Abstract
Introduction
The clinical relevance of Achromobacter xylosoxidans infection in cystic fibrosis (CF) remains controversial. This emerging agent in CF has been associated with increased lung inflammation, more frequent exacerbations and more severe lung disease. We describe a pair of CF siblings chronically colonized by the same multilocus genotype of A. xylosoxidans with different clinical courses, and assess whether this species may have developed any virulence traits and antimicrobial resistance that could have contributed to their singular outcomes.
Case presentation
Two siblings were positive for the F508del and Y1092X mutations, and were chronically colonized by Pseudomonas aeruginosa and Staphylococcus aureus. The female patient had a more severe CF phenotype and faster clinical deterioration than her brother. Her pulmonary function and computed tomography scan lesions were worse than those of her brother, and both parameters progressively declined. She died at 14 years of age, when he was 18. All isolates of A. xylosoxidans were biofilm producers. Achromobacter xylosoxidans showed less swarming motility in the female patient.
Conclusions
Biofilm production and diminution of motility allow persistence. Only swarming motility differed between the isolates recovered from the two siblings, but this finding is not sufficient to explain the different clinical outcomes despite their similar genotypes. Modifier genes, unknown environmental factors and female gender can partially explain differences between these siblings. We were unable to correlate any microbiological findings with their clinical courses, and more translational studies are necessary to decrease the gap of knowledge between laboratory and clinical data to promote better clinical interventions.
Keywords: Achromobacter spp., Achromobacter xylosoxidans, Cystic fibrosis
1. Introduction
Achromobacter spp. are emergent pathogens in cystic fibrosis (CF) patients. There are 16 species and 2 subspecies already described [1]. Recently there is a proposal to include 4 new species: Achromobacter agilis sp. nov., nom. rev., Achromobacter pestifer sp. nov., nom. rev., Achromobacter kerstersii sp. nov. and Achromobacter deleyi sp. nov. [2]. The most prevalent species in CF using discriminative molecular tools is Achromobacter xylosoxidans, which has been associated with increased lung inflammation [3], more frequent CF exacerbations, and more severe lung disease [4], [5]. However, evidence of the clinical relevance of these species remains controversial [6]. Achromobacter ruhlandii, Achromobacter insuavis, Achromobacter dolens and a few of other Achromobacter species may also chronically colonize CF patients, but most are sporadic [7]. The adaptation of Achromobacter species to the human host in chronic infection remains uncharacterized, and studies are needed to clarify the pathogenesis of this agent in CF lung disease [8].
We describe 2 cases of CF siblings chronically colonized with A. xylosoxidans for more than 10 years who had different clinical courses and assess whether this species may have developed any coping virulence traits and antimicrobial resistance that could have contributed to their singular outcomes. To measure differences in their clinical courses, we reviewed clinical data, computed tomography (CT) scans and lung function. To investigate the possible role of A. xylosoxidans in the etiopathology of lung disease, we investigated the presence of well-known virulence traits favouring bacterial colonization of the host mucosa, such as biofilm formation, bacterial motility and antibiotic resistance.
2. Case reports
Two siblings were diagnosed with CF in the same year, when the younger, a female patient, was 15 months old and her brother was 5 years and 10 months old. Both patients always lived with their parents in the same home where they were born. Both were positive for the F508del and Y1092X severe mutations, had exogenous pancreatic insufficiency and elevated sweat chloride concentrations and were chronically colonized by A. xylosoxidans, Pseudomonas aeruginosa (both mucoid and non-mucoid from the first colonization) and methicillin-susceptible Staphylococcus aureus for more than 10 years [9]. However, they had different clinical courses.
