Abstract
Purpose
It is not known if outflow facilities measured by pneumatonography and Schiøtz tonography are interchangeable. In this study we compared outflow facility measured by pneumatonography to outflow facility measured by digital Schiøtz tonography.
Methods
Fifty-six eyes from 28 healthy participants, ages 41 to 68 years, were included. Intraocular pressure (IOP) was measured in the sitting and supine positions with a pneumatonometer. With the subject in the supine position, IOP was recorded for 2 minutes by using a pneumatonometer with a 10-g weight and for 4 minutes by using a custom digital Schiøtz tonometer. Outflow facility was determined from the changes in pressure and intraocular volume and a standard assumed ocular rigidity coefficient for each instrument, respectively, and by using an ocular rigidity coefficient calculated by measuring pressure without and with a weight added to the pneumatonometer tip.
Results
The outflow facility was 0.29 ± 0.09 μL/min/mm Hg by Schiøtz tonography and 0.24 ± 0.08 μL/min/mm Hg by pneumatonography (P < 0.001) when using the standard assumed constant ocular rigidity coefficient. Mean calculated ocular rigidity coefficient was 0.028 ± 0.01 μL−1, and outflow facility determined by using this coefficient was 0.23 ± 0.08 μL/min/mm Hg by Schiøtz tonography and 0.21 ± 0.07 μL/min/mm Hg by pneumatonography (P = 0.003). Outflow facilities measured by the two devices were correlated when the ocular rigidity was assumed (r = 0.60, P < 0.001) or calculated (r = 0.70, P < 0.001).
Conclusions
Outflow facilities measured by pneumatonography were correlated with those measured by Schiøtz tonography, but Schiøtz tonography reported approximately 10% to 20% higher facilities when using the standard method. When ocular rigidity was determined for each eye, differences were smaller. Measurements from these devices cannot be compared directly.
Keywords: aqueous outflow facility, tonography, ocular rigidity, tonometry
Aqueous humor is formed by the ciliary processes, flows through the pupil and anterior chamber, and exits the eye at the anterior chamber angle. It returns to the venous system primarily through the trabecular or conventional pathway, but also exits by the uveoscleral or unconventional route.1,2 The ease of aqueous humor outflow through the conventional path is usually expressed as outflow facility in microliters per minute per millimeter of Hg pressure difference (μL/min/mm Hg).3 Impairment of aqueous humor outflow, which decreases outflow facility, elevates IOP and is a central tenet of pathophysiology and treatment of ocular hypertension and glaucoma.4,5 Although outflow facility is not routinely measured to diagnose glaucoma, it is used as a research tool to study physiological properties of the eye, and mechanisms of action of glaucoma medications and surgery.1,6,7
Outflow facility links the aqueous humor flow rate, Q, and intraocular pressure, IOP, as described by the modified Goldmann equation:
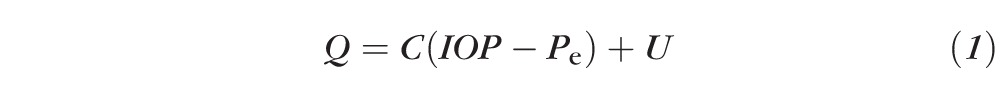 |
where C is the conventional outflow facility, Pe is the episcleral venous pressure, and U is the unconventional outflow.8 Outflow facility, aqueous humor flow rate, and intraocular and episcleral venous pressures can be measured directly, while unconventional outflow is calculated from Equation 1.
Outflow facility has been measured noninvasively by tonography, a method that determines the volume of fluid that leaves the eye in response to a known pressure elevation. Pressure is increased by applying to the cornea a weighted tonometer, such as the electronic Schiøtz tonometer originally described by Grant,9 or the pneumatonometer introduced by Langham et al.10 The force from the tonometer probe deforms the cornea and increases IOP. The increased IOP drives aqueous humor through the trabecular meshwork at a faster flow rate than at steady state as determined by outflow facility. The induced pressure change and the volume of aqueous humor loss during the 2 to 4 minutes that the tonometer is applied to the eye are used to estimate outflow facility. The volume of fluid loss is estimated from the pressures at the beginning and the end of tonography.
