Abstract
Background: Recently, intramedullary headless screw (IMHS) has shown promise as an alternative to dorsal plate fixation of metacarpal fractures. The purpose of this study was to assess the biomechanical performance of IMHS versus plating. We hypothesized that IMHS fixation provides inferior stability to plating. Methods: Metacarpal fracture model with 3-mm of volar gapping in forty-four human cadaveric metacarpals was created. The specimens were divided into 5 groups: Group 1, 1.5-mm non-locking plate; Group 2, 1.5-mm locking plate; Group 3, 2.0-mm non-locking plate; Group 4, 2.0-mm locking plate; and Group 5, 2.4-mm short cannulated IMHS. A 4-point bending model was used to assess load-to failure (LTF) and stiffness. Results: Mean LTF was 364 ± 130 N for 1.5-mm non-locking plates, 218 ± 94 N for 1.5-mm locking plates, 421 ± 86 N for 2.0-mm non-locking plates, 351 ± 71 N for 2.0-mm locking plates, and 75 ± 20 N for IMHS. Mean stiffness was 91 ± 12 N/mm for 1.5-mm non-locking plates, 110 ± 77 N/mm for 1.5-mm locking plates, 94 ± 20 N/mm for 2.0-mm non-locking plates, 135 ± 16 N/mm for 2.0-mm locking plates, and 55 ± 15 N/mm for IMHS. IMHS demonstrated significantly lower LTF and stiffness than plates. Conclusions: IMHS fixation of unstable metacarpal shaft fractures offers less stability compared to plating when loaded in bending. The LTF and stiffness of IMHS versus plating of metacarpal shaft fractures has not been previously quantified. Our results reveal that IMHS fixation is less favorable biomechanically and should be carefully chosen in regards to fracture stability.
Keywords: biomechanical, fracture, intramedullary, metacarpal, stiffness
Introduction
Metacarpal fractures are common upper extremity injuries accounting for up to 42% of hand fractures.1,8 While the majority of metacarpal fractures may be treated nonoperatively, closed reduction and percutaneous pinning as well as open reduction and internal fixation (ORIF) are recognized options for fixation of metacarpal fractures.10,13,18 Biomechanically, dorsal plate constructs have demonstrated superior fixation strength to other methods.2,9,16,20 However, such implants may interfere with extensor tendon gliding, cause stress shielding of the bone beneath the plate, or induce metallosis.10,14,15 Subsequent surgery to remove the plates is frequently required and may be particularly difficult in certain cases.
Recently, intramedullary headless screw (IMHS) fixation has been introduced as a novel fixation method for metacarpal neck fractures. These intramedullary implants allow for a less invasive surgical approach, minimal soft tissue disruption, and periosteal stripping, and have been reported to provide stable fracture fixation in clinical studies.3,17 In addition, ten Berg et al performed a 3-dimensional computed tomographic study supporting the use of IMHS via an articular starting point for fixation of metacarpal fractures.19 However, to our knowledge, no biomechanical studies comparing IMHS constructs with dorsal plate fixation of metacarpal shaft fractures have been performed.
The purpose of this study was to assess the biomechanical performance of IMHS and several dorsal plate fixation constructs in a cadaveric metacarpal shaft fracture model. Our aim was to provide recommendations for or against the use of IMHS as an alternative for metacarpal fracture fixation based on biomechanical performance as compared with commonly utilized fixation constructs. We hypothesized that IMHS fixation provides inferior fixation stability to dorsal plating.
Materials and Methods
Forty-eight cadaveric metacarpals from the second through fourth digits were initially harvested from 8 fresh-frozen human cadavers. However, 4 metacarpals sustained damage during the harvesting process and were excluded from analysis. The remaining 44 metacarpals underwent biomechanical analysis. Of the 8 cadavers, 4 were male and 4 were female. The average age at time of death was 73 years. The specimen were stored at −20°C and defrosted to room temperature (20°C) prior to fixation and testing. In each specimen, a 3-mm volar gapping mid-shaft osteotomy was created using a 1-mm oscillating saw. Each metacarpal was then randomly allocated into one of five fixation groups: group 1 (n = 9), group 2 (n = 9) group 3 (n = 9), group 4 (n = 8), and group 5 (n = 9).