Follow-up at the CF reference centre began when the girl was 15 months old, immediately after diagnosis. She had a past history of meconium ileus (MI), gastroesophageal reflux, failure to thrive, one hospitalization for oedema, hypoproteinaemia and anaemia and an episode of distal intestinal obstruction syndrome. Her parents also related daily productive cough in the previous 2 months, recurrent vomitus and 4 evacuations/day, with greasy malodorous stools. On physical examination, she weighed 6950 g, with a length of 72 cm and body mass index (BMI) of 13.4 kg/m2 (all less than the 3rd percentile for age and z score −2.01, −2.73 and −2.11 respectively), 44 breaths per minute, subcostal retractions, pulmonary rhonchi, 98% oxygen saturation and hepatomegaly. Oropharyngeal swab culture was positive for P. aeruginosa and S. aureus. Attempts to eradicate P. aeruginosa failed. After 1 year of P. aeruginosa and S. aureus chronic colonization, at 2 years and 3 months of age, she had the first positive culture for A. xylosoxidans, which also evolved to chronic infection. Regarding sporadic colonization, the girl had a single positive sputum culture for Haemophilus and one for Acinetobacter. In the first 2 years of follow-up in the CF care centre, she was hospitalized 2 times yearly because of CF exacerbation. The period between 3 and 10 years of age was almost free of bad events, except for persistent difficulty in weight gain, with one hospitalization because of CF exacerbation. Thereafter, her clinical condition steadily worsened. In the next 4 years, she was hospitalized 8 times for pulmonary exacerbations, of which 5 occurred during the last year, with progressive deterioration of lung disease and evolution to respiratory failure. She was referred to a lung transplant centre, but died before transplant at 14 years and 4 months of age.
Her brother, 4 years and 7 months older, was diagnosed with CF after her diagnosis. He was born at term without complications and had a past history of frequent vomiting and poor weight gain since his first months of life. He was hospitalized for dehydration at 3 months of age and for enterorrhagia by Meckel's diverticulum at 6 months of age. At 2 years of age, recurrent upper respiratory tract infections started. Despite a voracious appetite, his difficulty in weight gain worsened. In the first consultation at the CF reference centre, at 5 years and 10 months of age, his parents reported that he had frequent coughing. On physical examination, he exhibited pallor, with a weight of 15.7 kg (2nd percentile for age), height of 109 cm (11th percentile for age), BMI of 13.3 kg/m2 (3rd percentile for age), and z scores of −1.22, −1.94 and −1.81, respectively. From the first consultation, P. aeruginosa and S. aureus respiratory chronic colonization were detected and, from the following month, also A. xylosoxidans. Attempts to eradicate P. aeruginosa also failed. Regarding sporadic colonization, the boy had two positive cultures for Haemophilus. Until 18 years of age, he had 3 exacerbations of CF treated with hospitalization for 14 days each during the 7th and 8th years and another hospitalization for viral encephalitis at age 11. In the last year, at 17 years and 6 months old, liver disease was detected, and at 18 years of age he was admitted twice: once for vasculitis and another for exacerbation of CF. However, their mother claimed that she could not understand the greater debilitation of her daughter's health compared with her son's, despite the daughter's better compliance with the treatment regimen.