Electronic Schiøtz tonography was the standard method for measuring outflow facility for many years but this instrument is no longer commercially available. It indents the cornea with a weighted plunger and the depth of indentation can be directly related to intraocular pressure. Volumes that correspond to the degree of indentation have been estimated by Friedenwald11 from geometric considerations and a coefficient of ocular rigidity. The pneumatonometer is commercially available and can be adapted for tonography with the addition of a weight to the measurement tip. Similar to the Friedenwald tables for Schiøtz tonography, Langham et al.10 have developed tables that relate pressure to volume and used them to determine outflow facility with the pneumatonometer. Outflow facility measured by pneumatonography has been compared to that of Schiøtz in several studies.6,12,13 However, it is not known if outflow facilities measured by these two instruments are interchangeable. In this study we compared outflow facility measured by pneumatonography to outflow facility measured by digital Schiøtz tonography.
Methods
Study Subjects
Twenty-eight healthy participants were recruited from employees and patients of Mayo Clinic, or local area residents as part of a multicenter clinical trial (NCT01677507). Each participant underwent a general health interview and comprehensive ophthalmologic examination including visual acuity, IOP with pneumatonometry, gonioscopy, slit-lamp biomicroscopy, and fundoscopy. Subjects were excluded if they had a history or evidence of ocular pathology, intraocular surgery or trauma, laser treatment, narrow angles, or glaucoma. Each subject gave informed consent to participate after discussion of the nature and possible risks of the study. This study was approved by the Institutional Review Board at Mayo Clinic and followed the tenets of the Declaration of Helsinki.
Measurements
Intraocular Pressure.
Baseline IOP was measured in both eyes in the sitting position by using a pneumatonometer (Model 30 Classic; Medtronic Solan, Jacksonville, FL, USA). The subject was placed in a supine position and after 5 minutes, IOP was remeasured (P0). Calibration of the tonometer was verified according to the manufacturer's instruction and the tip was cleaned before each set of measurement. Topical proparacaine 0.5% was instilled before each IOP measurement and tonography. The right eye was always measured first.
Outflow Facility by Pneumatonography.
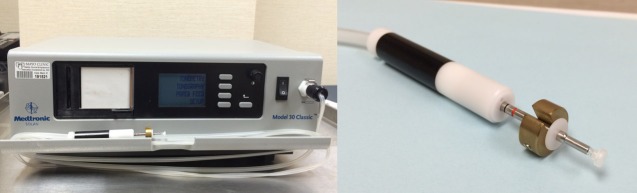
Outflow facility was measured by using a pneumatonometer with a tonography option. The subjects were placed in a supine position. Subjects were then asked to breathe normally and fixate on a target on the ceiling approximately 2 m from their eyes during the procedure. The eyelids were gently retracted by the examiner's fingers without applying additional pressure on the globe. The corneas were anesthetized by instilling proparacaine 0.5% topically. The pneumatonometer probe (tip diameter of 5.3 mm) with an added 10-g weight (Fig. 1) was placed on the center of the cornea and held perpendicularly to the corneal surface. Intraocular pressure was recorded for 2 minutes and the probe was removed from the eye. The right eye was always measured before the left eye.
Figure 1.
Pneumatonometer and its probe, which has a flat tip with an external diameter of 5.3 mm. The weight on the probe is used only for tonography.
Each 2-minute pressure tracing was printed by the pneumatonometer (Fig. 2), scanned, and digitized by using CurveSnap 1.1 (Fig. 3; http://xoofee.com/2012/12/curvesnap/). A second-order polynomial line was fitted to the pressure curve by least-squares regression and the IOP at the beginning (P1) and end of the interval (P2), while the eye was exposed to the weighted probe, was determined from the value of the line at 0 and 2 minutes. The outflow facility coefficient, C, was determined by using Grant's formula9:
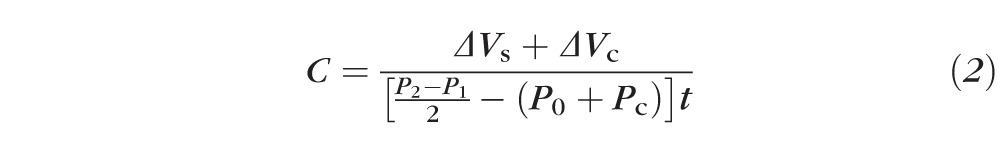 |
where ΔVs is the intraocular volume change from relaxation of tension in the sclera and corresponding to the pressure change during tonography, ΔVc is the change in intraocular volume by corneal indentation, P0 is pressure in the supine position, Pc is a correction of the steady-state pressure, and t is the duration of the contact, typically 2 or 4 minutes. Langham et al.10 have estimated that Pc is equal to −4 mm Hg because of an assumed indentation effect by the pneumatonometer, while Friedenwald11 has estimated a value of 1.25 mm Hg to account for episcleral venous pressure change during Schiøtz tonography, based on the measurement made by Linner.14
Figure 2.
