Abstract
Objectives
To evaluate the feasibility and safety of head-neck cooling in conscious normal volunteers (10) and patients with medically refractory epilepsy (5) without causing shivering.
Patients and methods
We used a non-invasive head-neck cooling system (CoolSystems Inc., Lincoln, CA, USA). The tympanic temperature (TT) and intestinal temperature (IT) were measured as two measurements of ‘core temperature’ (CT), and multi-site external temperatures, several physiologic variables and EEG were monitored. Seizure counts over 4-week precooling, treatment and follow-up phases were compared.
Results
All 15 participants completed all the cooling sessions without significant complaints. At the end of 60 min of cooling, scalp temperature fell on average by 12.2°C (P < 0.001), TT by 1.67°C (P < 0.001), and IT by 0.12°C (P = NS). Average weekly seizure frequency decreased from 2.7 to 1.7 events per patient per week (MANOVA: P < 0.05).
Conclusions
Non-invasive head-neck cooling is safe and well-tolerated. Initial pilot data in patients suggest that additional therapeutic studies are warranted.
Keywords: brain, core temperature, cortical excitability, epilepsy, non-invasive hypothermia, interictal cooling, seizures, treatment
Introduction
Significant advances in understanding epileptogenesis over the last two decades have not translated into effective treatments for many patients with medically refractory epilepsy who are not surgical candidates or who failed surgery (1). In spite of progress in pharmacotherapy (2) and surgery (3), at least one-third of patients continue to suffer from uncontrolled seizures and/or unwanted side effects of medications, underlining the need for new therapeutic approaches (1–4). Vagal nerve stimulation is an approved therapy (5), while transcranial magnetic stimulation (TMS) was ineffective in a controlled trial and more invasive modalities are in clinical trials (6).
Experimental and clinical evidence suggest that acute hypothermia may be a useful treatment option for ongoing seizures (7–11). Focal invasive approaches are being examined (11). Noninvasive whole head cooling without sedation (12) is an appealing alternative, but its feasibility and safety have not been established in non-sedated and/or non-anesthetized individuals, and lasting effects of interictal hypothermia are unknown.
We studied the feasibility and safety of noninvasive head and neck cooling without sedation in conscious normal volunteers without causing shivering. Here, we also describe the first exploratory interictal application of the same cooling method to initially asses its sustained therapeutic potential in a pilot study of five patients with medically refractory epilepsy.
However, this is an uncontrolled pilot study not designed to evaluate therapeutic effect of hypothermia on seizures and its therapeutic outcome has to be interpreted very cautiously.
Subjects and methods
Cooling was performed using a head-neck cooling system (CoolSystems, Inc., Lincoln, CA, USA) previously used for anesthetized and/or sedated patients with MS and head injury (12, 13). It includes a head/neck liner made of a lightweight, thin, flexible, laminated nylon urethane fabric, and a conditioning unit (temperature control, liquid pump and pressure control, air pump). We used the lowest temperature which the cooling device can produce. The cooling headgear was applied 30 min before the cooling started and removed at the end of cooling. During each session, temperature was measured every 5 min from the scalp, forearm, abdomen, and leg using a temperature transducer (Biolog, UFI, Morro Bay, CA, USA), from both ears [tympanic temperature (TT) (Braun PRO 3000 Thermometer, Braun GmbH, Germany)], and from the face and mouth using a hand-held infrared thermometer (D160 Infrared Thermometer Made In Japan, Addison, IL, USA). Intestinal temperature (IT) was measured using a temperature sensor pill (HQ, Inc., Palmetto, FL, USA) swallowed by each subject 5–6 h before cooling. These methods for measuring TT and IT were chosen for patients’ comfort and compliance. Temperature measurements were taken every 5 min from 30 min before until 30 min after cooling session. Peripheral oxygen saturation and pulse rates were monitored with a pulse oximeter.
