Abstract
Motor symptoms that define Parkinson’s disease (PD) are caused by the selective loss of nigral dopaminergic (DA) neurons. Cell replacement therapy for PD has been focused on midbrain DA neurons derived from human fetal mesencephalic tissue, human embryonic stem cells (hESC) or human induced pluripotent stem cells (iPSC). Recent development in the direct conversion of human fibroblasts to induced dopaminergic (iDA) neurons offers new opportunities for transplantation study and disease modeling in PD. The iDA neurons are generated directly from human fibroblasts in a short period of time, bypassing lengthy differentiation process from human pluripotent stem cells and the concern for potentially tumorigenic mitotic cells. They exhibit functional dopaminergic neurotransmission and relieve locomotor symptoms in animal models of Parkinson’s disease. In this review, we will discuss this recent development and its implications to Parkinson’s disease research and therapy.
Keywords: Parkinson’s disease, Induced dopaminergic neuron, Induced neuron, Induced pluripotent stem cell, Transcription factor
Graphical abstract
Highlights
-
•
Fibroblasts can be directly converted to induced dopaminergic neurons by transcription factors.
-
•
Many different types of cells can be converted to induced neurons in vitro and in vivo.
-
•
Appropriate cell culture conditions enhance the direct conversion to induced neurons.
-
•
The conversion to induced neurons is enhanced by G1 arrest and p53 attenuation.
-
•
iDA neurons is a promising tool for PD research and therapy.
1. Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, after Alzheimer’s disease. PD is characterized by its hallmark motor symptoms such as resting tremor, rigidity, bradykinesia and postural instability [1]. A variety of non-motor symptoms (NMSs), including dementia, depression, sensory dysfunction, dysautonomia, sleep disturbances, etc. are frequently comorbid with and sometimes precede the motor symptoms [1]. The pathology that defines PD, which is also the cause of its motor symptoms, is a rather selective and progressive loss of nigrostriatal dopaminergic (DA) neurons, while NMSs may result from degeneration of non-dopaminergic (e.g. serotonergic, noradrenergic and cholinergic) neurons in other parts of the brain [2]. Over 90% of PD cases are idiopathic, without a clear etiology. Extensive research on monogenic forms of Parkinson’s disease has shed significant insights into the molecular and cellular events that underlie the selective loss of nigral DA neurons in PD [3]. Current treatments for PD, including medication (e.g. levodopa, dopamine agonists, MAO-B inhibitors or COMT inhibitors) and surgery (e.g. deep brain stimulation of globus pallidus or subthalamic nucleus), only relieve symptoms and cannot significantly change disease progression [2]. A disease-modifying therapy for PD is long sought-after and will fundamentally change the care of PD patients.
Decades of research on the transplantation of DA neurons have demonstrated that human fetal ventral mesencephalic cells grafted in striatum can provide very long-term benefits (more than 15 years and ongoing) in a small number of patients so that they function independently without PD drugs [4], [5], although many factors contribute to the lack of therapeutic benefits in most patients [6]. Recent studies show that midbrain DA neurons differentiated from human ES cells with the floorplate-based method perform as well as human fetal ventral mesencephalic cells in 6-OHDA-lesioned rat model of Parkinson’s disease [7]. One main concern for the use of stem cell-derived dopamine neurons is the potential presence of mitotic cells, which may give rise to tumors after transplantation. The recent development of induced dopaminergic (iDA) neurons, which are directly converted from human fibroblasts [8], [9], may provide a useful alternative to stem cell-derived dopamine neurons in cell replacement therapy for PD. In this review, we will highlight the conversion methods, as well as the functional characteristics and utility of induced dopaminergic neurons in PD research and therapy (Fig. 1).
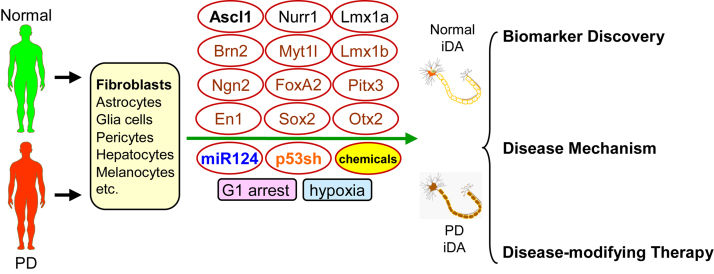
Fig. 1.
Direct conversion of patient-specific cells to induced dopaminergic neurons for Parkinson’s disease research and therapy. A variety of somatic cells, such as skin fibroblasts, from normal subjects or Parkinson’s disease (PD) patients, can be directly reprogrammed to induced dopaminergic (iDA) neurons using transcription factors and microRNAs. Acting as a pioneer transcription factor, Ascl1 drives the epigenetic conversion, which is guided to dopaminergic fate by transcription factors responsible for the specification of dopaminergic neurons, such as Nurr1 and Lmx1a. miRNAs, such as miR124, can reprogram fibroblasts to neurons in the absence of Ascl1. The conversion is enhanced by p53 knockdown, chemicals that activate suitable signaling pathways, cell cycle arrest at G1, and hypoxia. iDA neurons from PD patients and normal subjects are functional midbrain DA neurons that are useful for biomarker discovery, mechanistic study and disease-modifying therapies such as transplantation.
2. Direct conversion of fibroblasts to induced neurons
In 2010, Marius Wernig and colleagues made a significant breakthrough with the identification of transcription factors [Brn2, Ascl1 and Myt1l (BAM)] that directly convert mouse embryonic fibroblasts (MEFs) to induced neurons (iN) [10]. This method does not involve stem cells; the reprogramming of fibroblasts with BAM leads to rapid cell cycle exit within 24 h of turning on reprogramming factors [10]. The direct conversion takes about two weeks to generate induced neurons. This method has been successfully extended to the human system, where fetal and postnatal human fibroblasts are converted to iN with BAM plus NeuroD1 [11]. Many groups have successfully converted mouse, porcine, non-human primate [12] or human fibroblasts into iN, DA neuronal progenitors (iDPs) [13], [14], dopaminergic neurons [8], [15], [16], motor neurons (hiMNs) [17], [18], [19], peripheral sensory neurons [20], striatal medium spiny neurons (MSNs) [21], [22], cholinergic neurons [23], serotonergic neurons [24], [25], GABAergic neurons [26], glutamatergic neurons [27], neural precursor cells [28] and oligodendrocyte progenitor cells [29], [30], with different combinations of transcription factors and media additives (small molecule compounds and growth factors).