Paper tracing by pneumatonography.
Figure 3.
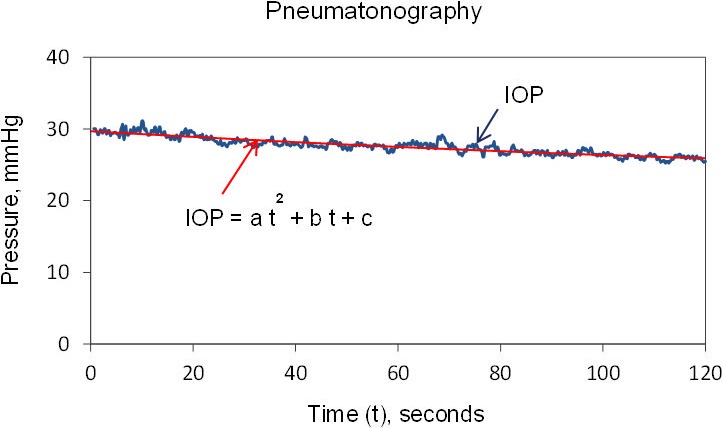
Digitized pressures by pneumatonography. A second-order polynomial was fitted to IOP, and outflow facility was calculated by using IOP determined from the fitted curve at 0 and 2 minutes. a, b, c: constants of second-order polynomial.
The relationship between pressure and indentation volume is based on the value of ocular rigidity for each instrument. A constant value for ocular rigidity is implicit in the tables by Langham et al.10 for pneumatonography (0.0126 μL−1) and Friedenwald11 for the Schiøtz tonometer (0.0215 μL−1). In addition, we calculated the ocular rigidity coefficient (K) from measurements of pressure without and with the weight added to the pneumatonometer probe and by using the equation of Friedenwald11:
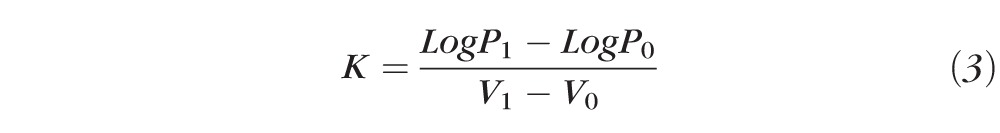 |
where V1 and V0 are the volumes indented in the eye by the tonometer when the pressure was P1 and P0, respectively. For pneumatonography, these volumes were extracted from data tabulated by Langham et al.10
Outflow Facility by Custom Digital Schiøtz Tonography.
Outflow facility was also measured 45 minutes after pneumatonography by using a digital Schiøtz tonographer designed by the Mayo Clinic Division of Engineering.15 This tonographer probe had a plunger with a concave tip and an external diameter of 10 mm (Fig. 4). The subjects were placed in the supine position for 5 minutes and fixated on a target on the ceiling, approximately 2 m in front of their eyes. The corneas were anesthetized by instilling proparacaine 0.5% topically, and the probe with a 5.5-g weight was placed on the center of cornea and held perpendicularly for 4 minutes. The right eye was always tested first followed by the left eye. A second-order polynomial was fitted to pressures recorded by the tonographer (Fig. 5) and outflow facility was determined from pressure on the fitted curve at 0 and 4 minutes by using Equation 2. The change in corneal indentation volume (ΔVc) was estimated from the 1955 tables by Friedenwald,16 and Pc was set to 1.25 mm Hg on the basis of the measurements made by Linner.14 The change in scleral distension volume (ΔVs) was determined by using the standard tables introduced by Friedenwald,11,16 which assumes a normal ocular rigidity coefficient (0.0215 μL−1), and by using the ocular rigidity coefficient estimated by our measurements with pneumatonometry.
Figure 4.
Custom digital Schiøtz tonographer and its probe, which has a plunger with a concave tip and an external diameter of 10 mm.
Figure 5.