Ten normal volunteers (age 21–47 years, five males and five females) underwent two cooling sessions (30 and 60 min) 3 days separately (Table 1). Each subject was comfortably seated in a reclining chair and covered with blankets to keep the body warm during cooling. To minimize electrode interference with the optimal fitting and cooling efficacy of an elastic cooling helmet, only nine-channel scalp EEG from parasagital chain (plus ECG) was recorded from 30 min before until 30 min after cooling. Following this session, the participants had their lunch and subsequently underwent 3 h of standard 21-channel video-EEG (V-EEG) monitoring before their discharge.
Table 1.
Temperature changes from baseline (°C) in healthy volunteers
| 30-Min cooling | 60-Min cooling | |||||||
|---|---|---|---|---|---|---|---|---|
| Scalp | Left ear |
Right ear |
IT | Scalp | Left ear |
Right ear |
IT | |
| Males | ||||||||
| Mean | −14.0 | −0.78 | −0.92 | −0.11 | −13.4 | −2.04 | −1.24 | −0.15 |
| SD | 2.1 | 0.27 | 0.58 | 0.15 | 1.8 | 0.75 | 0.84 | 0.3 |
| Females | ||||||||
| Mean | −10.7 | −1.14 | −1.52 | −0.01 | −11.0 | −1.58 | −1.88 | −0.08 |
| SD | 3.08 | 0.36 | 0.56 | 0.1 | 1.1 | 0.86 | 0.91 | 0.26 |
| Total mean | −12.4 | −0.96 | −1.22 | −0.06 | −12.2 | −1.81 | −1.56 | −0.12 |
The ’−’ sign indicates reduction and no sign indicates increase.
IT, intestinal temperature.
Five patients with medically refractory epilepsy having at least one seizure per week averaged over a 12-week precooling phase were recruited (Table 2). Patients were admitted to the hospital overnight for each of four 60-min weekly cooling sessions during a treatment phase. Cooling and subsequent V-EEG were performed using the same procedure as of volunteers. Patients were followed, and kept seizure calendars, at least for 4 weeks after cooling (a follow-up phase). No antiepileptic medications adjustments were allowed throughout the study period except for acute treatments with benzodiazepines and/or (fos)phenytoin as deemed necessary by emergency room personnel.
Table 2.
Patients’ clinical data and weekly seizure counts before, during and after cooling
| Patient | History | 12-Week baseline (seizures per week) |
Immediate 4 weeks before cooling (seizures per week) |
4-Week cooling phase (seizures per week) |
Immediate 4 weeks after cooling (seizures per week) |
|---|---|---|---|---|---|
| 1 (49 years, ♀) | Failed two temporal lobectomies | 1.25 | 1.75 | 1.50 | 0.75 |
| 2 (52 years, ♂) | With schizencephaly | 1.0 | 0.75 | 0 | 1.25 |
| 3 (29 years, ♂) | Post-traumatic seizures | 3.33 | 3.75 | 4.0 | 2.25 |
| 4 (35 years, ♂) | Multiple family members with epilepsy | 4.67 | 4.50 | 5.25 | 2.5 |
| 5 (26 years, ♂) | s/p right occipital heterotopia removal | 2.83 | 3.0 | 2.75 | 1.75 |
s/p, status post.
Paired Student’s t-test was used to compare the temperatures before and after cooling in healthy volunteers and patients. The MANOVA for repeated measurements was used to compare the seizure frequency in patients during the last 4 weeks of 12-week baseline phase (‘before’), a 4-week treatment phase (‘during’), and 4-week follow-up phase (‘after’).
The studies were approved by the FDA (IDE # G010202 for normal volunteers, G030058/S1 for patients) and National Institute of Neurological Disorders and Stroke (NINDS) IRB.