Group 1 was plated with a 6-hole 1.5-mm nonlocking plate (nonlocking adaptation plate) with bicortical nonlocking screws (DePuy-Synthes, Paoli, Pennsylvania). Group 2 was plated with a 6-hole 1.5-mm locking plate (locking compression plate) with bicortical locking screws (DePuy-Synthes). Group 3 was plated with a 6-hole 2.0-mm nonlocking plate (nonlocking limited contact-dynamic compression plate) with bicortical nonlocking screws (DePuy-Synthes). Group 4 was plated with a 6-hole 2.0-mm locking plate (locking compression plate) with bicortical locking screws (DePuy-Synthes). Group 5 was fixated with a 2.4-mm cannulated IMHS short-thread version (DePuy-Synthes). All instrumentation was made of stainless steel. Dorsal plates were centered at the fracture apex and fixated with a total of 3 screws on each side of the fracture. IMHS insertion technique was as follows: A 1.1-mm guide pin was inserted in a retrograde fashion through the dorsal metacarpal head, in line with the medullary canal, gaining purchase in the proximal subchondral bone. Next, cannulated drilling was performed over the pin, and the longest available screw (40 mm) was inserted, gaining isthmal purchase with the leading threads advanced proximal to the fracture line. As no image intensification was utilized, a ruler was used to confirm that the implant is equidistant from the fracture line on both sides. Each screw was buried beneath the chondral surface.
The specimens were tested to failure in a 4-point bending test apparatus, mounted in a servohydraulic testing machine (MTS 858 Mini Bionix, MTS Systems, Corp, Eden Prairie, Minnesota) as performed in similar studies.4,6,9 The construct was positioned so that the dorsal cortex was always facing down (Figure 1). The loading pins of the outer span were positioned at a set length and supported the construct at 2 points beyond the ends of the plate. Testing loads were applied at 2 points, equidistant from the osteotomy site at a rate of 10-mm/min to the central portion of the metacarpal until failure occurred. Load, displacement, and failure data were collected from each test. Specimens were visually examined during testing to assess mechanism of failure.
Figure 1.
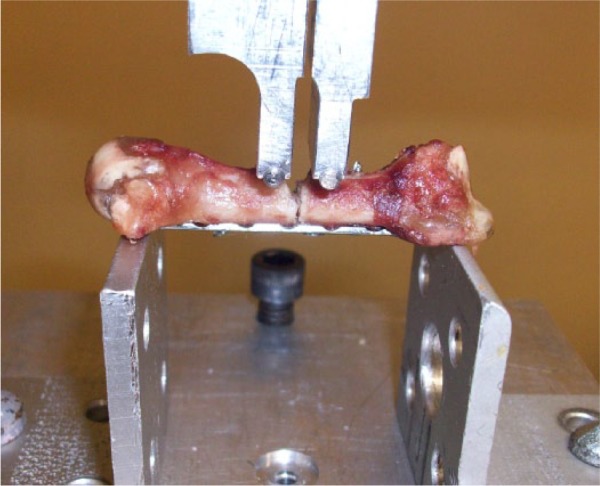
A 2.0-mm nonlocking plate construct positioned for testing in the biomechanical jig.
Statistical Analysis
Failure was defined as a marked change in the load versus displacement curve. Stiffness was determined from the slope of the elastic region of the load versus displacement curve (Figure 2). A one-way analysis of variance (ANOVA) test was used to identify statistically significant differences in load to failure (LTF) and stiffness among groups with Wilcoxon tests utilized for pairwise comparisons. The level of significance for all tests was P < .05.
Figure 2.