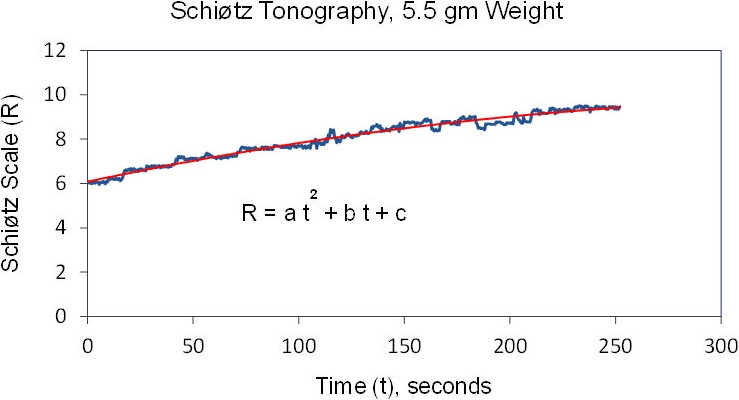
Outflow facility from Schiøtz tonography. A second-order polynomial was fitted to IOP, and outflow facility was calculated by using IOP determined from the fitted curve at 0 and 4 minutes. a, b, c: constants of second-order polynomial.
Statistical Analysis
The difference in outflow facility between instruments and significance of correlations between instruments were examined by using generalized estimating equation models to account for possible correlation between fellow eyes. Relationships between instruments were illustrated by Pearson correlation. Limits of agreement between measurements were defined as the mean difference ± 2 standard deviations (SDs) of the difference (Bland and Altman).17 All statistical tests were two sided and were calculated by using SPSS statistical software (version 21.0; SPSS, Inc., Chicago, IL, USA). Differences were considered significant if P was less than 0.05.
Results
Fifty-six eyes from 28 healthy participants (6 males and 22 females; 27 Caucasians and 1 African American), ages 41 to 68 years (51 ± 8 years, mean ± SD) were included in the study.
The mean outflow facility was 0.29 ± 0.09 μL/min/mm Hg (± SD) by Schiøtz tonography and 0.24 ± 0.08 μL/min/mm Hg by pneumatonography (P < 0.001) when using the assumed ocular rigidity coefficient for each device (Table). Outflow facilities by the two devices were correlated (r = 0.6, P < 0.001) (Fig. 6). The mean difference between instruments was 0.05 ± 0.08 μL/min/mm Hg and limits of agreement ranged from −0.1 to 0.21 μL/min/mm Hg (Fig. 7).
Table.
Outflow Facility Measurements by Pneumatonography and Schiøtz Tonography
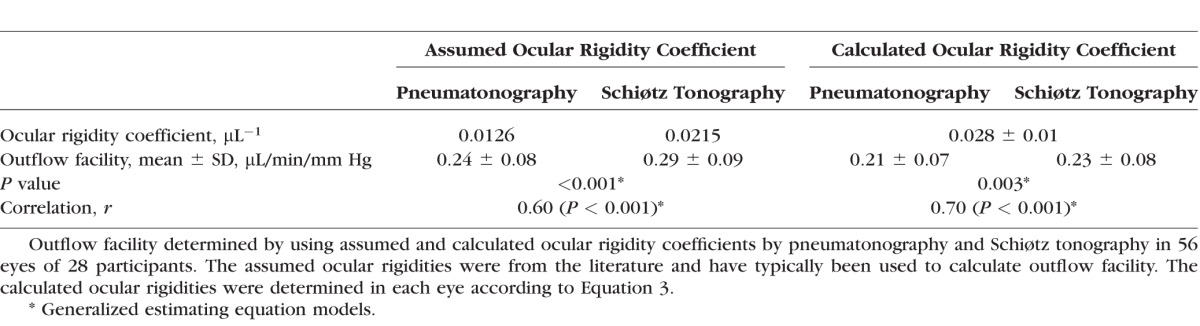
Figure 6.