Results
Volunteers
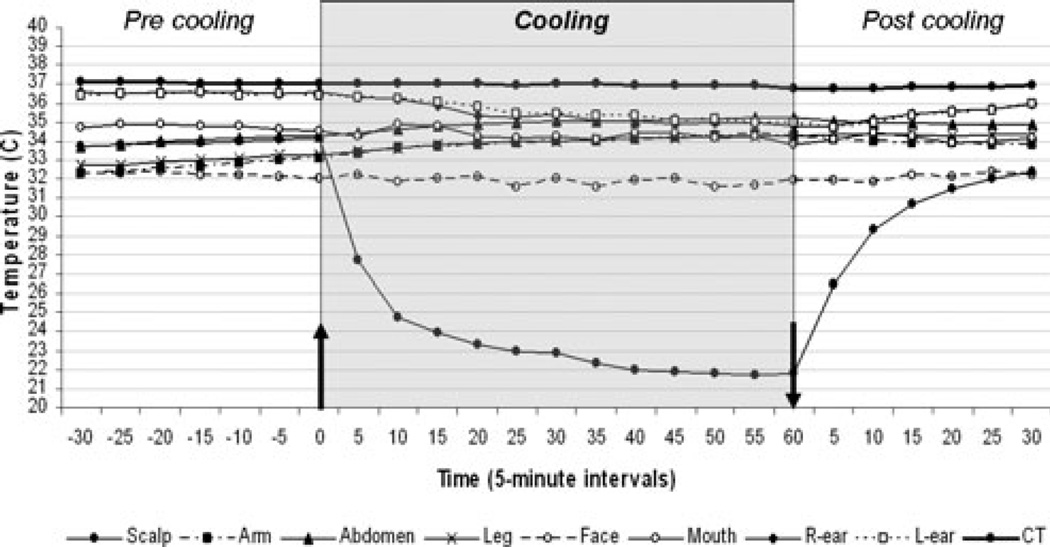
Output temperature of the cooling system (the temperature of circulating water in the system) at the beginning of cooling was approximately 1.5°C and rose to 4.5–5°C at the end of cooling. Just before cooling began, the average scalp temperature was 34.1°C, the right ear 36.6°C, the left ear 36.5°C, and IT 37.1°C. The overall mean reduction of IT at the end of 30 min of cooling was 0.06°C and at the end of 60 min of cooling was 0.12°C (Table 1). A statistically significant difference was seen between the start and the end of cooling in both scalp temperature (P < 0.001) and TT in both ears (P < 0.001), but not in IT measured intestinally (P = NS). There was no statistical difference between men and women in degrees of temperature changes except for scalp temperature at the end of the 60-min cooling sessions (P < 0.05). Temperatures from arm, abdomen, and leg were unchanged throughout both 30- and 60-min cooling sessions, as were heart rate and oxygen saturation. Fig. 1 shows average temperature changes before, during, and after 60 min of cooling in volunteers. All volunteers remained mentally alert and coherent throughout cooling. Discomforts during cooling included mild ‘ice cream’ headache (1), mild nausea (1), mild headache and nauseated feeling (1), and ‘chilly sensation’ (1). No interruption of cooling, however, was necessary in any of these cases. One female subject awakened with a mild headache on the morning of the experiment, but reported improvement during cooling.
Figure 1.
Temperature changes before, during and after 60-min cooling of healthy volunteers. Arrows indicate start and end of cooling.
Patients
During the cooling sessions of all patients, there were no significant differences between patients (data not shown) and control values (Table 1). There were no changes in the patients’ physical examination after cooling, and none of the patients reported any complaints that necessitated interruption or adjustment of cooling.