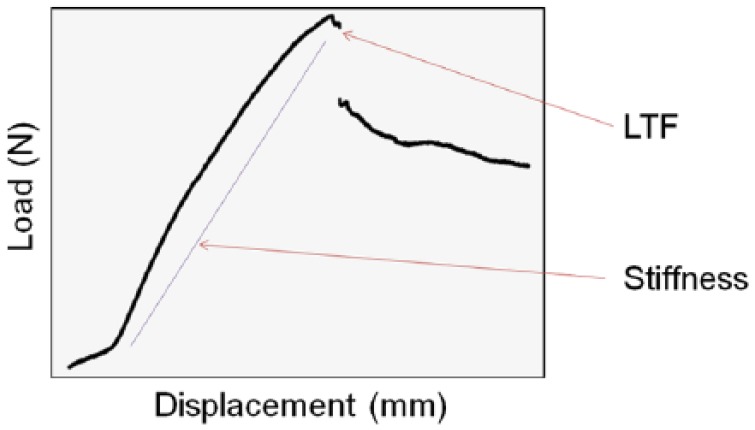
Example of a typical load versus displacement graph.
Note. LTF was defined a marked change in the load versus displacement curve as indicated in this figure. Stiffness was determined from the slope of the elastic region of the load versus displacement curve. LTF, load to failure.
Results
All IMHS constructs failed at the screw-bone interface. A single specimen in both the 1.5-mm nonlocking plate and 2.0-mm nonlocking plate groups failed at the screw-bone interface. Three specimens in both 1.5-mm locking plate and 2.0-mm plate locking groups failed at the screw-bone interface. Failure in all remaining fixation constructs occurred with bending of the plate, though no plate fractures were noted (Figure 3).
Figure 3.
(a) 1.5-mm nonlocking plate constructs, (b) 1.5-mm locking plate constructs, (c) 2.0-mm nonlocking plate constructs, (d) 2.0-mm locking plate constructs, and (e) 2.4-mm intramedullary headless screws after biomechanical testing to failure.
Note. Values are displayed as mean and standard deviation. IMHS, intramedullary headless screw.
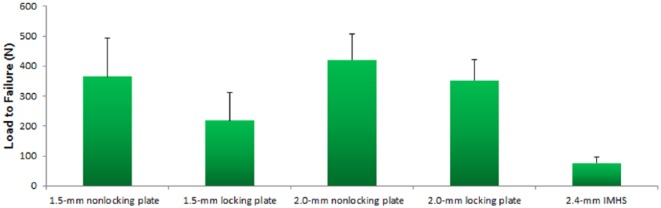
The mean LTF was 364 ± 130 N for 1.5-mm nonlocking plates, 218 ± 94 N for 1.5-mm locking plates, 421 ± 86 N for 2.0-mm nonlocking plates, 351 ± 71 N for 2.0-mm locking plates, and 75 ± 20 N for IMHS constructs. Statistical analysis revealed a significant difference in LTF between constructs (P < .001). Pairwise comparison demonstrated that 1.5-mm locking plates had a lower LTF than 2.0-mm nonlocking plates (218 vs 421; P = .01) and 2.0-mm locking plates (218 vs 351; P = .023). IMHS constructs had significantly lower LTF than 1.5-mm nonlocking plates (75 vs 364; P = .003), 1.5-mm locking plates (75 vs 218; P = .002), 2.0-mm nonlocking plates (75 vs 421; P = .003), and 2.0-mm locking plates (75 vs 351; P = .003). Figure 4 illustrates LTF data.
Figure 4.
Chart displaying load-to-failure data.
Note. Values are displayed as mean and standard deviation. IMHS, intramedullary headless screw.