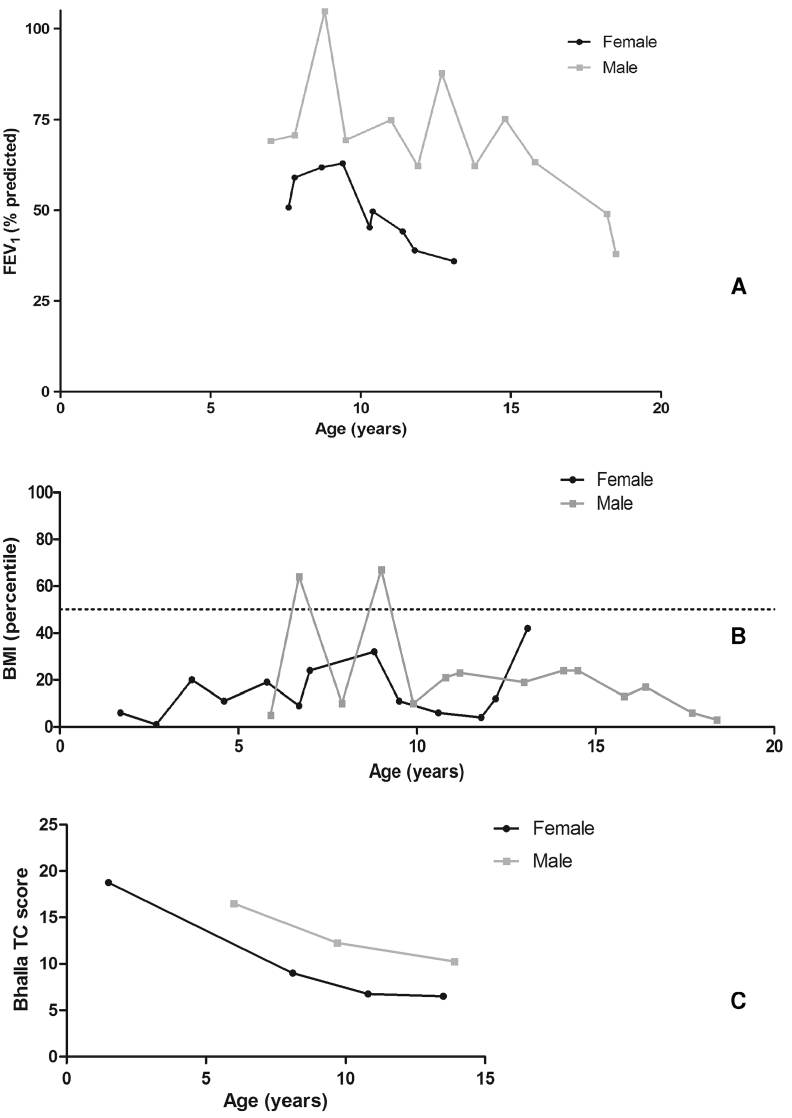
The comparisons of lung function (forced expiratory volume in 1 s - FEV1), BMI and Bhalla CT score in two siblings were done at the same age and are presented in Fig. 1. Although both siblings exhibited some improvement in their absolute BMI during follow-up at the reference centre, the female's values were significantly lower than the male's (Mann-Whitney test, p < 0.0001; interquartile Range (IQR) - male: 2.5 kg/m2, female: 1.1 kg/m2).
Fig. 1.
Evolution of forced expiratory volume in 1 s (FEV1% predicted) (A), body mass index (BMI) (B) and Bhalla computed tomography score (C) in two siblings with cystic fibrosis. The target of 50th percentile of BMI for age is showed on the dotted line (B). She never achieved the target of 50th percentile of BMI for age, which he passed eventually in the first 5 years of follow-up. The female's FEV1 was 50.9% at her first spirometry at 7 years and 7 months of age. Her FEV1 improved to 62.9% in 2 years and declined to 36% in the next 42 months. At 7 years of age, his FEV1 was 69.1%, and in the next 2 years, it improved to the normal range. However, between 15 and 18 years of age, there was a faster FEV1 decline to 38%. During the 6 years we were able to measure the lung function of both siblings, his FEV1 measures were 35% higher than hers. However, the female also exhibited a faster decline of FEV1 in the last 4 years, to 32%.
To measure lung structural damage, 2 radiologists who were blinded to any other information reviewed the CT scans separately. They assigned modified Bhalla scores [10], [11] for each CT scan and reassigned the scores 1 month later. The median of the final Bhalla scores was obtained for each available CT scan. The female had lower Bhalla scores than her brother: 18.7 (1 year and 6 months), 9 (7 year and 2 months), 6.7 (10 years and 11 months) and 6.5 (13 years and 6 months). His scores were 16.5 (5 years and 10 months), 12.2 (9 years and 7 months) and 10.2 (14 years).
Their bacteriological backgrounds were studied between 3.3 and 6.5 years of age for the female and 7.7 and 9.9 years of age for her brother. Species identification was performed by conventional methods, and genotype analysis was performed by multi-locus sequence typing (MLST) of 7 housekeeping genes (nusA, rpoB, eno, gltB, lepA, nuoL, nrdA), as previously described [12], [13]. Allelic profiles and ST were analysed according to the PubMLST Website (http://pubmlst.org/achromobacter/). All isolates were A. xylosoxidans and belonged to the same sequence type (ST 201). This is a new ST previously identified in Brazilian CF patients by our group and added to the PubMLST Website [13].