3. Direct conversion of fibroblasts to induced dopaminergic neurons
As shown in Table 1, many groups independently identify various transcription factor combinations that can be used to convert fibroblasts to induced dopaminergic (iDA) neurons [8], [9], [15], [16], [31], [32], [33], [34], [35]. This is accomplished by overexpression of transcription factors involved in the specification of dopaminergic neurons during development, together with a combination of small molecule compounds and neurotrophic factors to facilitate the survival and maturation of converted iDA neurons. Despite the differences in reprogramming factors and various other conditions used, the iDA neurons that are converted from fibroblasts share many similar properties. For example, they all express neuronal markers such as β3-tubulin, MAP2 and NeuN, as well as the dopaminergic markers TH, AADC, VMAT2, ALDH1A1, Nurr1, Pitx3, DAT, midbrain markers such as FoxA2 and En1, and synaptic markers such as PSD95 and Syntaxin1. In addition, qRT-PCR and RNAseq analysis of iDA neurons confirmed the enriched expression of dopaminergic genes, such as TH, Vmat2, AADC, Ret, Gfra1, Foxa1, GDNF, Drd2 and synaptic markers such as synaptotagmin I and synapsins. Dopamine release and reuptake are observed in iDA neurons. They exhibit electrophysiological activities such as action potentials and excitatory postsynaptic currents. Finally, transplantation of iDA neurons generated from MEF cells in 6-OHDA-lesioned mice alleviated motor deficits [16].
Table 1.
Summary of iDA neurons directly converted from fibroblasts.
| Source Cells | Transcription factors | Media | Efficiency (%) | Time | Ref. |
|---|---|---|---|---|---|
| (days) | |||||
| HEF; HPF | Ascl1, Brn2, Myt1l, Foxa2, Lmx1a | insulin, transferrin, sodium selenite, progesterone, putrescine | 1.6; 0.4 | 20–24 | [15] |
| MEF; IMR90; HAF | Ascl1, Nurr1, Lmx1a | insulin, transferrin, sodium selenite, progesterone, putrescine | 18; 6; 3 | 6–16 | [8] |
| TTFs | Ascl1, Nurr1, Lmx1a, Pitx3, Foxa2, En1 | insulin, transferrin, sodium selenite, progesterone, putrescine, FGF8, Shh | 9.1 | 4–18 | [16] |
| IMR90 | Ascl1, Nurr1, Pitx3, Ngn2, Sox2 | B27, Shh, FGF-8, NEAA | 1–2 | 10–20 | [31] |
| hEF, hFL1 | Ascl1, Brn2, Myt1l, Lmx1a, Lmx1b, Foxa2, Otx2 | N2, B27, BDNF, GDNF, NT3, db-cAMP, CHIR99021, SB431542, Noggin, LDN-193189 | N/A | 15 | [32] |
| MEF | Nurr1; Ascl1 | ITS, N2, B27, ascorbic acid, bFGF, EGF, FGF8b, Shh | 33 | 31 | [33] |
| MEF; TTFs, REF; | Ascl1, Brn2, Myt1l, Foxa2, Nurr1 | BDNF, GDNF, db-cAMP, ascorbic acid, N2, bFGF, FGF8, LIF | 3.88; 19.63 | 20 | [34] |
| MEF | Ascl1, Pitx3, Nurr1, Lmx1a | N3 medium, nanogrooved substrate | N/A | 10 | [35] |
| MRC5 | Ascl1, Nurr1, Lmx1a, miR124, p53 shRNA | N2, B27, Y27632, CHIR, VC, DM, SB, Pur, NGF, GDNF, BDNF, TGFβ3, serum-free | 59.2 | 9 | [9] |
Abbreviations: HEF, human embryonic fibroblasts; HPF, human postnatal fibroblasts; MEF, mouse embryonic fibroblasts; IMR90, human fetal fibroblasts; HAF, human adult fibroblasts; TTFs, adult mouse tail tip fibroblasts; hFL1, human fetal lung fibroblasts; MRC5, human fetal lung fibroblast.
4. Many different types of cells can be converted to induced neurons in vitro and in vivo
The discovery of the transdifferentiation of fibroblasts to induced neurons stimulates a plethora of studies on the conversion of various cell types to neurons. As summarized in Table 2, a diverse array of cell types have been used in the conversion experiments, with different combinations of transcription factors and media additives, yielding a variety of induced neurons at varying efficiency. For example, astrocytes can be converted to neurons using a single transcription factor such as Neurog2, Brn4, NeuroD1, Ascl1 or Dlx2 in vitro and in vivo [36], [37], [38], [39], [40]. Sox2 alone can reprogram astrocytes and NG2+ glia cells to doublecortin+ neuroblasts and neurons in vivo [41], [42], [43]. Using Lmx1a, Nurr1 and Ascl1, striatal NG2+ glia cells can be converted in vivo to functional neurons with synaptic connections [44]. Mammalian Müller cells – the retinal glial cells – are directly reprogrammed to retinal progenitors and neurons by deletion of p53 [45]. Primary mouse liver cells can be converted into iN cells with Brn2, Ascl1 and Myt1l (BAM) [46]. Adult mouse liver cells and B lymphocytes can be converted to neural stem cells by transient overexpression of 8 transcription factors [47]. Cochlear non-sensory epithelial cells can be reprogrammed to functional neurons by Ascl1 alone or Ascl1 and NeuroD1 [48]. Human blood cells from umbilical cord blood or peripheral blood can be converted to neurons or neural stem cells (iNSCs) by transduction of various transcription factors [49], [50], [51], [52], [53]. Adult human pigmented epidermal-derived melanocytes can be converted into iNSCs by the active intracellular form of Notch1 [54]. Myoblasts can be converted to a neuronal phenotype by REST-VP16, which directly binds to and activates target genes of the transcriptional repressor REST [55], [56]. Pericytes, which accounts for almost half of the cells in adult human cerebral cortex, can be converted to induced neurons by retrovirus-mediated coexpression of Sox2 and Ascl1 for 5 weeks [57], [58]. C. elegans germ cells can be directly converted to glutamatergic, cholinergic, or GABAergic neurons by the removal of the histone chaperone LIN-53 (RbAp46/48 in humans), which turns on neuron type-specific terminal selector transcription factors to drive the transdifferentiation to various types of neurons [59]. Consistent with this, rat Sertoli cells can be converted to iNSCs with nine transcription factors (Ascl1, Ngn2, Hes1, Id1, Pax6, Brn2, Sox2, c-Myc, and Klf4) [60]. Urinary tract cells flushed out in urine can be reprogrammed to induced neural progenitor cells (iNPC) by different combinations of transcription factors or only with small-molecule compounds [61], [62], [63]. It remains unclear whether any of these cell types can be efficiently converted to iDA neurons. The relative ease in obtaining human fibroblasts, which can be efficiently converted to iDA neurons, appears to have made human fibroblasts the favored source material for the generation of iDA neurons.