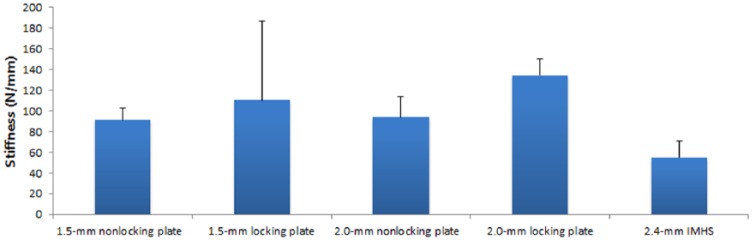
Mean stiffness was 91 ± 12 N/mm for 1.5-mm nonlocking plates, 110 ± 77 N/mm for 1.5-mm locking plates, 94 ± 20 N/mm for 2.0-mm nonlocking plates, 135 ± 16 N/mm for 2.0-mm locking plates, and 55 ± 15 N/mm for IMHS constructs. There was a significant difference in stiffness between constructs (P = .005). Pairwise comparison revealed that 2.0-mm locking plates had greater stiffness than 1.5-mm nonlocking plates (135 vs 91; P = .012) and 2.0-mm nonlocking plates (135 vs 94; P = .022). IMHS constructs had significantly less stiffness than 1.5-mm nonlocking plates (55 vs 91; p = .005), 2.0-mm nonlocking plates (55 vs 94; P = .003), and 2.0-mm locking plates (55 vs 135; P = .003). Figure 5 illustrates stiffness data.
Figure 5.
Chart displaying stiffness data.
Note. Values are displayed as mean and standard deviation. IMHS, intramedullary headless screw.
Discussion
The current study assessed the mechanical performance of IMHS in comparison with several dorsal plate fixation constructs in a cadaveric metacarpal shaft fracture model. Our hypothesis that IMHS fixation would provide inferior fixation stability than dorsal plating was supported by our results. The IMHS was found to have significantly lower LTF than all dorsal plating constructs and was also significantly less stiff than all dorsal plating constructs except 1.5-mm locking plates.
In contrast to our biomechanical findings, the clinical use of metacarpal IMHS fixation has demonstrated favorable outcomes in the literature for select fracture types. Boulton et al reported their use of a 3.0-mm IMHS for a treatment of a comminuted metacarpal neck fracture of the little finger in a 54-year-old patient.3 They noted that the use of the construct allowed for early range of motion, resulting in uneventful healing and excellent functional results. Ruchelsman et al also reviewed 39 patients with a mean age 28 years treated with an IMHS and reported that all fractures healed uneventfully at 6 weeks with no hardware-related complications.17 Etiology of the discrepancy between our biomechanical findings and the previously described clinical studies may be due to differences in IMHS purchase in the isthmus of the medullary canal versus the metacarpal neck. Our investigation utilized a 2.4-mm IMHS during biomechanical testing, whereas a 3.0-mm IMHS was used in many of the reported clinical cases. We chose the smaller diameter 2.4-mm screw due to our concern that the 3-mm screw might get incarcerated in the narrow isthmus of the fourth metacarpal. This is not to suggest that the bigger biomechanically favorable screw should not be used after proper drilling. In addition, only second through fourth metacarpals were fixated in our biomechanical study. However, the fifth metacarpal was the primary site of fixation in both clinical studies and may represent an improved ratio of IMHS diameter to metacarpal intramedullary diameter. Furthermore, the comparatively young age of patients presented in the clinical studies suggests relatively good bone quality and may have also contributed to their good outcomes.
IMHS fixation of metacarpal fractures is reported to have several theoretical strengths. Using a cannulated headless screw design and a limited-open extensor-splitting approach, IMHS fixation is less invasive than dorsal plating and may allow for early postoperative joint motion.3,17 The intramedullary technique also allows for direct visualization of the starting point, which eliminates the need for multiple attempts to achieve the correct starting point. In addition, an IMHS addresses several of the limitations associated with intramedullary fixation of metacarpal fractures performed with smooth k-wires including pin track sepsis, k-wire migration, and need for implant removal after bone healing.7,11,12 However, despite these potential strengths, our findings overwhelmingly demonstrate the biomechanical inferiority of IMHS as compared with dorsal plating constructs.