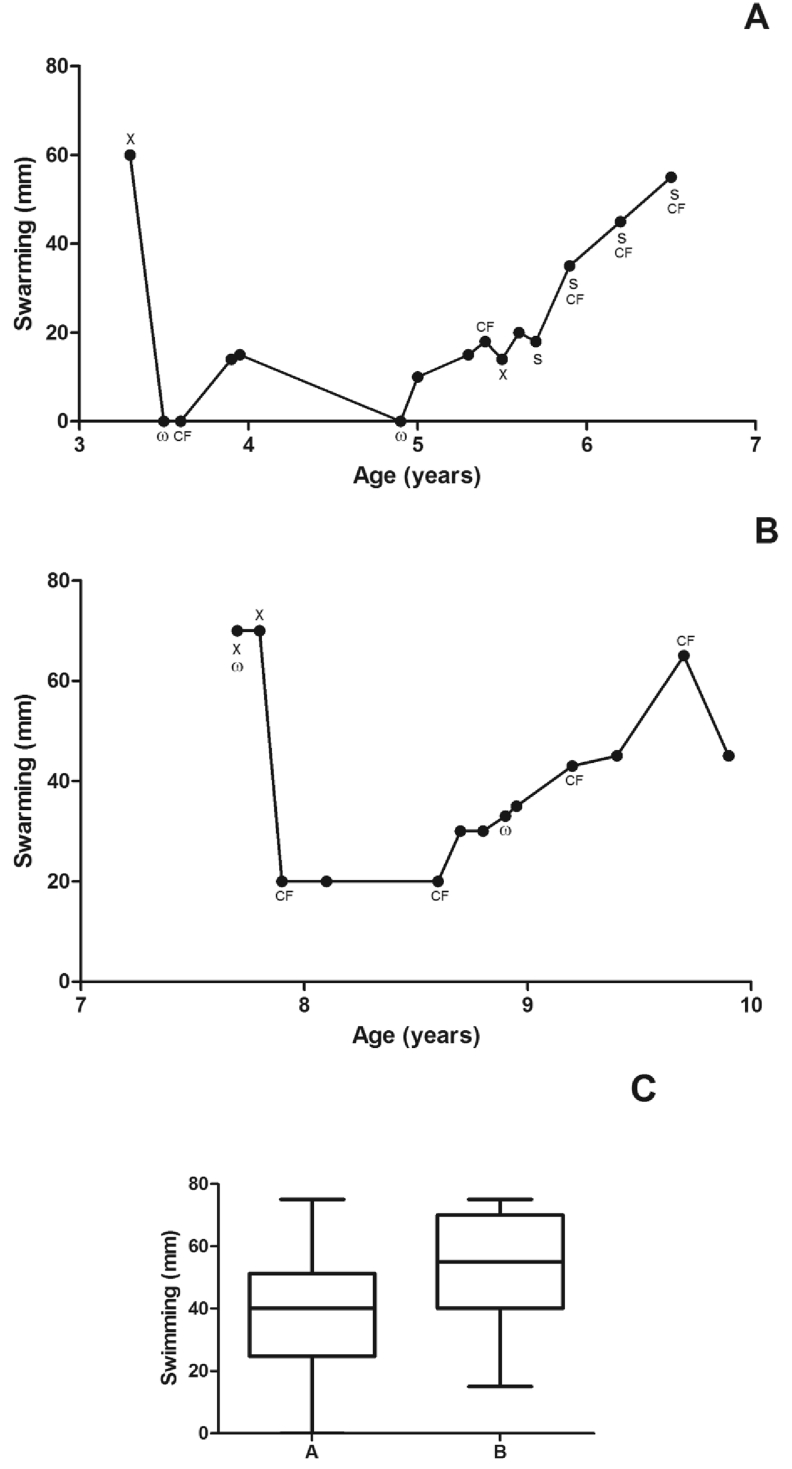
The determination of minimal inhibitory concentrations (MIC) of antibiotics [ceftazidime (CAZ), ciprofloxacin (CIP), imipenem (IMP) and thimethoprim-sulfamethoxazole (TMP-SXT)] was performed using E-test strips (AB Biodisk, Solna, Sweden). The breakpoints used were those recommended by CLSI for non-Enterobacteriaceae. The isolates were considered resistant when MIC ≥32 μg/mL for CAZ; MIC ≥4 μg/mL for CIP; MIC ≥16 μg/mL for IMP and MIC ≥4/76 μg/mL for TMP-SXT [14]. The majority of isolates (58.3%) were susceptible to all antibiotics tested (95%CI: 36.5–80.1%). All isolates were susceptible to IMP and CAZ (Fig. 2).