Table 2.
Cell types that can be converted to induced neurons.
| Source Cells | Species | Transcription Factors | Media Additives | Induced cells | Efficiency (%) | Time (days) | Ref. |
|---|---|---|---|---|---|---|---|
| Astrocyte | Mouse | Neurog2; Ascl1 and/or Dlx2 | B27, BDNF, 10% CO2, no change medium | Glutamatergic neurons; GABAergic neurons | 85.4; 33.7 | 26; 22 | [36], [37] |
| Astrocyte; NG2 cells | Mouse; Human | NeuroD1 | In vivo; EGF, FGF2 | Glutamatergic neurons; Glutamatergic & GABAergic neurons | In vivo; human 90 | 7 | [38] |
| Astrocyte | Mouse (adult) | Sox2 | In vivo, BDNF, Noggin, histone deacetylase inhibitor | Neuroblasts (iANBs) | In vivo N/A | 21 | [41] |
| Astrocyte | Mouse | Brn4 | None | Neuron | 2.5–3.5 | 20 | [40] |
| Astrocyte | Mouse | Ascl1 | In vivo | Neuron | 76.8± 6.4 | 10–21 | [39] |
| Astrocyte | Mouse | Sox2 | In vivo, valproic acid | Neuroblasts neuron | 6–7 | 4 weeks | [42] |
| Astrocyte | Rat | None | Defined medium: 5C | Tuj+ neuronal like cells | 22–40 | 16 | [64] |
| Astrocyte | Mouse | None | VPA, CHIR99021, Repsox | Neuronal cells | 20–30 | 12–18 | [65] |
| Glioma | Rat | None | Taxol | Neuron, astrocyte, oligodendrocytes | 20 | 48 h | [66] |
| NG2 glia | Mouse | Sox2 | In vivo | Neuron | 13.9 ± 3.5 | 12 | [43] |
| NG2 glia | Mouse | Ascl1, Lmx1a, Nurr1 | In vivo | Neuron | 20.8 ±5.9; 6.8 ±2.9 | 4–12 weeks | [44] |
| Muller glia cells | Mouse | P53 (RNAi) | None | Retinal neuronal cell | 80 | 5–10 | [45] |
| Germ cell | C. | LIN-53 RNAi, other TFs | In vivo | Neuron | 60 of 200 (30%) | 6 hrs | [59] |
| elegans | |||||||
| Hepatocyte | Mouse | Ascl1, Brn2, Myt1l | N3 medium | Neuron | Postnatal: 6; Adult: 2.7 | 13 | [46] |
| Cochlear non-sensory epithelial cells | Mouse | Ascl1/ NeuroD1 | None | Neurons | 43±7; 91±2 | 7 | [48] |
| CD133+ cord blood cells | Human | Sox2, | None | Neuron | 25–80 | 15–30 | [49] |
| c-Myc | |||||||
| Myoblasts | Mouse | REST-VP16 | None | Neuronal phenotype | N/A | 20 | [55], [56] |
| Pericyte | Human | Ascl1, Sox2 | 5% O2 | Neurons | 48 | 28–35 | [57], [58] |
| Human urine cells | Human | Ascl1, Brn2, NeuroD, c-Myc, Myt1l | None | Neurons | 1.55 ±0.01 | 14 | [62] |
iANBs, induced adult neuroblasts; iNPCs, induced neural precursor cells.
5. Appropriate cell culture conditions enhance the direct conversion to induced neurons
Although the epigenetic reprogramming of fibroblasts to neurons is driven by transcription factors, many small molecule compounds and neurotrophic factors significantly enhance the transdifferentiation. For example, activin-like kinase 5 (ALK5) inhibitor SB-43 1542 (SB), which blocks SMAD2/3 and SMAD1/5/8 signaling pathways, noggin and the GSK-3β inhibitor CHIR99021 (CHIR) greatly facilitate the derivation of human induced neurons by Ascl1 and Ngn2 [67]. SB and dorsomorphin (DM), which inhibit BMP type 1 receptors ALK2, ALK3 and ALK6 and thus block SMAD1/5/8, enhance the efficiency in the direct reprogramming of human mesenchymal stem cells into neuronal-like cells [68]. SB431542, noggin, CHIR99021, dibutyryl-cAMP, VPA and unoprostone increase the conversion of human fibroblasts to induced neurons by BAM [69]. Forskolin dramatically enhances reprogramming efficiency while DM promotes the survival and maturation of induced cholinergic neurons converted from human fibroblasts by Neurogenin 2 [23]. SHH and FGF8b promote the reprogramming of mouse embryonic fibroblasts to iN and iDA neurons by Ascl1 and Nurr1 [33]. LDN, SB431542, CHIR99021, purmorphamine, and the histone deacetylase inhibitor VPA increase the conversion of human fibroblasts to induced dopaminergic neural progenitor-like cells in a 3D sphere culture by TAT-mediated transduction of SOX2 and LMX1a proteins [13].
