent with the existing literature, we did not find any covariation between any measures of loneliness and CAR. A few reasons might lay behind this null effect. First, the directionality of the relationship between loneliness and CAR is not very clear, as evidence for both a positive association (i.e. greater loneliness/larger CAR) (Steptoe et al., 2004; Adam et al., 2006; Doane and Adam, 2010) and a negative association (i.e. greater loneliness/smaller CAR) have accumulated (Sladek and Doane, 2015). Second, previous work suggests that CAR might be less accentuated in youth at risk, especially during the early phase of puberty (Quevedo et al., 2012). This explanation, along with potential problems with compliance might explain why the effect of CAR in our sample was found to be in the opposite direction that expected. These aspects might have contributed to decrease the variability and range in CAR and/or inflate the error variance associated with it, and consequently obscured any modulatory effects of third variables (e.g., loneliness) on CAR.
Turning to the sleep results, we found that children with high levels of trait loneliness reported lower sleep quality and a higher number of night awakenings. Daily loneliness also predicted a greater number of night awakenings. These findings were independent of individual differences in self-reported health, recent stressful events, and other demographic variables, and corroborate the well-established link between loneliness and diminished sleep quality (Cacioppo et al., 2002b; Steptoe et al., 2004; Pressman et al., 2005) and augmented night awakenings (Cacioppo et al., 2002a). The general consensus in the field is that trait loneliness does not impair sleep duration among older adolescents and adults (Mahon, 1994; Hawkley et al., 2010; Kurina et al., 2011 but see, Cacioppo et al., 2000, for a positive relationship between the two). In contrast with this literature, children in our study who scored higher on trait loneliness also reported more time in bed. One possible explanation for the positive association between trait loneliness and sleep duration is that the former causes distress and fatigue (Jaremka et al., 2013) and heighten sensitivity to pain (Oishi et al., 2013), and may lead the individual to sleep longer in the attempt to recover from chronic loneliness-related (e.g., ostracism) demands. Longer sleep duration might also be a way to offset the physiological (Pressman et al., 2005) and immune (Jaremka et al., 2013) costs associated with loneliness. Another possibility is that sleep duration is simply a byproduct of a coping strategy that relies on social withdraw as the main behavior to deal with the emotional adversities experienced during the day (Cacioppo et al., 2000). However, in our study we also found that high levels of daily loneliness were associated with less time in bed, suggesting that transient (i.e., daily) feelings of loneliness might lead to shorter sleep duration. One possibility is that daily loneliness ratings partially overlapped with the frequency and severity of daily stressors, which have been showed to lead to shorter sleep duration in precarious family environments (Hanson and Chen, 2010).
An important caveat about our sleep results was that, except for the trait loneliness/number of awakenings link and the trait loneliness/time in bed link, the significance of the presented associations disappeared after controlling for psychological constructs overlapping with loneliness, namely depression and daily negative affect. Interestingly, however, neither negative affect nor depression was associated with sleep disturbances, suggesting a multicollinearity issue. Future research is needed to more comprehensively understand the specific contribution of each construct by collecting data across a wider span (e.g., weeks) than the current study (i.e., days) as a way to reduce multicollinearity.
Both dysregulation of the HPA axis, in the form of morning hypocortisolism and flattening of the diurnal cortisol rhythm, and disruptions of restorative processes lead to adverse health outcomes (Goodyer et al., 1996; Gunnar and Vazquez, 2001; Bower et al., 2005; King et al., 2008; Matthews et al., 2006), including mortality (Kripke et al., 2002; Kumari et al., 2011). Accumulating research among adults and older adolescents point at these two mechanisms as the main pathways through which loneliness leads to medical morbidity and mortality. However, prospective studies have showed that the cumulative detrimental effects of loneliness on physical health start at a young age (Caspi et al., 2006; Harris et al., 2013). Notably, our study provides the first empirical evidence that some of these negative effects of loneliness might be mediated by alterations in cortisol secretion and sleep.