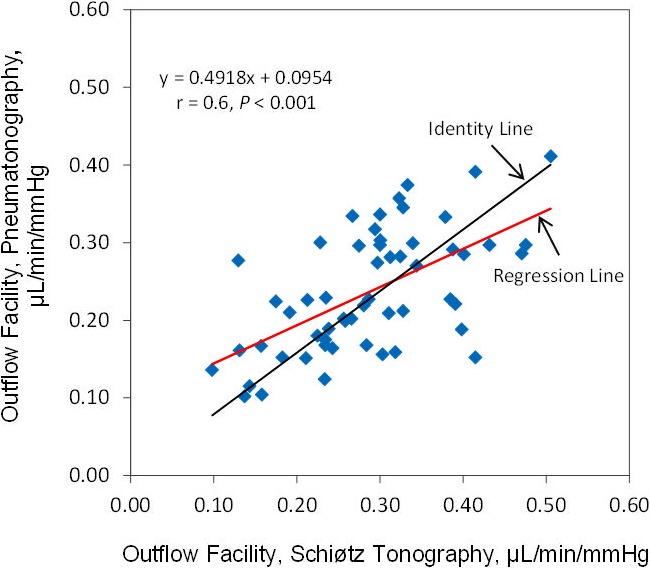
Correlation of outflow facility measured by pneumatonography and Schiøtz tonography with assumed ocular rigidity coefficient.
Figure 7.
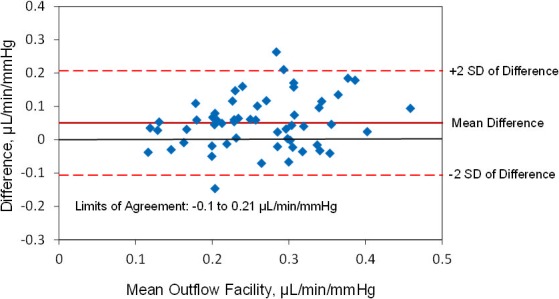
Limits of agreement, outflow facility measured by pneumatonography and Schiøtz tonography with assumed ocular rigidity coefficient.
The mean calculated ocular rigidity coefficient was 0.028 ± 0.01 μL−1 (± SD, n = 56 eyes). When the same calculated ocular rigidity coefficient was used to calculate outflow facility for both Schiøtz tonography and pneumatonography, the mean outflow facility was 0.23 ± 0.08 μL/min/mm Hg by Schiøtz tonography and 0.21 ± 0.07 μL/min/mm Hg by pneumatonography (P = 0.003). There was a strong correlation between outflow facility calculated by the two devices (r = 0.7, P < 0.001) (Fig. 8). The mean difference between instruments was 0.02 ± 0.06 μL/min/mm Hg and limits of agreement ranged from −0.09 to 0.14 μL/min/mm Hg (Fig. 9).
Figure 8.
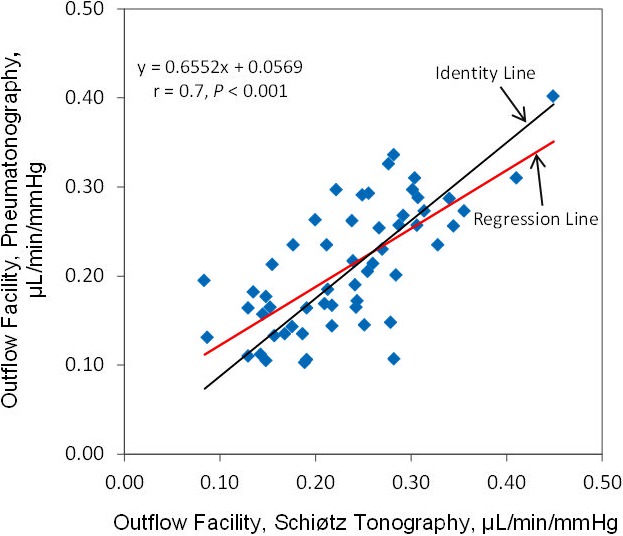
Correlation of outflow facility measured by pneumatonography and Schiøtz tonography with calculated ocular rigidity coefficient.
Figure 9.
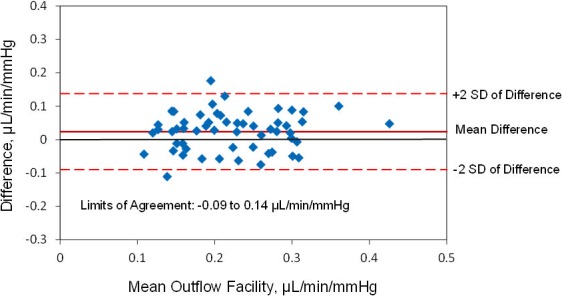
Limits of agreement, outflow facility measured by pneumatonography and Schiøtz tonography with calculated ocular rigidity coefficient.