Remarkably, combinations of small molecule compounds can directly convert various cell types to neurons without the need for transcription factors. For example, human fibroblasts from patients with Alzheimer’s disease have been converted to induced neurons at an efficiency of 20% in 7 days with 7 compounds (VPA, CHIR99021, TGFβR-1/ALK5 inhibitor Repsox, forskolin, JNK inhibitor SP600125, PKC inhibitor GO6983 and Y-27632) [27]. In another study, human fibroblasts are efficiently converted to neuronal cells by a cocktail of six compounds (SB-431542, LDN-193189, CHIR99021, PD0325901, Pifithrin-α and Forskolin) [70]. Furthermore, astrocytes are converted to neuronal cells by VPA, CHIR99021 and Repsox [65]. Different types of somatic cells have been converted to neural stem cells, neural progenitor cells or neural crest cells by various combinations of small molecular compounds [63], [71], [72], [73].
Many studies have shown that hypoxia (5% O2) promotes cellular reprogramming including the direct conversion of various cell types to neurons. For example, the conversion of human fibroblasts by BAM and NeuroD1 to MAP2+ neurons is increased 2.4 folds by hypoxia [74]. Hypoxia promotes the conversion of different somatic cells to neural progenitor cells [63]. Our studies have shown that hypoxia increases the transdifferentiation of human fibroblasts to induced dopaminergic neurons [9] and induced serotonergic neurons [25].
It appears that cell culture conditions, including small molecule compounds, growth factors and hypoxia, impinge on the epigenetic landscape of the genome by activating various signal transduction pathways in the cell. These signaling events, either by themselves, or in conjunction with transcription factors used in reprogramming, must change the epigenome so that the genome is read differently to produce various types of neurons. Understanding the mechanistic details of transdifferentiation will not only reveal fundamental insights into the molecular definition of cell types, but also enable the development of more efficient and useful reprogramming methods.
6. Mechanism of transdifferentiation
The key transcription factor in the direct conversion of fibroblasts to various types of neurons is Ascl1 [10], [75]. By itself, Ascl1 reprograms MEF cells to immature neurons, which can maturate by coculturing with glia cells. Although Brn2 and Myt11 enhance the efficiency in generating mature neurons, no neuron is produced in the absence of Ascl1 [10], [75]. Ascl1, as known as Mash1, belongs to the proneural bHLH gene family, which perform important functions in the induction of neural lineages in early developments [76], [77], [78], and in the late differentiation stage. Ascl1 is a pioneer transcription factor, which can access heterochromatin in fibroblasts to turn on neuronal genes that are not expressed in fibroblasts [79]. Brn2 is recruited by Ascl1 to genomic targets of Ascl1. The engagement of Ascl1 with its genomic targets brings a trivalent chromatin state (H3K4me1, H3K27acetyl, and H3K9me3) that leads to the activation of these target genes [79].
Within 24 h of reprogramming, Ascl1 drives cell cycle exit [9], [10] through the induction of the cyclin-dependent kinase inhibitor p27 [80]. The fibroblasts that are converted to neurons never enter S phase. Thus, cell cycle arrest at the G1/S checkpoint by a variety of mechanism including serum withdrawal significantly enhances the transdifferentiation of fibroblasts to neurons [9]. As mitotic cells integrate extracellular and intracellular information during G1 to determine whether it is suitable to commit to the replication of genomic DNA, cells synchronized at G1 offers the optimal timing for conversion into post-mitotic cells such as neurons [81], [82].
Several recent studies have shown the critical role of p53 in the direct conversion of fibroblasts to various types of induced neurons. Depletion of p53 alone converts 70–85% fibroblasts to induced neurons after 3–4 weeks. The addition of Ascl1, Brn2 and Neurod2 greatly increases the conversion, as p53 binds the promoter of Neurod2 and regulates its expression during fibroblast-to-neuron conversion [83]. Depletion of p53 targets p16Ink4a or p19Arf also reprograms human fibroblasts to induced neurons [84]. Blocking the activity of p53 with a dominant-negative mutant p53 significant improves the conversion of human fibroblasts to iDA neurons by Ascl1, Nurr1 and Lmx1a [85]. We show that p53 attenuation significantly enhances the transdifferentiation of human fibroblasts to iDA neurons by inducing the expression of Tet1, a DNA hydroxylase [9] that oxidizes 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC) [86], a DNA modification particularly abundant in neurons [87]. Tet1 is also induced when human fibroblasts are arrested at G1 or upon the expression of reprogramming factors (Ascl1, Nurr1 and Lmx1a). The synergistic induction of Tet1 by p53 knockdown, G1 arrest and expression of reprogramming factors appears to drive the conversion of the epigenome from a fibroblast state to a neuronal state. Overexpression of Tet1 significantly enhances the conversion, whereas Tet1 knockdown during conversion is toxic to the cell [9]. The important role of p53 is corroborated in the direct conversion of human fibroblasts to induced serotonergic neurons by Ascl1, FoxA2, Lmx1b and FEV [25].
Although the conversion of fibroblasts to neurons is driven by Ascl1, transcription factors that specify dopaminergic neurons must be present during conversion in order to generate induced dopaminergic neurons [8], [15], [16]. Lmx1a is a critical transcription factor in the initial specification of mesodiencephalic DA neurons [88]. In proliferating precursor cells of mesodiencephalic DA neurons, Lmx1a acts as an early activator of the DA differentiation program by inducing the expression of Msx1 [89]. Both of them cooperate to induce Ngn2 and support neuronal differentiation to generate midbrain DA neurons [89]. Overexpression of Lmx1a in embryonic stem cells increases the generation of midbrain DA neurons [89]. Nurr1 is a key transcription factor controlling the terminal differentiation of midbrain DA neurons [90]. Along with Pitx3 and other transcription factors, Nurr1 drives the development of postmitotic DA neurons to mature midbrain DA neurons by helping these neurons acquire their neurotransmitter identity. Nurr1 induces the expression of a battery of genes that determine the identity of midbrain DA neurons, including TH, DAT, VMAT2, AADC, etc. [90]. Nurr1 knockout mice die at birth and lack midbrain DA neurons [91].