Fig. 2.
Patients' A. xylosoxidans characteristics (ST201). Female (A), male (B) and box plot diagram showing that swimming did not reach a statistical significant difference (C). Only one colony per sputum of different visits was included for analysis. ω: a weak biofilm producer isolate (all remaining isolates were strong biofilm producers); CF: an isolate resistant to ciprofloxacin; S: an isolate resistant to thimethoprim-sulfamethoxazole (most isolates were susceptible to all antibiotics tested); X: susceptibility data not available.
The swimming and swarming motility abilities were determined as described by Rashid et al. [15]. The results are presented as the median diameters obtained in 3 independent assays performed in triplicate (Fig. 2). There were no differences in swimming phenotype between the isolates (unpaired t-test, p = 0.07; 95%CI - male: 38.1–58.2 mm, female: 27.2–45.4 mm), but the swarming phenotype was significantly higher for the A. xylosoxidans isolates recovered from the male (Mann-Whitney test, p = 0.004; IQR - male: 23 mm, female: 20 mm).
Biofilm formation on abiotic plastic surfaces was determined as described by Tendolkar et al. [16]. Then, the A. xylosoxidans isolates were divided into different biofilm-producer classes: N, no biofilm producer; W, weak biofilm producer; M, moderate biofilm producer; and S, strong biofilm producer, as described by Stepanovic et al. [17]. All isolates produced biofilms, and the majority were strong producers, but no significant difference in the amount of biofilm formation was detected when isolates from both patients were compared.
Data were analysed with Graph Pad Prism software, version 5.0. The specific tests used are described in the text. Significance was accepted at the p < 0.05 level.
3. Discussion
The main strength of this study is the attempt to connect microbiological and clinical data to promote the integration of these areas of knowledge and understanding of this complexity. The rapid and deep progress of knowledge are so remarkable that such integration is imperative to reduce doubts and enable effective strategies for clinical interventions. These siblings had the same genetic mutations, lived in the same environment, and were colonized by the same bacterial species, seemingly promising characteristics to “control” possible study bias.
Studies of A. xylosoxidans chronic colonization have revealed the persistence of a unique ST revealed by MLST analysis [18], [19]. The two siblings shared the same ST, suggesting the possibility of family spread or a common source of acquisition. Transmission in the same family has been described previously by others [18], [20], [21].
To cause chronic infection, bacteria must overcome the heterogeneous, hostile and stressful lung environment through adaptation mechanisms [22]. Although little is known about the mechanisms of virulence and adaptation of Achromobacter spp., studies of P. aeruginosa in CF patients have suggested that phenotypes that confer mobility (swimming, swarming and twitching) and toxin production are important for acute infection, whereas biofilm production, the reduction of virulence factors and development of resistance are mechanisms of adaptation that favour chronic infection [15], [22]. In our study, more than 50% of isolates from both patients were strong biofilm producers and were susceptible to all antibiotics tested. The significantly lower values of swarming motility of A. xylosoxidans isolated from the female patient suggests a loss of motility as a bacterial strategy to reduce virulence and to avoid host immune attack in favour of chronicity [23]. Similar adaptive mechanisms have been observed in studies focused on P. aeruginosa and other species [15], [22], [23]. It is interesting to note the variability observed between successive isolates for some of the characteristics studied, such as changes in drug resistance and swarming. Since only one colony per specimen was studied, intra-specimen diversity could probably be one hypothesis to explain these observations.