Our work is not without limitations. First, the restricted number of sampling days (i.e., 3) precluded us from modeling within-person changes in loneliness and testing its relations to HPA axis functioning and sleep. Likewise, the decision to implement two-level multilevel models prevented us from modeling daily predictors (e.g., weekday) in cortisol analyses. Future studies should include loneliness, cortisol, and sleep assessments over a greater number of time days to fully capture the complex relationships among our study variables. That said, the limited sampling regime was offset by a large sample, which lends increased confidence about the current findings. Second, because of the correlational nature of our study, some of the relationships between loneliness and sleep outcomes we observed could be explained in terms of sleeping habits influencing loneliness. For example, it is possible that spending more time in bed, either sleeping or engaging in ludic activities, may lead to more opportunities for children to socially isolate themselves. Thus, future studies involving multiple and longitudinal sleep and loneliness measures will be necessary before stronger causal claims could be made. Third, one trade-off in our study was the reliance on self-reported measures of sleep in favor of a large sample size. Despite being widely used, subjective reports of sleep disturbances might deviate from objective measures of sleep duration and quality (Bertocci et al., 2005; Tremaine et al., 2010). For example, in their study, Bertocci and colleagues (2005) found no difference in objective EEG-recorded number of awakenings between children with major depressive disorder and matched controls, despite the fact that the former group self-reported a greater number of awakenings than the latter group. Thus, it is important for future studies to replicate our findings by using more sophisticated sleep measures, such as actigraphy (Sadeh et al., 1995). Fourth, because the age range of our sample was broad (8–15 years old), children at presumably different stages of development (e.g., older youths undergoing puberty) were included. This weakness of our study should be considered in future research aiming at understanding the role played by developmental switch points on cortisol modulation. A last caveat to consider in our study concerns the uniqueness of our sample, which might limit the generalizability to other subject populations.
To summarize, in a sample of 645 children affected by parental HIV, we found that high levels of trait loneliness predicted lower morning cortisol levels, longer time in bed, lower sleep quality, and a higher number of night awakenings, daily loneliness was associated with a flatter diurnal cortisol slope and shorter time in bed. Although the association between trait loneliness and daily loneliness with HPA activity remained significant after controlling for psychological constructs that overlap with loneliness (i.e., depression and daily negative affect), some of the associations between loneliness and sleep measures disappeared after including these additional covariates. We hope that the results of our study can help guide future intervention studies and stimulate further research among high-risk populations on the biobehavioral pathways through which loneliness exerts its effects on health across the lifespan.
Highlights.
Among 645 children, high trait loneliness predicted low morning cortisol.
Children reporting high daily loneliness experienced a flatter diurnal cortisol slope.
Trait loneliness was negatively associated with time in bed.
Trait loneliness was negatively associated with sleep quality.
Trait loneliness was negatively associated with number of night awakenings.
Acknowledgments
Role of funding sources
Data collection was supported by the National Institute on Aging (Grant R01NINR013466).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SOL was strongly skewed, so analyses were re-run using log-transformed values instead of raw data. Notably, results did not change.
Author Contributions
SZ, RBS, PC, XL, JZ, GZ: study conceptualization. SZ: data analyses. SZ and RBS: writing. SZ, RBS, PC, XL: editing.
Conflict of Interest
None declared.