Another route for the transdifferentiation of fibroblasts to neurons is through microRNAs, without the need for Ascl1. Initially, miR-124 and miR-9/9* are shown to facilitate the transdifferentiation of human fibroblasts to induced neurons by Ascl1, NeuroD2 and Myt1l [92]. Subsequently, it is found that miR124, Myt1l and Brn2, in the absence of Ascl1, convert human fibroblasts to induced neurons [93]. It appears that miR-124 or miR-9/9* may promote a neuronal fate by regulating the neural progenitor BAF chromatin-remodelling complex. These miRNAs are also implicated in controlling numerous genes that regulate neuronal differentiation and function, such as components of the REST complex including REST and CoREST, and polypyrimidine-tract-binding (PTB) protein [92]. Overexpression of pluripotency stem cell-specific miRNA-302/367 cluster and the neuron-specific miRNAs miRNA-9/9* and miRNA-124 converts human fibroblasts to neurons [94]. Forced expression of such miRNAs [21], [22], [84], [95] or repression of PTB protein [96] reprograms a diverse array of cell types to neurons.
7. iDA neurons for PD research and therapy
There are three unmet needs in Parkinson’s disease: finding a predicative biomarker, elucidating disease mechanism, and discovering a disease-modifying therapy. Patient-specific iDA neurons and iPSC-derived DA neurons are highly valuable in meeting these challenges. While iPSCs provide a permanent and replenishable resource to generate patient-specific midbrain DA neurons, their drawbacks are the long time required to differentiate iPSCs to mature midbrain DA neurons [97] and the resetting of epigenetic information during reprogramming [98]. The differentiation process reestablishes the epigenetic information to that of embryonic DA neurons, which are useful to study the intrinsic, genetically-determined vulnerabilities important to PD. But it might miss the epigenetic information imparted by age. In contrast, the transdifferentiation of human fibroblasts to iDA neurons preserves the epigenetic information associated with the age of the fibroblasts [99]. The added benefit is that transdifferentiation is much faster, taking a few days to see neurons and about 30 days to generate iDA neurons with mature synaptic physiology [9]. Thus, iDA neurons are a very useful addition to the toolset in using PD patients to study Parkinson’s disease, considering the pros and cons of iDA neurons and iPSC-derived DA neurons (Table 3). For example, patient-specific iDA neurons can be generated from a large number of PD patients and normal subjects for biomarker discovery. These iDA neurons share many properties similar to nigral DA neurons in the brain of the subjects, including sophisticated regulation of dopaminergic transmission, such as the regulation of dopamine release by autoreceptors [9]. The patient-specific iDA neurons would thus be a very close surrogate for proteomic and transcriptomic studies that may identify a molecular signature for idiopathic Parkinson’s disease, which so far has defied invasive experimental analyses. Mechanistic studies on PD can also significantly benefit from patient-specific iDA neurons, because it is much easier for more researchers to adopt the highly efficient transdifferentiation protocols to generate patient-specific DA neurons, as compared to the more challenging technical requirement for the differentiation of iPSCs to midbrain DA neurons.
Table 3.
Pros and Cons of iPSC-derived DA neurons vs. iDA neurons.
| Attributes | iDA Neurons | iPSC-derived DA Neurons |
|---|---|---|
| Technical Challenges | Can be readily implemented without stem cell experience; only need good viruses. | Needs expertise on stem cell culture and differentiation; technically demanding and labor intensive. |
| Length of Derivation | Neuronal markers within 10 days; mature neurons in ~ 30 days or more. | Neuronal markers within 30 days; mature neurons in ~50 days or more. |
| Source Durability | Source fibroblasts may be limiting; embryonic fibroblasts are more expandable. | iPSCs are stable lines that can be differentiated to unlimited amount of DA neurons. |
| Epigenetics of Age | Better preservation of age-associated epigenetic information. | Reversion of age to embryonic state in iPSCs, may be reestablished in differentiation. |
| Mitotic Cells | Small percentage of unreprogrammed mitotic cells with low proliferative potential. | May contain undifferentiated cells with high proliferative potential. |
Perhaps the most anticipated development for iDA neurons is to test its potentials in cell replacement therapy of Parkinson’s disease. Transplantation of mouse iDA neurons in 6-OHDA-lesioned mice restores locomotor deficits [16]. Similarly, mouse iDA neurons transplanted in 6-OHDA-lesioned rats functionally integrate into the rat neuronal network and alleviate motor symptoms [100]. Further studies are needed to evaluate human iDA neurons grafted in animal PD models. The key questions are whether iDA neurons can survive in animal brain, integrate with existing neuronal network, extend significant axon arbors and reduce locomotor deficits. It has taken many years of intensive efforts to develop effective methods to transplant human pluripotent stem cell-derived midbrain DA neurons for cell replacement therapy of Parkinson’s disease. We are still at a very early stage in the development of iDA neurons for transplantation studies. There are signs that this new tool can be very useful. First, the direct conversion does not involve any stem cell intermediates, which means less worries for mitotic cells that can potentially be tumorigenic. Indeed, our study has shown that 93% of the cells at day 10 are neurons (including 59% that are TH+). The remaining 7% cells that are not converted to neurons do not express markers for fibroblasts [9]. The very high reprogramming efficiency makes it possible to sort the cells further so that only neurons or DA neurons are transplanted. Second, the use of fibroblasts obviates ethical concerns associated with fetal tissue or embryonic stem cells. Third, the ease to generate iDA neurons from any subject makes autologous transplantation more feasible. The disadvantage of using iDA neurons for cell replacement studies include the difficulty to significantly expand dermal fibroblasts as the starting material; the epigenetic memory of the age of the fibroblasts; and potential DNA damage in dermal fibroblasts in patient of advanced age. Future development is needed for a footprint-free system to reliably convert human dermal fibroblasts to iDA neurons at a high efficiency. The current system, in which viral transgenes are integrated in the iDA neurons, is unlikely to be suitable for clinical applications.
8. Conclusion
The explosive growth in cell reprogramming in the past ten years since the discovery of mouse iPSCs has fundamentally transformed biomedical research. With the ability to convert easily accessible cells, such as skin fibroblasts, to inaccessible cells that are lost in diseases (e.g. midbrain DA neurons in PD), it is possible to generate cells in vitro that are increasingly similar to their in vivo counterparts. Although there are still many questions on iDA neurons, the ability of this technology to capture the intrinsic properties of nigral DA neurons in PD patients will lead to useful applications that will address the three unmet needs for Parkinson’s disease, with the ultimate goal of finding a disease-modifying therapy (Fig. 1).