A few translational studies have attempted to connect microbiological and clinical data. Trancassini et al. [8] correlated strong A. xylosoxidans biofilm producers with severe obstruction in lung function. Although the present study design did not permit the inference of a cause-and-effect relationship, it supports the chronicity of A. xylosoxidans colonization and subsequent deterioration of pulmonary function and structural pulmonary damage. Pulmonary function worsened to severe obstruction in both patients, and both also gradually lost points in the Bhalla CT score.
Various CT scores can be used to assess the presence, severity and extent of pulmonary structural damage and have been correlated with lung function [23]. The Bhalla score is one of the most studied. We used only the final (total) Bhalla score based on its higher levels of reproducibility compared with each component of the score [24]. In addition, the available CT scans were acquired using different protocols, which could favour intra- and interobserver bias for fine details, thus further supporting the use of only the final score.
The combination of the F508del and Y1092X genetic mutations is expected to cause pancreatic insufficiency, as observed in these siblings [25]. However, the CF phenotype of the female patient was more severe since birth, and she had a markedly worse outcome than her brother. Siblings with CF often have different phenotypes and clinical courses, even in the presence of the same disease-causing genes, because the CF phenotype is affected not only by the CFTR genotype but also by environmental and other genetic factors [26]. MI, for example, occurs in almost 20% of CF patients and is more frequent with some CFTR mutations (F508del, G542X, W1282X, R553X, and G551D) but does not necessarily affect multiple siblings. This phenotypic variability has been partially attributed to modifier genes [27], [28]. The female gender may also have influenced the outcome as a negative prognostic factor. Despite an equal prevalence of CF, females have a lower median life expectancy than males and a higher risk of respiratory infections [29]. Females acquire P. aeruginosa earlier and exhibit a more rapid decline of lung function once infected [30]. The mechanisms underlying this gender disparity has not been fully elucidated, but sex hormones play a role. Estrogen enhances the conversion of P. aeruginosa from the non-mucoid to mucoid phenotype, which is more resistant to antibiotics [31]. Studies also suggest that women have more frequent CF exacerbations during ovulation, a period of high estrogen levels. Harness-Bronley et al. [30] analysed a large cohort of patients from the United States Cystic Fibrosis Foundation Patient Registry and found that not only P. aeruginosa but also methicillin-susceptible S. aureus, methicillin-resistant S. aureus, Haemophilus influenzae, A. xylosoxidans, Burkholderia cepacia, Aspergillus species, and non-tuberculous mycobacterium are acquired by females at earlier ages, often even before puberty.
Like any study, ours has limitations. First, there was no overlap of periods of microbiological follow-up for the two siblings. Only a period of microbiological data was available that were obtained prior to objective clinical data, such as data related to CT scans and pulmonary function, thus limiting the potential for translational conclusions. Second, we were unable to determine the precise timing of the initial A. xylosoxidans infection. In addition, these sibling patients were also chronically colonized by P. aeruginosa, which may be a cause of poor outcome. Thus, more translational studies are needed to better understand the relationships between microbiological and clinical data and potentially improve patient management.
Funding source
This study was supported by the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ; grant number E-110.742/2012) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; grant number 471326/2012-7), Brazil.
Conflict of interest
The authors have no potential conflicts of interest to disclose.