Discussion
With tonography, we calculate the outflow facility of aqueous humor from the volume of aqueous humor forced out of the eye by a transiently elevated pressure. The pressure is increased by placing a weighted tonometer probe on the cornea, and the volume of fluid loss is estimated from the change in pressure from the beginning to the end of application of the weighted probe. The pressure change is recorded through the tonometer, but the volume change must be estimated from the indentation volume of the tonometer during contact with the eye and the change in ocular volume associated with the reduced pressure during tonography. However, differences in the way that indentation volume is calculated, along with the subsequent calculation of ocular rigidity, may result in slightly different values for outflow facility from the two devices.
The two tonographers used here both measure IOP but the approach to deriving the change in volume from pressure is somewhat different between instruments. The indentation volume from the Schiøtz tonometer has been tabulated and is based on geometric considerations of the probe plunger as it indents the cornea.11 The indentation volume is directly related to the height of the plunger below the probe contact position on the cornea, and this is directly related to the measured pressure. These relationships are unique for each weight added to the plunger and have been tabulated for direct conversion to outflow facility.11,16 The pneumatonometer does not have a plunger and the indentation volume is associated with the indentation of the entire probe, and because there is not a way to measure the indentation of the probe directly, this relationship has been determined empirically and published by Langham et al.10 However, the derivation of this relationship for the pneumatonometer has not been explicitly described.
The relationship between volume and pressure for both instruments is dependent on the ocular rigidity coefficient, a parameter determined by the resistance that the eye exerts against distending forces and described by Equation 3.11 This relationship is implicit in the calculation of outflow facility, and the most commonly used pressure–volume relationship for the calculation of ocular rigidity has been the equation of Friedenwald.11 Friedenwald found a mean value of 0.0215 μL−1 as derived from measurements on cadaveric human eyes and this has been used in the calibration data for conversion of Schiøtz tonometer scale readings to IOP measurements in millimeters of Hg, as well as tables for estimation of ocular volume change (microliters). Since the Schiøtz scale is based on the assumption that the eye has an average normal rigidity, large deviations in rigidity from the average normal eye will result in erroneous estimates of IOP or outflow facility.18 Clearly, accuracy of the outflow facility estimates from each device is dependent on the accuracy of the calibration data,19 but it is unclear if either the Schiøtz or pneumatonography calibration data are superior to the other in accuracy.
Another potential difference between the two devices is the reported difference in the pressure–volume relationship of living eyes compared with enucleated eyes.10 Several studies have concluded that the ocular rigidity of enucleated eyes is higher than that in living human eyes. The difference may be related to lack of active choroidal blood flow that leads to smaller volume of displaced fluid and postmortem changes in enucleated eyes.10,20–22 While the ocular rigidity coefficient in Friedenwald's tables for Schiøtz tonography was calculated based on enucleated eyes, the assumed ocular rigidity coefficient for pneumatonography in Langham's tables was developed based on living eyes.
Measurement of ocular rigidity in living human eyes has been reported previously, based on injection of small volumes of saline solution into the anterior chamber and measurement of the resulting pressure change with a transducer.23,24 This method has been reported by Prijot and Weekers,25 Prijot,26 Ytteborg,20,21,27,28 and Eisenlohr et al.22 More recently, Pallikaris et al.23 calculated the ocular rigidity coefficient as the slope of the line fitted by linear regression, to the pressure–volume relationship as measured during cataract surgery. The mean ocular rigidity coefficient was 0.0126 μL−1 with their method. Karyotakis et al.29 and Dastiridou et al.5,30 used the same manometric method and fitted an exponential curve to the pressure-volume relationship over a range of IOP from 15 to 40 mm Hg. The mean ocular rigidity coefficients were 0.028 μL−1 and 0.022 μL,−1 respectively. This variability in directly measured ocular rigidity coefficient in different study populations suggests that assumption of a constant value may not be ideal for calculation of outflow facility.
Because the Friedenwald and Langham tables assume a constant (normal) ocular rigidity coefficient, calculation of outflow facility can be different on eyes with high or low ocular rigidity. We accounted for these differences by calculating the ocular rigidity coefficient for each eye from IOP measured by using a pneumatonometer without and with a 10-g weight added to the tonometer probe, based on the Friedenwald's equation (Equation 3),11 and by using Langham's tables for determining ocular volume change.10 Notably, our mean ocular rigidity coefficient, 0.028 ± 0.01 μL−1, was higher than both assumed values. One possible reason is that we assumed that the ocular displacement volume was zero when the probe was not weighted on the initial supine measurement (P0). However, the inherent weight of the tonometer probe would have indented the cornea slightly, and this would have somewhat reduced the volume change used to determine the ocular rigidity coefficient. This coefficient therefore may have been slightly overestimated. Although our estimate was somewhat higher than that reported for living eyes by Friedenwald11 (0.021 μL−1) and Dastiridou et al.5,30 (0.022 μL−1), it was similar to that of Karyotakis et al. (0.028 μL−1),29 who used a manometric technique to determine the ocular rigidity coefficient.