Acknowledgement
The work was supported by the Department of Veterans Affairs Merit Award I01BX002452, NYSTEM contracts C028129, C029556 and C026714, SUNY REACH, National Program of Basic Research Fund of China [grant numbers 2010CB945200, 2011CB504104] and National Natural Science Fund [grant numbers 81430022, 81371407, 91332107].
References
- 1.Lang A., Lozano A. Medical progress: Parkinson's disease (first of two parts) N. Engl. J. Med. 1998;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- 2.Lange A., Lozano A. Parkinson’s disease: second of two parts. N. Engl. J. Med. 1998;339:1130–1143. doi: 10.1056/NEJM199810153391607. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J. Genetic analysis of pathways to Parkinson disease. Neuron. 2010;68(2):201–206. doi: 10.1016/j.neuron.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kefalopoulou Z. Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol. 2014;71(1):83–87. doi: 10.1001/jamaneurol.2013.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker R.A. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson's disease. Lancet Neurol. 2013;12(1):84–91. doi: 10.1016/S1474-4422(12)70295-8. [DOI] [PubMed] [Google Scholar]
- 6.Barker R.A., Drouin-Ouellet J., Parmar M. Cell-based therapies for Parkinson disease [mdash] past insights and future potential. Nat. Rev. Neurol. 2015;11(9):492–503. doi: 10.1038/nrneurol.2015.123. [DOI] [PubMed] [Google Scholar]
- 7.Grealish S. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson’s disease. Cell Stem Cell. 2014;15(5):653–665. doi: 10.1016/j.stem.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caiazzo M. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476(7359):224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- 9.Jiang H. Cell cycle and p53 gate the direct conversion of human fibroblasts to dopaminergic neurons. Nat. Commun. 2015;6:10100. doi: 10.1038/ncomms10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vierbuchen T. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463(7284):1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pang Z.P. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476(7359):220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Z. Reprogramming non-human primate somatic cells into functional neuronal cells by defined factors. Mol. Brain. 2014;7(1):1–11. doi: 10.1186/1756-6606-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirakhori F. Direct conversion of human fibroblasts into dopaminergic neural progenitor-like cells using TAT-mediated protein transduction of recombinant factors. Biochem. Biophys. Res. Commun. 2015;459(4):655–661. doi: 10.1016/j.bbrc.2015.02.166. [DOI] [PubMed] [Google Scholar]
- 14.Tian C. Selective generation of dopaminergic precursors from mouse fibroblasts by direct lineage conversion. Sci. Rep. 2015:5. doi: 10.1038/srep12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfisterer U. Direct conversion of human fibroblasts to dopaminergic neurons. Proc. Natl. Acad. Sci. 2011;108(25):10343–10348. doi: 10.1073/pnas.1105135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J. Functional integration of dopaminergic neurons directly converted from mouse fibroblasts. Cell Stem Cell. 2011 doi: 10.1016/j.stem.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzoni E.O. Synergistic binding of transcription factors to cell-specific enhancers programs motor neuron identity. Nat. Neurosci. 2013;16(9):1219–1227. doi: 10.1038/nn.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu M.L., Zang T., Zhang C.L. Direct lineage reprogramming reveals disease-specific phenotypes of motor neurons from human ALS patients. Cell Rep. 2016;14(1):115–128. doi: 10.1016/j.celrep.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Son E.Y. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011;9(3):205–218. doi: 10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanchard J.W. Selective conversion of fibroblasts into peripheral sensory neurons. Nat. Neurosci. 2015;18(1):25–35. doi: 10.1038/nn.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Victor M.B. Generation of human striatal neurons by MicroRNA-dependent direct conversion of fibroblasts. Neuron. 2014;84(2):311–323. doi: 10.1016/j.neuron.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richner M. MicroRNA-based conversion of human fibroblasts into striatal medium spiny neurons. Nat. Protoc. 2015;10(10):1543–1555. doi: 10.1038/nprot.2015.102. [DOI] [PubMed] [Google Scholar]
- 23.Liu M.-L. Small molecules enable neurogenin 2 to efficiently convert human fibroblasts into cholinergic neurons. Nat. Commun. 2013:4. doi: 10.1038/ncomms3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vadodaria K.C. Generation of functional human serotonergic neurons from fibroblasts. Mol. Psychiatry. 2016;21(1):49–61. doi: 10.1038/mp.2015.161. [DOI] [PubMed] [Google Scholar]
- 25.Xu Z. Direct conversion of human fibroblasts to induced serotonergic neurons. Mol. Psychiatry. 2016;21(1):62–70. doi: 10.1038/mp.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu H. Direct conversion of mouse fibroblasts to GABAergic neurons with combined medium without the introduction of transcription factors or miRNAs. Cell Cycle. 2015;14(15):2451–2460. doi: 10.1080/15384101.2015.1060382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu W. Direct conversion of normal and Alzheimer’s disease human fibroblasts into neuronal cells by small molecules. Cell Stem Cell. 2015;17(2):204–212. doi: 10.1016/j.stem.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Xu X.-L. Direct reprogramming of porcine fibroblasts to neural progenitor cells. Protein Cell. 2014;5(1):4–7. doi: 10.1007/s13238-013-0015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang N. Generation of oligodendroglial cells by direct lineage conversion. Nat. Biotechnol. 2013;31(5):434–439. doi: 10.1038/nbt.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Najm F.J. Transcription factor-mediated reprogramming of fibroblasts to expandable, myelinogenic oligodendrocyte progenitor cells. Nat. Biotechnol. 2013;31(5):426–433. doi: 10.1038/nbt.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X. Direct reprogramming of human fibroblasts into dopaminergic neuron-like cells. Cell Res. 2011 doi: 10.1038/cr.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pereira M. Highly efficient generation of induced neurons from human fibroblasts that survive transplantation into the adult rat brain. Sci. Rep. 2014:4. doi: 10.1038/srep06330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh S.-i. Efficient reprogramming of mouse fibroblasts to neuronal cells including dopaminergic neurons. Sci. World J. 2014:2014. doi: 10.1155/2014/957548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim M.-S. Generation of dopamine neurons from rodent fibroblasts through the expandable neural precursor cell stage. J. Biol. Chem. 2015;M114:629808. doi: 10.1074/jbc.M114.629808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoo J. Nanogrooved substrate promotes direct lineage reprogramming of fibroblasts to functional induced dopaminergic neurons. Biomaterials. 2015;45:36–45. doi: 10.1016/j.biomaterials.2014.12.049. [DOI] [PubMed] [Google Scholar]