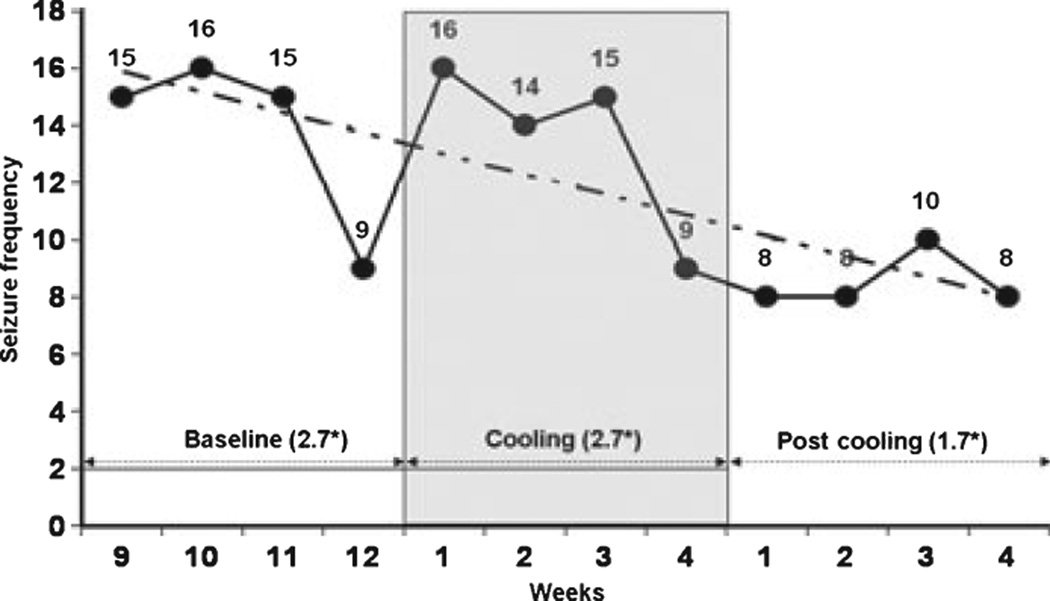
Cooling appeared to reduce seizure frequency during the 4-week follow-up phase (Table 2, Fig. 2). The difference between average weekly seizure frequency averaged over the respective 4-week intervals before, during, and after the treatment phase was statistically significant at a 5% threshold level using MANOVA (F = 15.202, DF = 2, Significance < 0.05).
Figure 2.
Weekly group seizure frequency (thick line) with a linear trend (broken line) and overall average* [*weekly 4-week average seizure frequency = all seizures for the group over the respective 4 weeks/20 · (five patients × 4 weeks)] during 4-week of baseline phase immediately prior to cooling, 4-week cooling phase and 4-week follow-up phase. The decrease in a weekly group 4-week average seizure frequency was statistically significant at 5% threshold level using the MANOVA (F = 15.202, DF = 2, Significance < 0.05)].
Discussion
Human brain cooling typically has been performed under sedation or general anesthesia with or without brain exposure (7, 12, 14, 15). We show the feasibility and safety of head-neck cooling in conscious normal volunteers and patients with medically refractory epilepsy without causing shivering.
Potential acute neurological effects of hypothermia were noticed more than a century ago, while major advances in elucidating potential mechanisms were made relatively recently [for review, see (16)]. Hyperthermia decreases seizure threshold acutely (7), while cortical cooling alters brain electrical behavior leading to increased seizure threshold (8–10, 15, 17, 18) and may acutely stop seizures in animal models and patients (7–10). Cold saline application stops ongoing after-discharges induced by electrical stimulation, and spontaneous interictal epileptiform discharges (19), and stimulation-evoked seizures (13).
Although the output temperature from the cooling system was considerably lower in our study (1.1–1.7°C) than in a recent MS study (12.7°C) (20), we encountered no side effects requiring cooling discontinuation. Shivering was prevented by keeping the torso warm during cooling (13). Cooling efficacy may depend on headgear fit and amount of hair; a 35-year-old man with very short hair whose cooling helmet fit exceptionally well had the lowest IT after 30 min of cooling and the second lowest after 60 min of cooling; head shaving may be beneficial (12). Monitored parameters and clinical observations revealed no physiological abnormalities or signs of distress, confirming earlier observations (11, 21). The method appears to have adequate safety and tolerability.
The best method for assessing brain temperature non-invasively is uncertain. TT may not be a valid measure of intracerebral temperature or core temperature (CT) because of reading contamination caused by direct cooling of the contiguous skin (22–25). Directly measured brain temperatures are always higher than CT (26). Thus, the correlation of the CT with brain temperature and the best noninvasive methods for measuring the former are still uncertain (22–25, 27). Intestinal readings obtained by a temperature sensor did not reveal statistically significant changes in IT. This may be a consequence of technological limitations of the method we used (28) or measurement errors (29).