References
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience–cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Asher SR, Hymel S, Renshaw PD. Loneliness in children. Child Dev. 1984:1456–1464. [Google Scholar]
- Barth J, Schneider S, von Känel R. Lack of social support in the etiology and the prognosis of coronary heart disease: a systematic review and meta-analysis. Psychosom Med. 2010;72:229–238. doi: 10.1097/PSY.0b013e3181d01611. [DOI] [PubMed] [Google Scholar]
- Bertocci MA, Dahl RE, Williamson DE, Iosif AM, Birmaher B, Axelson D, Ryan ND. Subjective sleep complaints in pediatric depression: a controlled study and comparison with EEG measures of sleep and waking. J Am Acad Child Adolesc Psychiatry. 2005;44:1158–1166. doi: 10.1097/01.chi.0000179057.54419.17. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30:92–100. doi: 10.1016/j.psyneuen.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol Levels in preschool-aged foster children: Differential effects of maltreatment type. Dev Psychobiol. 2009;51:14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Ernst JM, Burleson MH, McClintock MK, Malarkey WB, Hawkley LC, Kowalewski RB, Paulsen A, Hobson JA, Hugdahl K. Lonely traits and concomitant physiological processes: the MacArthur social neuroscience studies. Int J Psychophysiol. 2000;35:143–154. doi: 10.1016/s0167-8760(99)00049-5. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Berntson GG, Ernst JM, Gibbs AC, Stickgold R, Hobson JA. Do lonely days invade the nights? Potential social modulation of sleep efficiency. Psychol Sci. 2002a;13:384–387. doi: 10.1111/1467-9280.00469. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Crawford LE, Ernst JM, Burleson MH, Kowalewski RB, Malarkey WB, Van Cauter E, Berntson GG. Loneliness and health: Potential mechanisms. Psychosom Med. 2002b;64:407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Cairns RB, Leung MC, Buchanan L, Cairns BD. Friendships and social networks in childhood and adolescence: Fluidity, reliability, and interrelations. Child Dev. 1995;66:1330–1345. [PubMed] [Google Scholar]
- Caspi A, Harrington H, Moffitt TE, Milne BJ, Poulton R. Socially isolated children 20 years later: risk of cardiovascular disease. Arch Pediatr Adolesc Med. 2006;160:805–811. doi: 10.1001/archpedi.160.8.805. [DOI] [PubMed] [Google Scholar]
- Chen X, He Y, Oliveira AMD, Coco AL, Zappulla C, Kaspar V, Schneider B, Valdivia IA, Tse HCH, DeSouza A. Loneliness and social adaptation in Brazilian, Canadian, Chinese and Italian children: a multi-national comparative study. Journal of Child Psychology and Psychiatry. 2004;45:1373–1384. doi: 10.1111/j.1469-7610.2004.00844.x. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang L, Li D, Liu J. Loneliness in Chinese children across contexts. Dev Psychol. 2014;50:2324–2333. doi: 10.1037/a0037689. [DOI] [PubMed] [Google Scholar]
- Chi P, Li X. Impact of parental HIV/AIDS on children’s psychological well-being: a systematic review of global literature. AIDS Behav. 2013;17:2554–2574. doi: 10.1007/s10461-012-0290-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi P, Li X, Zhao J, Zhao G. Vicious circle of perceived stigma, enacted stigma and depressive symptoms among children affected by HIV/AIDS in China. AIDS Behav. 2014;18:1054–1062. doi: 10.1007/s10461-013-0649-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluver LD, Gardner F, Operario D. Effects of stigma on the mental health of adolescents orphaned by AIDS. J Adolesc Health. 2008;42:410–417. doi: 10.1016/j.jadohealth.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM. Social ties and susceptibility to the common cold. JAMA. 1997;277:1940–1944. [PubMed] [Google Scholar]
- Collins LM, Schafer JL, Kam CM. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychological methods. 2001;6:330–351. [PubMed] [Google Scholar]
- Cornman JC, Goldman N, Glei DA, Weinstein M, Chang MC. Social Ties and Perceived Support Two Dimensions of Social Relationships and Health among the Elderly in Taiwan. J Aging Health. 2003;15:616–644. doi: 10.1177/0898264303256215. [DOI] [PubMed] [Google Scholar]
- De Jong-Gierveld J, Raadschelders J. Types of loneliness. Loneliness: A sourcebook of current theory, research and therapy. 1982:105–119. [Google Scholar]