- 36.Heinrich C. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 2010;8(5) doi: 10.1371/journal.pbio.1000373. (e1000373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heinrich C. Generation of subtype-specific neurons from postnatal astroglia of the mouse cerebral cortex. Nat. Protoc. 2011;6(2):214–228. doi: 10.1038/nprot.2010.188. [DOI] [PubMed] [Google Scholar]
- 38.Guo Z. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell. 2014;14(2):188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y. Ascl1 converts dorsal midbrain astrocytes into functional neurons in vivo. J. Neurosci. 2015;35(25):9336–9355. doi: 10.1523/JNEUROSCI.3975-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potts M.B. Analysis of Mll1 deficiency identifies neurogenic transcriptional modules and Brn4 as a factor for direct astrocyte-to-neuron reprogramming. Neurosurgery. 2014;75(4):472–482. doi: 10.1227/NEU.0000000000000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niu W. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat. Cell Biol. 2013;15(10):1164–1175. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su Z. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat. Commun. 2014:5. doi: 10.1038/ncomms4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heinrich C. Sox2-mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem Cell Rep. 2014;3(6):1000–1014. doi: 10.1016/j.stemcr.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torper O. In vivo reprogramming of striatal NG2 Glia into functional neurons that integrate into local host circuitry. Cell Rep. 2015;12(3):474–481. doi: 10.1016/j.celrep.2015.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao J.J. Induction of retinal progenitors and neurons from mammalian Muller glia under defined conditions. J. Biol. Chem. 2014;289(17):11945–11951. doi: 10.1074/jbc.M113.532671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marro S. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell. 2011;9(4):374–382. doi: 10.1016/j.stem.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cassady J.P. Direct lineage conversion of adult mouse liver cells and B lymphocytes to neural stem cells. Stem Cell Rep. 2014;3(6):948–956. doi: 10.1016/j.stemcr.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishimura K. Generation of induced neurons by direct reprogramming in the mammalian cochlea. Neuroscience. 2014;275:125–135. doi: 10.1016/j.neuroscience.2014.05.067. [DOI] [PubMed] [Google Scholar]
- 49.Castaño J. Fast and efficient neural conversion of human hematopoietic cells. Stem Cell Rep. 2014;3(6):1118–1131. doi: 10.1016/j.stemcr.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu K.-R. Rapid and efficient direct conversion of human adult somatic cells into neural stem cells by HMGA2/let-7b. Cell Rep. 2015;10(3):441–452. doi: 10.1016/j.celrep.2014.12.038. [DOI] [PubMed] [Google Scholar]
- 51.Liao W. Direct Conversion of cord blood CD34+ cells into neural stem cells by OCT4. Stem Cells Transl. Med. 2015 doi: 10.5966/sctm.2014-0289. (2014-0289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang T. Direct induction of human neural stem cells from peripheral blood hematopoietic progenitor cells. J. Vis. Exp.: JoVE. 2014;(95) doi: 10.3791/52298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang X. Conversion of adult human peripheral blood mononuclear cells into induced neural stem cell by using episomal vectors. Stem Cell Res. 2016;16(2):236–242. doi: 10.1016/j.scr.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 54.Zabierowski S.E. Direct Reprogramming of melanocytes to neural crest stem‐like cells by one defined factor. Stem Cells. 2011;29(11):1752–1762. doi: 10.1002/stem.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gopalakrishnan V. Myoblast‐derived neuronal cells form glutamatergic neurons in the mouse cerebellum. Stem Cells. 2010;28(10):1839–1847. doi: 10.1002/stem.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watanabe Y. Conversion of myoblasts to physiologically active neuronal phenotype. Genes Dev. 2004;18(8):889–900. doi: 10.1101/gad.1179004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karow M. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell. 2012;11(4):471–476. doi: 10.1016/j.stem.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 58.Karow M. Lineage-reprogramming of pericyte-derived cells of the adult human brain into induced neurons. J. Vis. Exp. 2014;(87) doi: 10.3791/51433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tursun B. Direct conversion of C. elegans germ cells into specific neuron types. Science. 2011;331(6015):304–308. doi: 10.1126/science.1199082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheng C. Direct reprogramming of Sertoli cells into multipotent neural stem cells by defined factors. Cell Res. 2011 doi: 10.1038/cr.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang L. Generation of integration-free neural progenitor cells from cells in human urine. Nat. Methods. 2013;10(1):84–89. doi: 10.1038/nmeth.2283. [DOI] [PubMed] [Google Scholar]
- 62.Zhang S.Z. Modeling neurological disease by rapid conversion of human urine cells into functional neurons. Stem Cells Int. 2016;2016:2452985. doi: 10.1155/2016/2452985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng L. Generation of neural progenitor cells by chemical cocktails and hypoxia. Cell Res. 2014;24(6):665–679. doi: 10.1038/cr.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He S. Reprogramming somatic cells to cells with neuronal characteristics by defined medium both in vitro and in vivo. Cell Regen. 2015;4:12. doi: 10.1186/s13619-015-0027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng L. Direct conversion of astrocytes into neuronal cells by drug cocktail. Cell Res. 2015 doi: 10.1038/cr.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chao C.C. Induction of neural differentiation in rat C6 glioma cells with taxol. Brain Behav. 2015;5:12. doi: 10.1002/brb3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ladewig J. Small molecules enable highly efficient neuronal conversion of human fibroblasts. Nat. Methods. 2012;9(6):575–578. doi: 10.1038/nmeth.1972. [DOI] [PubMed] [Google Scholar]