It is uncertain whether a statistically significant change in CT is required to achieve acute or lasting therapeutic CNS effects (11, 30). There seems to be a wide temperature safety window before normal brain function is significantly affected by cooling (11, 21, 31, 32). Using the same cooling system, as in our study, brain temperature was monitored invasively using an intracerebral temperature sensor (18). An average reduction in intracerebral temperature of 1.84°C (range 0.9–2.4°C) within 1 h of applying the cooling helmet was not associated with a significant change in CT measured in bladder. Thus, brain temperature and CT in normal volunteers and patients in our study may have been lower than the IT measured, particularly, as there was a statistically significant drop in the TT in both the groups (33).
Our study was designed to assess safety, tolerability, and acceptability and not therapeutic efficacy. The reduction in mean seizure frequency during the follow-up phase may have been due to ‘random’ fluctuations, the placebo effect, ‘the effect of informed-consent procedures, the effect of medical and nursing care (the ‘Hawthorne’ and ‘halo’ effects), and the patient–doctor relationship’ (34) – that may influence the perception of therapeutic benefit (35, 36) in our unblinded trial, although it is interesting that there was no reduction during the cooling period itself, perhaps related to acute effects of rewarming (37). However, cooling might have prolonged effects (38). A controlled trial could be performed by regulating helmet output to produce a mild scalp sensation but no CT change.
References
- 1.Stefan H, Lopes da Silva FH, Loscher W, et al. Epileptogenesis and rational therapeutic strategies. Acta Neurol Scand. 2006;113:139–155. doi: 10.1111/j.1600-0404.2005.00561.x. [DOI] [PubMed] [Google Scholar]
- 2.Klitgaard H. Antiepileptic drug discovery: lessons from the past and future challenges. Acta Neurol Scand Suppl. 2005;181:68–72. doi: 10.1111/j.1600-0404.2005.00513.x. [DOI] [PubMed] [Google Scholar]
- 3.Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128:1188–1198. doi: 10.1093/brain/awh449. [DOI] [PubMed] [Google Scholar]
- 4.Brodie MJ. Diagnosing and predicting refractory epilepsy. Acta Neurol Scand Suppl. 2005;181:36–39. doi: 10.1111/j.1600-0404.2005.00507.x. [DOI] [PubMed] [Google Scholar]
- 5.Tecoma ES, Iragui VJ. Vagus nerve stimulation use and effect in epilepsy: what have we learned? Epilepsy Behav. 2006;8:127–136. doi: 10.1016/j.yebeh.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Theodore WH, Fisher R. Brain stimulation for epilepsy. Acta Neurochir Suppl. 2007;2:261–272. doi: 10.1007/978-3-211-33081-4_29. [DOI] [PubMed] [Google Scholar]
- 7.Vastola EF, Homan R, Rosen A. Inhibition of focal seizures by moderate hypothermia: a clinical and experimental study. Arch Neurol. 1969;20:430–439. doi: 10.1001/archneur.1969.00480100106015. [DOI] [PubMed] [Google Scholar]
- 8.Yang X-F, Duffy DW, Morley RE, Rothman SM. Neocortical seizure termination by focal cooling: temperature dependence and automated seizure detection. Epilepsia. 2002;43:240–245. doi: 10.1046/j.1528-1157.2002.33301.x. [DOI] [PubMed] [Google Scholar]
- 9.Yang X-F, Chang JH, Rothman SM. Intracerebral temperature alterations associated with focal seizures. Epilepsy Res. 2002;52:97–105. doi: 10.1016/s0920-1211(02)00193-6. [DOI] [PubMed] [Google Scholar]