References
- 1.LPSN http://www.bacterio.net/achromobacter.html (Accessed 10 January 2017)
- 2.Vandamme P.A., Peeters C., Inganäs E., Cnockaert M., Houf K., Spilker T. Taxonomic dissection of Achromobacter denitrificans Coenye et al. 2003 and proposal of Achromobacter agilis sp. nov., nom. rev., Achromobacter pestifer sp. nov., nom. rev., Achromobacter kerstersii sp. nov. and Achromobacter deleyi sp. nov. Int. J. Syst. Evol. Microbiol. 2016 Sep;66(9):3708–3717. doi: 10.1099/ijsem.0.001254. [DOI] [PubMed] [Google Scholar]
- 3.Hansen C.R., Pressler T., Nielsen K.G., Jensen P.Ø., Bjarnsholt T., Høiby N. Inflammation in Achromobacter xylosoxidans infected cystic fibrosis patients. J. Cyst. Fibros. 2010 Jan;9(1):51–58. doi: 10.1016/j.jcf.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Lambiase A., Catania M.R., Del Pezzo M., Rossano F., Terlizzi V., Sepe A. Achromobacter xylosoxidans respiratory tract infection in cystic fibrosis patients. Eur. J. Clin. Microbiol. Infect. Dis. 2011 Aug;30(8):973–980. doi: 10.1007/s10096-011-1182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Firmida M.C., Pereira R.H.V., Silva E.A.S.R., Marques E.A., Lopes A.J. Clinical impact of Achromobacter xylosoxidans colonization/infection in patients with cystic fibrosis. Braz. J. Med. Biol. Res. 2016;49(4):e5097. doi: 10.1590/1414-431X20155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zemanick E.T., Hoffman L.R. Cystic fibrosis: microbiology and host response. Pediatr. Clin. North Am. 2016 Aug;63(4):617–636. doi: 10.1016/j.pcl.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dupont C., Michon A.-L., Jumas-Bilak E., Nørskov-Lauritsen N., Chiron R., Marchandin H. Intrapatient diversity of Achromobacter spp. involved in chronic colonization of Cystic Fibrosis airways. Infect. Genet. Evol. 2015 Jun;32:214–223. doi: 10.1016/j.meegid.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Trancassini M., Iebba V., Citerà N., Tuccio V., Magni A., Varesi P. Outbreak of Achromobacter xylosoxidans in an Italian Cystic fibrosis center: genome variability, biofilm production, antibiotic resistance, and motility in isolated strains. Front. Microbiol. 2014 Apr 3;5:138. doi: 10.3389/fmicb.2014.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee T.W.R., Brownlee K.G., Conway S.P., Denton M., Littlewood J.M. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J. Cyst. Fibros. 2003 Mar;2(1):29–34. doi: 10.1016/S1569-1993(02)00141-8. [DOI] [PubMed] [Google Scholar]
- 10.de Jong P.A., Ottink M.D., Robben S.G.F., Lequin M.H., Hop W.C.J., Hendriks J.J.E. Pulmonary disease assessment in cystic fibrosis: comparison of CT scoring systems and value of bronchial and arterial dimension measurements. Radiology. 2004 May;231(2):434–439. doi: 10.1148/radiol.2312021393. [DOI] [PubMed] [Google Scholar]
- 11.Bhalla M., Turcios N., Aponte V., Jenkins M., Leitman B.S., McCauley D.I. Cystic fibrosis: scoring system with thin-section CT. Radiology. 1991 Jun;179(3):783–788. doi: 10.1148/radiology.179.3.2027992. [DOI] [PubMed] [Google Scholar]
- 12.Spilker T., Vandamme P., LiPuma J.J. A multilocus sequence typing scheme implies population structure and reveals several putative novel Achromobacter species. J. Clin. Microbiol. 2012 Sep;50(9):3010–3015. doi: 10.1128/JCM.00814-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigues E.R.A., Ferreira A.G., Leão R.S., Leite C.C.F., Carvalho-Assef A.P., Albano R.M. Characterization of Achromobacter species in cystic fibrosis patients: comparison of bla(OXA-114) PCR amplification, multilocus sequence typing, and matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2015 Dec;53(12):3894–3896. doi: 10.1128/JCM.02197-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CSLI . Clinical and Standards Laboratory Institute; Wayne, PA: 2014. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-fourth Informational Supplement, CLSI Document M100–S24.http://shop.clsi.org/site/Sample_pdf/M100S25_sample.pdf (Accessed 20 October 2016) [Google Scholar]