- 68.Alexanian A.R., Liu Q.-s., Zhang Z. Enhancing the efficiency of direct reprogramming of human mesenchymal stem cells into mature neuronal-like cells with the combination of small molecule modulators of chromatin modifying enzymes, SMAD signaling and cyclic adenosine monophosphate levels. Int. J. Biochem. Cell Biol. 2013;45(8):1633–1638. doi: 10.1016/j.biocel.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 69.Passeri E. Enhanced conversion of induced neuronal cells (iN cells) from human fibroblasts: utility in uncovering cellular deficits in mental illness-associated chromosomal abnormalities. Neurosci. Res. 2015;101:57–61. doi: 10.1016/j.neures.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dai P., Harada Y., Takamatsu T. Highly efficient direct conversion of human fibroblasts to neuronal cells by chemical compounds. J. Clin. Biochem. Nutr. 2015;56(3):166–170. doi: 10.3164/jcbn.15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mirakhori F. Brief azacytidine step allows the conversion of suspension human fibroblasts into neural progenitor-like cells. Cell J. 2015;17(1):153–158. doi: 10.22074/cellj.2015.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han Y.C. Direct reprogramming of mouse fibroblasts to neural stem cells by small molecules. Stem Cells Int. 2016;2016:4304916. doi: 10.1155/2016/4304916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gabel S. Inflammation promotes a conversion of astrocytes into neural progenitor cells via NF-kappaB activation. Mol. Neurobiol. 2015 doi: 10.1007/s12035-015-9428-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davila J. Acute reduction in oxygen tension enhances the induction of neurons from human fibroblasts. J. Neurosci. Methods. 2013;216(2):104–109. doi: 10.1016/j.jneumeth.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chanda S. Generation of induced neuronal cells by the single reprogramming factor Ascl1. Stem Cell Rep. 2014;3(2):282–296. doi: 10.1016/j.stemcr.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lo L., Sommer L., Anderson D.J. MASH1 maintains competence for BMP2-induced neuronal differentiation in post-migratory neural crest cells. Curr. Biol. 1997;7(6):440–450. doi: 10.1016/s0960-9822(06)00191-6. [DOI] [PubMed] [Google Scholar]
- 77.Parras C.M. Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity. Genes Dev. 2002;16(3):324–338. doi: 10.1101/gad.940902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li J., Dani J.A., Le W. The role of transcription factor Pitx3 in dopamine neuron development and Parkinson's disease. Curr. Top. Med. Chem. 2009;9(10):855. [PMC free article] [PubMed] [Google Scholar]
- 79.Wapinski O.L. Hierarchical mechanisms for direct reprogramming of fibroblasts to neurons. Cell. 2013;155(3):621–635. doi: 10.1016/j.cell.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Farah M.H. Generation of neurons by transient expression of neural bHLH proteins in mammalian cells. Development. 2000;127(4):693–702. doi: 10.1242/dev.127.4.693. [DOI] [PubMed] [Google Scholar]
- 81.Feng J. Kinetic barriers in transdifferentiation. Cell Cycle. 2016;15(8):1019–1020. doi: 10.1080/15384101.2016.1151730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Soufi A., Dalton S. Cycling through developmental decisions: how cell cycle dynamics control pluripotency, differentiation and reprogramming. Development. 2016;143(23):4301–4311. doi: 10.1242/dev.142075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou D. Conversion of fibroblasts to neural cells by p53 depletion. Cell Rep. 2014;9(6):2034–2042. doi: 10.1016/j.celrep.2014.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun C.K. Senescence impairs direct conversion of human somatic cells to neurons. Nat. Commun. 2014;5:4112. doi: 10.1038/ncomms5112. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85.Liu X. Enhancing the efficiency of direct reprogramming of human primary fibroblasts into dopaminergic neuron-like cells through p53 suppression. Sci. China Life Sci. 2014;57(9):867–875. doi: 10.1007/s11427-014-4730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu H., Zhang Y. Mechanisms and functions of Tet protein-mediated 5-methylcytosine oxidation. Genes Dev. 2011;25(23):2436–2452. doi: 10.1101/gad.179184.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kriaucionis S., Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324(5929):929–930. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smidt M.P., Burbach J.P.H. How to make a mesodiencephalic dopaminergic neuron. Nat. Rev. Neurosci. 2007;8(1):21–32. doi: 10.1038/nrn2039. [DOI] [PubMed] [Google Scholar]
- 89.Andersson E. Identification of intrinsic determinants of midbrain dopamine neurons. Cell. 2006;124(2):393–405. doi: 10.1016/j.cell.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 90.Flames N., Hobert O. Transcriptional control of the terminal fate of monoaminergic neurons. Annu. Rev. Neurosci. 2011;34:153–184. doi: 10.1146/annurev-neuro-061010-113824. [DOI] [PubMed] [Google Scholar]
- 91.Zetterstrom R.H. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997;276(5310):248–250. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- 92.Yoo A.S. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476(7359):228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ambasudhan R. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell stem Cell. 2011;9(2):113–118. doi: 10.1016/j.stem.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou C. MicroRNA 302/367 cluster effectively facilitates direct reprogramming from human fibroblasts into functional neurons. Stem Cells Dev. 2015;24(23):2746–2755. doi: 10.1089/scd.2015.0123. [DOI] [PubMed] [Google Scholar]
- 95.Lau S. Direct neural conversion from human fibroblasts using self-regulating and nonintegrating viral vectors. Cell Rep. 2014;9(5):1673–1680. doi: 10.1016/j.celrep.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 96.Xue Y. Direct conversion of fibroblasts to neurons by reprogramming PTB-regulated MicroRNA circuits. Cell. 2013 doi: 10.1016/j.cell.2012.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiang H. Parkin controls dopamine utilization in human midbrain dopaminergic neurons derived from induced pluripotent stem cells. Nat. Commun. 2012;3:668. doi: 10.1038/ncomms1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Young R.A. Control of the embryonic stem cell state. Cell. 2011;144(6):940–954. doi: 10.1016/j.cell.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mertens J. Directly reprogrammed human neurons retain aging-associated transcriptomic signatures and reveal age-related nucleocytoplasmic defects. Cell Stem Cell. 2015;17(6):705–718. doi: 10.1016/j.stem.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dell’Anno M.T. Remote control of induced dopaminergic neurons in parkinsonian rats. J. Clin. Investig. 2014;124(7):3215–3229. doi: 10.1172/JCI74664. [DOI] [PMC free article] [PubMed] [Google Scholar]