- Doane LD, Adam EK. Loneliness and cortisol: Momentary, day-to-day, and trait associations. Psychoneuroendocrinology. 2010;35:430–441. doi: 10.1016/j.psyneuen.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier M, Manni M, Gordon MK, Peloso E, Gunnar MR, Stovall-McClough KC, Eldreth D, Levine S. Foster children’s diurnal production of cortisol: An exploratory study. Child Maltreat. 2006;11:189–197. doi: 10.1177/1077559505285779. [DOI] [PubMed] [Google Scholar]
- Du H, Chi P, Li X, Zhao J, Zhao G. Relational self-esteem, psychological well-being, and social support in children affected by HIV. Journal of health psychology. 2014;20:1568–1578. doi: 10.1177/1359105313517276. [DOI] [PubMed] [Google Scholar]
- Edwards KM, Bosch JA, Engeland CG, Cacioppo JT, Marucha PT. Elevated macrophage migration inhibitory factor (MIF) is associated with depressive symptoms, blunted cortisol reactivity to acute stress, and lowered morning cortisol. Brain, Behav, Immun. 2010;24:1202–1208. doi: 10.1016/j.bbi.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Eng PM, Rimm EB, Fitzmaurice G, Kawachi I. Social ties and change in social ties in relation to subsequent total and cause-specific mortality and coronary heart disease incidence in men. Am J Epidemiol. 2002;155:700–709. doi: 10.1093/aje/155.8.700. [DOI] [PubMed] [Google Scholar]
- Fees BS, Martin P, Poon LW. A model of loneliness in older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 1999;54:P231–P239. doi: 10.1093/geronb/54b.4.p231. [DOI] [PubMed] [Google Scholar]
- Fendrich M, Weissman MM, Warner V. Screening for depressive disorder in children and adolescents: validating the center for epidemiologic studees depression scale for children. Am J Epidemiol. 1990;131:538–551. doi: 10.1093/oxfordjournals.aje.a115529. [DOI] [PubMed] [Google Scholar]
- Garde AH, Hansen A. Long- term stability of salivary cortisol. Scand J Clin Lab Invest. 2005;65:433–436. doi: 10.1080/00365510510025773. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Altham PME, Pearson J, Secher SM, Shiers HM. Adrenal secretion during major depression in 8-to 16-year-olds, I. Altered diurnal rhythms in salivary cortisol and dehydroepiandrosterone (DHEA) at presentation. Psychol Med. 1996;26:245–256. doi: 10.1017/s0033291700034644. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Dev Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hanson MD, Chen E. Daily stress, cortisol, and sleep: the moderating role of childhood psychosocial environments. Health Psychol. 2010;29:394. doi: 10.1037/a0019879. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Dodsworth RO, Harlow MK. Total social isolation in monkeys. Proc Natl Acad Sci USA. 1965;54:90–97. doi: 10.1073/pnas.54.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA, Qualter P, Robinson SJ. Loneliness trajectories from middle childhood to pre-adolescence: Impact on perceived health and sleep disturbance. J Adolesc. 2013;36:1295–1304. doi: 10.1016/j.adolescence.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Cacioppo JT. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. nn Behav Med. 2010;40:218–227. doi: 10.1007/s12160-010-9210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley LC, Masi CM, Berry JD, Cacioppo JT. Loneliness is a unique predictor of age-related differences in systolic blood pressure. Psychol Aging. 2006;21:152–164. doi: 10.1037/0882-7974.21.1.152. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Preacher KJ, Cacioppo JT. Loneliness impairs daytime functioning but not sleep duration. Health Psychol. 2010;29:124–129. doi: 10.1037/a0018646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Psychology. 2015;66:143–172. doi: 10.1146/annurev-psych-010213-115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaremka LM, Fagundes CP, Glaser R, Bennett JM, Malarkey WB, Kiecolt-Glaser JK. Loneliness predicts pain, depression, and fatigue: Understanding the role of immune dysregulation. Psychoneuroendocrinology. 2013;38:1310–1317. doi: 10.1016/j.psyneuen.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Ricker D, George J, Messick G, Speicher CE, Garner W, Glaser R. Urinary cortisol levels, cellular immunocompetency, and loneliness in psychiatric inpatients. Psychosom Med. 1984;46:15–23. doi: 10.1097/00006842-198401000-00004. [DOI] [PubMed] [Google Scholar]