- 15.Rashid M.H., Kornberg A. Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 2000;97(9):4885–4890. doi: 10.1073/pnas.060030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tendolkar P.M., Baghdayan A.S., Gilmore M.S., Shankar N. Enterococcal surface protein, Esp, enhances biofilm formation by Enterococcus faecalis. Infect. Immun. 2004 Oct;72(10):6032–6039. doi: 10.1128/IAI.72.10.6032-6039.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stepanović S., Vuković D., Hola V., Di Bonaventura G., Djukić S., Cirković I. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS. 2007 Aug;115(8):891–899. doi: 10.1111/j.1600-0463.2007.apm_630.x. [DOI] [PubMed] [Google Scholar]
- 18.Amoureux L., Bador J., Siebor E., Taillefumier N., Fanton A., Neuwirth C. Epidemiology and resistance of Achromobacter xylosoxidans from cystic fibrosis patients in Dijon, Burgundy: first French data. J. Cyst. Fibros. 2013 Mar;12(2):170–176. doi: 10.1016/j.jcf.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Ridderberg W., Bendstrup K.E.M., Olesen H.V., Jensen-Fangel S., Nørskov-Lauritsen N. Marked increase in incidence of Achromobacter xylosoxidans infections caused by sporadic acquisition from the environment. J. Cyst. Fibros. 2011 Dec;10(6):466–469. doi: 10.1016/j.jcf.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Pereira R.H., Carvalho-Assef A.P., Albano R.M., Folescu T.W., Jones M.C.M.F., Leão R.S. Achromobacter xylosoxidans: characterization of strains in Brazilian cystic fibrosis patients. J. Clin. Microbiol. 2011 Oct;49(10):3649–3651. doi: 10.1128/JCM.05283-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanellopoulou M., Pournaras S., Iglezos H., Skarmoutsou N., Papafrangas E., Maniatis A.N. Persistent colonization of nine cystic fibrosis patients with an Achromobacter (Alcaligenes) xylosoxidans clone. Eur. J. Clin. Microbiol. Infect. Dis. 2004 Apr;23(4):336–339. doi: 10.1007/s10096-004-1105-9. [DOI] [PubMed] [Google Scholar]
- 22.Winstanley C., O'Brien S., Brockhurst M.A. Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol. 2016 May;24(5):327–337. doi: 10.1016/j.tim.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cullen L., McClean S. Bacterial Adaptation during chronic respiratory infections. Pathogens. 2015 Mar 2;4(1):66–89. doi: 10.3390/pathogens4010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calder A.D., Bush A., Brody A.S., Owens C.M. Scoring of chest CT in children with cystic fibrosis: state of the art. Pediatr. Radiol. 2014 Dec;44(12):1496–1506. doi: 10.1007/s00247-013-2867-y. [DOI] [PubMed] [Google Scholar]
- 25.CFTR2 Website http://cftr2.org/mutation/general/F508del/Y1092X (Accessed 07 October 2016)
- 26.Castellani C., Cuppens H., Macek M., Cassiman J.J., Kerem E., Durie P. Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. J. Cyst. Fibros. 2008 May;7(3):179–196. doi: 10.1016/j.jcf.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlyle B.E., Borowitz D.S., Glick P.L. A review of pathophysiology and management of fetuses and neonates with meconium ileus for the pediatric surgeon. J. Pediatr. Surg. 2012 Apr;47(4):772–781. doi: 10.1016/j.jpedsurg.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 28.Li W., Soave D., Miller M.R., Keenan K., Lin F., Gong J. Unraveling the complex genetic model for cystic fibrosis: pleiotropic effects of modifier genes on early cystic fibrosis-related morbidities. Hum. Genet. 2014 Feb;133(2):151–161. doi: 10.1007/s00439-013-1363-7. [DOI] [PubMed] [Google Scholar]
- 29.Raghavan D., Jain R. Increasing awareness of sex differences in airway diseases. Respirology. 2016 Apr;21(3):449–459. doi: 10.1111/resp.12702. [DOI] [PubMed] [Google Scholar]
- 30.Harness-Brumley C.L., Elliot A.C., Rosenbluth D.B., Raghavan D., Jain R. Gender differences in outcomes in patients with cystic fibrosis. J. Womens Health. 2014 Dec;23(12):1013–1020. doi: 10.1089/jwh.2014.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chotirmall S.H., Smith S.G., Gunaratnam C., Cosgrove S., Dimitrov B.C., O'Neil S.J. Effect of estrogen on pseudomonas mucoidy and exacerbations in cystic fibrosis. N. Engl. J. Med. 2012 May 24;366(21):1978–1986. doi: 10.1056/NEJMoa1106126. [DOI] [PubMed] [Google Scholar]