- 10.Yang X-F, Chang JH, Rothman SM. Long-lasting anticonvulsant effect of focal cooling on experimental neocortical seizures. Epilepsia. 2003;44:1500–1505. doi: 10.1111/j.0013-9580.2003.23003.x. [DOI] [PubMed] [Google Scholar]
- 11.Rothman SM, Smyth MD, Yang X-F, Peterson GP. Focal cooling for epilepsy: an alternative therapy that might actually work. Epilepsy Behav. 2005;7:214–221. doi: 10.1016/j.yebeh.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Olivero W, Lanzino G, et al. Rapid and selective cerebral hypothermia achieved using a cooling helmet. J Neurosurg. 2004;100:272–277. doi: 10.3171/jns.2004.100.2.0272. [DOI] [PubMed] [Google Scholar]
- 13.Ku YTE, Montgomery LD, Webbon BW. Hemodynamic and thermal responses to head and neck cooling in men and women. Am J Phys Med Rehabil. 1996;75:443–450. doi: 10.1097/00002060-199611000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Sourek K, Travnicek V. General and local hypothermia of the brain in the treatment of intractable epilepsy. J Neurosurg. 1970;33:253–259. doi: 10.3171/jns.1970.33.3.0253. [DOI] [PubMed] [Google Scholar]
- 15.Sartorius CJ, Berger MS. Rapid termination of intraoperative stimulation-evoked seizures with application of cold Ringer’s lactate to the cortex. Technical Note. J Neurosurg. 1998;88:349–351. doi: 10.3171/jns.1998.88.2.0349. [DOI] [PubMed] [Google Scholar]
- 16.Yang X-F, Ouyang Y, Kennedy BR, Rothman SM. Cooling blocks rat hippocampal neurotransmission by a presynaptic mechanism: observations using 2-photon microscopy. J Physiol. 2005;567:215–224. doi: 10.1113/jphysiol.2005.088948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill MW, Wong M, Amarakone A, Rothman SM. Rapid cooling aborts seizure-like activity in rodent hippocampal-entorhinal slices. Epilepsia. 2000;41:1241–1248. doi: 10.1111/j.1528-1157.2000.tb04601.x. [DOI] [PubMed] [Google Scholar]
- 18.Yang X-F, Rothman SM. Focal cooling rapidly terminates experimental neocortical seizures. Ann Neurol. 2001;49:721–726. doi: 10.1002/ana.1021. [DOI] [PubMed] [Google Scholar]
- 19.Karkar KM, Garcia PA, Bateman LM, Smyth MD, Barbaro NM, Berger M. Focal cooling suppresses spontaneous epileptiform activity without changing the cortical motor threshold. Epilepsia. 2002;43:932–935. doi: 10.1046/j.1528-1157.2002.03902.x. [DOI] [PubMed] [Google Scholar]
- 20.NASA/MS Cooling Study Group. A randomized controlled study of the acute and chronic effects of cooling therapy for MS. Neurology. 2003;60:1955–1960. doi: 10.1212/01.wnl.0000070183.30517.2f. [DOI] [PubMed] [Google Scholar]
- 21.Bakken HE, Kawasaki H, Oya H, Greenlee JD, Howard MA., III A device for cooling localized regions of human cerebral cortex. J Neurosurg. 2003;99:604–608. doi: 10.3171/jns.2003.99.3.0604. [DOI] [PubMed] [Google Scholar]
- 22.Fulbrook P. Core body temperature measurement: a comparison of axilla, tympanic membrane and pulmonary artery blood temperature. Intensive Crit Care Nurs. 1997;13:266–272. doi: 10.1016/s0964-3397(97)80425-9. [DOI] [PubMed] [Google Scholar]
- 23.Mariak Z, White MD, Lyson T, Lewko J. Tympanic temperature reflects intracranial temperature changes in humans. Pflugers Arch. 2003;446:279–284. doi: 10.1007/s00424-003-1021-3. [DOI] [PubMed] [Google Scholar]