The effect of the ocular rigidity coefficient on outflow facility was apparent from the results of our study. When we used the traditional assumed constant ocular rigidity coefficients, the outflow facilities calculated by pneumatonometry were significantly lower than those by Schiøtz tonography. However, when we calculated K, based on Friedenwald's equation and the estimated indentation volumes from Langham et al.,10 our estimate of outflow facility by pneumatonography was much closer to that by Schiøtz tonography.
Another potential error in the outflow facility calculation is in the measurement of the initial unweighted supine IOP (P0). The ocular volume parameters vary with pressure, and an error of a few millimeters of Hg in P0 can cause large errors in the outflow facility coefficient. This may be particularly important for the Schiøtz tonometer, since it cannot measure P0 directly (because a weight is a necessary component of the device) and consequently, values of P0 are determined by using an average value of the ocular rigidity coefficient. Several studies have reported that current Schiøtz readings based on Friedenwald's 1955 calibration scale16 are in error. Friedenwald used applanation tonometry on seated patients as a means of checking the indentation calibration scale, based on the assumption that the mean IOP of normal eyes increased by 1 mm Hg upon lying down, owing to an identical pressure increment in the episcleral vein.31 However, subsequent studies32–39 report that this postural effect exceeds 1 mm Hg and is between 1.8 and 5 mm Hg. These studies suggest that the Schiøtz values may be significantly low, with the Schiøtz calibration in error by as much as 3 to 5 mm Hg.
In contrast to Schiøtz tonometry, pneumatonometry can measure initial unweighted supine IOP (P0) directly without the need to use the ocular rigidity relationship. Langham et al.10 report that pneumatonometry measurements are 2.6 ± 0.26 mm Hg greater than Goldmann applanation tonometry measurements from normal and glaucomatous eyes in the sitting position. They state that this difference is caused by a slight corneal indentation by the pneumatonometer. Measurements in the supine position increases an additional 1 mm Hg owing to the weight of the piston when the tonometer is held vertically. Hence, for pneumatonography, Langham et al.10 suggest the addition of a correction factor of −4 mm Hg to the P0 in Equation 2.
The outflow facility estimated by pneumatonometry with default ocular rigidity in our study subjects (0.24 ± 0.08 μL/min/mm Hg) was close to that estimated by others. Langham et al.10 report an outflow facility of 0.28 ± 0.01 μL/min/mm Hg by 4-minute pneumatonography, which is not significantly different from 0.29 ± 0.01 μL/min/mm Hg by 2-minute pneumatonography. They conclude that the 2-minute tonography could be used as a standard for pneumatonography. Liu et al.40 report 0.23 ± 0.05 μL/min/mm Hg for normal subjects aged 37 to 74 years and in another study Toris et al.41 obtained 0.25 ± 0.11 μL/min/mm Hg for subjects aged 21 to 30 years and 0.20 ± 0.09 μL/min/mm Hg for subjects aged 60 to 79 years. The difference between age groups is not significant.
Our outflow facility measured by digital Schiøtz tonography (0.29 ± 0.09 μL/min/mm Hg) with assumed constant ocular rigidity was also similar to measurements of others by the same method. Grant9,19 reports values of 0.23 μL/min/mm Hg and 0.24 μL/min/mm Hg for outflow facility by tonography in normal human living eyes, and Becker42 reports a mean value of 0.28 ± 0.05 μL/min/mm Hg in his study on 909 normal eyes. Selvadurai et al.,15 Nau et al.,43 and Sit et al.44 recently performed several studies on normal subjects. They report an outflow facility of 0.28 ± 0.09 μL/min/mm Hg in one study, and in two separate studies report 0.28 ± 0.09 μL/min/mm Hg in younger group (age 30–45 years) and 0.23 ± 0.06 μL/min/mm Hg in older subjects (age 47–76 years).