- King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300:2859–2866. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- Kumari M, Shipley M, Stafford M, Kivimaki M. Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: findings from the Whitehall II study. The Journal of Clinical Endocrinology & Metabolism. 2011;96:1478–1485. doi: 10.1210/jc.2010-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurina LM, Knutson KL, Hawkley LC, Cacioppo JT, Lauderdale DS, Ober C. Loneliness is associated with sleep fragmentation in a communal society. Sleep. 2011;34:1519–1526. doi: 10.5665/sleep.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Barnett D, Fang X, Lin X, Zhao G, Zhao J, Hong Y, Zhang L, Naar-King S, Stanton B. Lifetime incidences of traumatic events and mental health among children affected by HIV/AIDS in rural China. Journal of Clinical Child & Adolescent Psychology. 2009;38:731–744. doi: 10.1080/15374410903103601. [DOI] [PubMed] [Google Scholar]
- Mahon NE. Loneliness and sleep during adolescence. Percept Motor Skills. 1994;78:227–231. doi: 10.2466/pms.1994.78.1.227. [DOI] [PubMed] [Google Scholar]
- Matthews K, Schwartz J, Cohen S, Seeman T. Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosom Med. 2006;68:657–661. doi: 10.1097/01.psy.0000244071.42939.0e. [DOI] [PubMed] [Google Scholar]
- Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyblade L, Stangl A, Weiss E, Ashburn K. Combating HIV stigma in health care settings: what works? Journal of the international AIDS Society. 2009;12:15. doi: 10.1186/1758-2652-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi S, Schiller J, Gross EB. Felt understanding and misunderstanding affect the perception of pain, slant, and distance. Social psychological and personality science. 2013;4:259–266. [Google Scholar]
- Parkhurst JT, Asher SR. Peer rejection in middle school: Subgroup differences in behavior, loneliness, and interpersonal concerns. Dev Psychol. 1992;28:231–241. [Google Scholar]
- Patterson AC, Veenstra G. Loneliness and risk of mortality: A longitudinal investigation in Alameda County, California. Soc Sci Med. 2010;71:181–186. doi: 10.1016/j.socscimed.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Peng CYJ, Harwell M, Liou SM, Ehman LH. Advances in missing data methods and implications for educational research. Real data analysis. 2006:31–78. [Google Scholar]
- Pressman SD, Cohen S, Miller GE, Barkin A, Rabin BS, Treanor JJ. Loneliness, social network size, and immune response to influenza vaccination in college freshmen. Health Psychol. 2005;24:297–306. doi: 10.1037/0278-6133.24.3.297. [DOI] [PubMed] [Google Scholar]
- Qiao S, Li X, Zhao G, Zhao J, Stanton B. The role of perceived social support in loneliness and self-esteem among children affected by HIV/AIDS: a longitudinal multilevel analysis in rural China. AIDS. 2014;28:S369–S377. doi: 10.1097/QAD.0000000000000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo K, Johnson AE, Loman ML, LaFavor TL, Gunnar M. The confluence of adverse early experience and puberty on the cortisol awakening response. International journal of behavioral development. 2012;36:19–28. doi: 10.1177/0165025411406860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, Hauri PJ, Kripke DF, Lavie P. The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18:288–302. doi: 10.1093/sleep/18.4.288. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychological methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Sladek MR, Doane LD. Daily diary reports of social connection, objective sleep, and the cortisol awakening response during adolescents’ first year of college. Journal of youth and adolescence. 2015;44:298–316. doi: 10.1007/s10964-014-0244-2. [DOI] [PubMed] [Google Scholar]
- Slatcher RB, Chi P, Li X, Zhao J, Zhao G, Ren X, Zhu J, Stanton B. Associations Between Coping and Diurnal Cortisol Among Children Affected by Parental HIV/AIDS. Health Psychol. 2015 doi: 10.1037/hea0000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Owen N, Kunz-Ebrecht SR, Brydon L. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middle-aged men and women. Psychoneuroendocrinology. 2004;29:593–611. doi: 10.1016/S0306-4530(03)00086-6. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proceedings of the National Academy of Sciences. 2013;110:5797–5801. doi: 10.1073/pnas.1219686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler C, Dickerson SS, Miller GE. Uncoupling of social zeitgebers and diurnal cortisol secretion in clinical depression. Psychoneuroendocrinology. 2004;29:1250–1259. doi: 10.1016/j.psyneuen.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Tremaine RB, Dorrian J, Blunden S. Subjective and objective sleep in children and adolescents: Measurement, age, and gender differences. Sleep and Biological Rhythms. 2010;8:229–238. [Google Scholar]