- 24.Farnell S, Maxwell L, Tan S, Rhodes A, Philips B. Temperature measurement: comparison of non-invasive methods used in adult critical care. J Clin Nurs. 2005;14:632–639. doi: 10.1111/j.1365-2702.2004.00916.x. [DOI] [PubMed] [Google Scholar]
- 25.Nimah MM, Bshesh K, Callahan JD, Jacobs BR. Infrared tympanic thermometry in comparison with other temperature measurement techniques in febrile children. Pediatr Crit Care Med. 2006;7:48–55. doi: 10.1097/01.pcc.0000185476.35550.b2. [DOI] [PubMed] [Google Scholar]
- 26.Mcilvoy L. Comparison of brain temperature to core temperature: a review of the literature. J Neurosci Nurs. 2004;36:23–31. doi: 10.1097/01376517-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Childs C, Vail A, Protheroe R, King AT, Dark PM. Differences between brain and rectal temperatures during routine critical care of patients with severe traumatic brain injury. Anaesthesia. 2005;60:759–765. doi: 10.1111/j.1365-2044.2005.04193.x. [DOI] [PubMed] [Google Scholar]
- 28.Sparling PB, Snow TK, Millard-Stafford ML. Monitoring core temperature during exercise: ingestible sensor vs. rectal thermistor. Aviat Space Environ Med. 1993;64:760–763. [PubMed] [Google Scholar]
- 29.Sund-Levander M, Grodzinsky E, Loyd D, Wahren LK. Errors in body temperature assessment related to individual variation, measuring technique and equipment. Int J Nurs Pract. 2004;10:216–223. doi: 10.1111/j.1440-172X.2004.00483.x. [DOI] [PubMed] [Google Scholar]
- 30.Jessen C. Selective brain cooling in mammals and birds. Jpn J Physiol. 2001;51:291–301. doi: 10.2170/jjphysiol.51.291. [DOI] [PubMed] [Google Scholar]
- 31.Galuske RA, Schmidt KE, Goebel R, Lomber SG, Payne BR. The role of feedback in shaping neural representations in cat visual cortex. Proc Natl Acad Sci USA. 2002;99:17083–17088. doi: 10.1073/pnas.242399199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lomber SG. Learning to see the trees before the forest: reversible deactivation of the superior colliculus during learning of local and global visual features. Proc Natl Acad Sci USA. 2002;99:4049–4054. doi: 10.1073/pnas.062551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purssell E, Commentary on Farnell S, Maxwell L, Tan S, Rhodes A, Phillips A. Temperature measurement: comparison of non-invasive methods used in adult critical care. J Clin Nurs. 2007;14:632–639. doi: 10.1111/j.1365-2702.2004.00916.x. [DOI] [PubMed] [Google Scholar]
- 34.Kaptchuk TJ, Goldman P, Stone DA, Stason WB. Do medical devices have enhanced placebo effects? J Clin Epidemiol. 2000;53:786–792. doi: 10.1016/s0895-4356(00)00206-7. [DOI] [PubMed] [Google Scholar]
- 35.Kaptchuk TJ. Powerful placebo: the dark side of the randomised controlled trial. Lancet. 1998;351:1722–1725. doi: 10.1016/S0140-6736(97)10111-8. [DOI] [PubMed] [Google Scholar]
- 36.De la Fuente-Ferna´ndez R, Schulzer M, Stoessl AJ. The placebo effect in neurological disorders. Lancet Neurol. 2002;1:85–91. doi: 10.1016/s1474-4422(02)00038-8. [DOI] [PubMed] [Google Scholar]
- 37.Battin M, Bennet L, Gunn AJ. Rebound seizures during rewarming. Pediatrics. 2004;114:1369. doi: 10.1542/peds.2004-1695. [DOI] [PubMed] [Google Scholar]
- 38.Vosler PS, Logue ES, Repine MJ, Callaway CW. Delayed hypothermia preferentially increases expression of brain-derived neurotrophic factor exon III in rat hippocampus after asphyxial cardiac arrest. Brain Res Mol Brain Res. 2005;135:21–29. doi: 10.1016/j.molbrainres.2004.11.006. [DOI] [PubMed] [Google Scholar]