Selection of the optimal fixation construct for metacarpal fractures depends on many factors including fracture location, fracture stability, and the potential for adequate soft tissue coverage. In our study, the 3-mm volar gap fracture model simulated metacarpal fractures with volar comminution. Although our findings indicate that IMHS fixation is biomechanically inferior to dorsal plating of comminuted metacarpal shaft fractures, it is unclear how an IMHS would perform biomechanically in the fixation of transverse metacarpal fractures without comminution. The average bending moment during metacarpophalangeal joint flexion has been reported to be 0.35 Nm, which is several magnitudes below the IMHS LTF demonstrated in our study.20 This finding suggests that IMHS fixation may be suitable for the fixation of select metacarpal fractures. However, an assessment of the biomechanical performance of IMHS for the fixation of transverse metacarpal fractures without volar comminution was beyond the scope of our study.
Although not statistically significant, our study demonstrated greater LTF for 1.5-mm nonlocking plates and 2.0-mm nonlocking plates compared with their corresponding locking plates. Conversely, 2.0-mm locking plates had significantly greater stiffness than 2.0-mm nonlocking plates. The 1.5-mm locking plates also demonstrated greater stiffness than 1.5-mm nonlocking plates, though this difference was not statistically significant. Similar findings have been reported in the literature. Doht et al utilized a porcine model to assess biomechanical stability of locked metacarpal fixation and found greater maximum load in linear nonlocking constructs versus corresponding linear locking constructs.5 In that study, linear locking constructs were also found to have greater stiffness than their corresponding linear nonlocking constructs.
There are limitations to the current study. Our biomechanical study used cadaveric metacarpals, which were not of a standardized size or bone density and may have introduced variability to our results. In addition, we did not subject the specimen to cyclic loading but instead utilized a quasi-static 4-point bending model to assess biomechanical properties. Thus, our findings should not be interpreted in terms of repetitive stress to the construct over time. However, there are also strengths to our study. The current investigation was the first to biomechanically compare IMHS fixation with dorsal plating of metacarpal shaft fractures, providing a quantitative assessment of an alternative fixation strategy.
Based on our results, we conclude that IMHS fixation of metacarpal shaft fractures with volar comminution offers significantly less stable fixation then dorsal plating. However, the biomechanical performance of IMHS fixation of metacarpal fractures without comminution is unknown. Future investigations, both biomechanical studies and long-term clinical comparisons, are needed to assess IMHS use in metacarpal shaft fractures without comminution.
Footnotes
Authors’ Note: Investigation Performed at NYU Hospital for Joint Diseases.
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Statement of Informed Consent: Informed consent was not obtained as this study did not involve human subjects.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.T.C. is on the Speakers Bureau for Integra Life Sciences and is a consultant for Wright Medical Technology.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Afshar R, Fong TS, Latifi MH, Kanthan SR, Kamarul T. A biomechanical study comparing plate fixation using unicortical and bicortical screws in transverse metacarpal fracture models subjected to cyclic loading. J Hand Surg Eur Vol. 2012;37(5):396-401. [DOI] [PubMed] [Google Scholar]
- 2. Black D, Mann RJ, Constine R, Daniels AU. Comparison of internal fixation techniques in metacarpal fractures. J Hand Surg Am. 1985;10(4):466-472. [DOI] [PubMed] [Google Scholar]
- 3. Boulton CL, Salzler M, Mudgal CS. Intramedullary cannulated headless screw fixation of a comminuted subcapital metacarpal fracture: case report. J Hand Surg Am. 2010;35(8):1260-1263. [DOI] [PubMed] [Google Scholar]
- 4. Bozic KJ, Perez LE, Wilson DR, Fitzgibbons PG, Jupiter JB. Mechanical testing of bioresorbable implants for use in metacarpal fracture fixation. J Hand Surg Am. 2001;26(4):755-761. [DOI] [PubMed] [Google Scholar]
- 5. Doht S, Jansen H, Meffert R, Frey S. Higher stability with locking plates in hand surgery? biomechanical investigation of the TriLock system in a fracture model. Int Orthop. 2012;36(8):1641-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dona E, Gillies RM, Gianoutsos MP, Walsh WR. Plating of metacarpal fractures: unicortical or bicortical screws? J Hand Surg Br. 2004;29(3):218-221. [DOI] [PubMed] [Google Scholar]
- 7. Downing ND, Davis TR. Intramedullary fixation of unstable metacarpal fractures. Hand Clin. 2006;22(3):269-277. [DOI] [PubMed] [Google Scholar]
- 8. Feehan LM, Sheps SB. Incidence and demographics of hand fractures in British Columbia, Canada: a population-based study. J Hand Surg Am. 2006;31(7):1068-1074. [DOI] [PubMed] [Google Scholar]
- 9. Firoozbakhsh KK, Moneim MS, Howey T, Castaneda E, Pirela-Cruz MA. Comparative fatigue strengths and stabilities of metacarpal internal fixation techniques. J Hand Surg Am. 1993;18(6):1059-1068. [DOI] [PubMed] [Google Scholar]
- 10. Gajendran VK, Szabo RM, Myo GK, Curtiss SB. Biomechanical comparison of double-row locking plates versus single- and double-row non-locking plates in a comminuted metacarpal fracture model. J Hand Surg Am. 2009;34(10):1851-1858. [DOI] [PubMed] [Google Scholar]
- 11. Gonzalez MH, Hall RF., Jr. Intramedullary fixation of metacarpal and proximal phalangeal fractures of the hand. Clin Orthop Relat Res. 1996;327:47-54. [DOI] [PubMed] [Google Scholar]
- 12. Henry MH. Fractures of the proximal phalanx and metacarpals in the hand: preferred methods of stabilization. J Am Acad Orthop Surg. 2008;16(10):586-595. [DOI] [PubMed] [Google Scholar]
- 13. Kollitz KM, Hammert WC, Vedder NB, Huang JI. Metacarpal fractures: treatment and complications. Hand (N Y). 2014;9(1):16-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lionelli GT, Korentager RA. Biomechanical failure of metacarpal fracture resorbable plate fixation. Ann Plast Surg. 2002;49(2):202-206. [DOI] [PubMed] [Google Scholar]
- 15. Mahmood B, Hammert WC. Metal implant allergy. J Hand Surg Am. 2015;40(4):831-833. [DOI] [PubMed] [Google Scholar]
- 16. Mann RJ, Black D, Constine R, Daniels AU. A quantitative comparison of metacarpal fracture stability with five different methods of internal fixation. J Hand Surg Am. 1985;10(6, pt 2):1024-1028. [DOI] [PubMed] [Google Scholar]
- 17. Ruchelsman DE, Puri S, Feinberg-Zadek N, Leibman MI, Belsky MR. Clinical outcomes of limited-open retrograde intramedullary headless screw fixation of metacarpal fractures. J Hand Surg Am. 2014;39(12):2390-2395. [DOI] [PubMed] [Google Scholar]
- 18. Sohn RC, Jahng KH, Curtiss SB, Szabo RM. Comparison of metacarpal plating methods. J Hand Surg Am. 2008;33(3):316-321. [DOI] [PubMed] [Google Scholar]
- 19. ten Berg PW, Mudgal CS, Leibman MI, Belsky MR, Ruchelsman DE. Quantitative 3-dimensional CT analyses of intramedullary headless screw fixation for metacarpal neck fractures. J Hand Surg Am. 2013;38(2):322-330.e2. [DOI] [PubMed] [Google Scholar]
- 20. Vanik RK, Weber RC, Matloub HS, Sanger JR, Gingrass RP. The comparative strengths of internal fixation techniques. J Hand Surg Am. 1984;9(2):216-221. [DOI] [PubMed] [Google Scholar]