Other investigators compared Schiøtz tonography to pneumatonography in the same subjects and found that the two methods produce similar mean outflow facilities, although outflow facility measured by pneumatonography shows more variability. Feghali et al.12 found no significant difference in outflow facility between methods on 13 normal subjects and 3 glaucoma patients (0.24 ± 0.11 μL/min/mm Hg with pneumatonography and 0.22 ± 0.07 μL/min/mm Hg with Schiøtz tonography). However, both intersubject and interobserver variabilities were significantly higher with pneumatonography. Wheeler et al.6 estimate a mean outflow facility of 0.22 ± 0.09 μL/min/mm Hg and 0.23 ± 0.07 μL/min/mm Hg with pneumatonography and Schiøtz tonography, respectively, in eight normal subjects. Although these mean outflow facilities are not significantly different, pneumatonography had high standard deviation and poor reproducibility. The study is limited by a small sample size. Lim et al.13 studied 30 subjects and found a mean of tonographic outflow facility of 0.23 ± 0.09 μL/min/mm Hg and 0.22 ± 0.08 μL/min/mm Hg with Schiøtz and 2-minute pneumatonography, respectively, for right eye and 0.26 ± 0.07 μL/min/mm Hg and 0.20 ± 0.08 μL/min/mm Hg with Schiøtz and 2-minute pneumatonography, respectively, for left eye.
The higher variability of outflow facilities by pneumatonography may be associated with a greater mobility of the smaller probe tip, which is flat and can slide across the cornea. This tip design could allow more transmission of eye and hand movements to the transducer and cause variable indentation and transient changes in IOP. In contrast, the tip of the Schiotz tonometer is concave, has larger diameter, and rests more steadily on the cornea. The greater mechanical stability of this design may result in less variability of IOP than with pneumatonography.
It is not completely clear why our study found a difference between Schiøtz tonography and pneumatonography, while previous investigators did not. Smaller sample size in previous studies may be one of the reasons. One significant difference in the analysis methods was the use of data extraction by fitting of a second-order polynomial to the data in our study. It is possible that this technique accentuates differences by eliminating the temptation to manually smooth a paper tonography tracing. Further research is required to determine if the differences in outflow facility between the two devices persist in other populations, such as glaucoma patients.
Summary
Outflow facilities measured by using the pneumatonography are correlated with those measured by Schiøtz tonography, but Schiøtz tonography reports approximately 10% to 20% higher facilities. Consequently, the measurements from these devices cannot be directly compared to determine differences in outflow facility, and the devices cannot be used interchangeably. Differences in shape, size, and weight of the two tonographer tips, method of IOP measurement, and using different calibration tables may be responsible for the differences in outflow facility measurement. When the ocular rigidity coefficient for each eye was calculated and the same value used for both instruments, outflow facilities were much closer between methods. Each method has several advantages and disadvantages, and which method is more accurate cannot be determined from our noninvasive study. Each of the methods appears to provide reasonable estimates of outflow facility, and while the indicated values are not identical, they are tightly correlated. Therefore, the instruments cannot be used interchangeably, but either is a reasonable choice for assessing differences in outflow facilities in human subjects.
Acknowledgments
This study was presented in part at the annual meeting of the Association for Research in Vision and Ophthalmology (ARVO), Denver, Colorado, United States, May 2015.
Supported by National Institutes of Health (NIH) EY022124 (SEM), NIH EY007003 (University of Michigan National Eye Institute Core Grant), and unrestricted departmental grants from Research to Prevent Blindness, New York, New York, United States (Mayo Clinic and University of Michigan). AJS is a recipient of a Schaub Special Scholar Award from Research to Prevent Blindness. VG has received funding from NEI-K23EY023266.
Disclosure: A. Kazemi, None; J.W. McLaren, None; S.-C. Lin, None; C.B. Toris, None; V. Gulati, None; S.E. Moroi, None; A.J. Sit, None
References
- 1. Johnstone MA. Aqueous humor outflow system overview. : Stamper RL,, Lieberman MF,, Drake MV, Becker-Shaffer's Diagnosis and Therapy of the Glaucomas. Edinburgh, UK: Mosby Elsevier; 2009: 25–46. [Google Scholar]
- 2. Johnson M,, McLaren JW,, Overby DR. Unconventional aqueous humor outflow: a review [published online ahead of print February 2, 2016] Exp Eye Res. doi:10.1016/j.exer.2016.01.017. [DOI] [PMC free article] [PubMed]
- 3. Becker B,, Friedenwald JS. Clinical aqueous outflow. AMA Arch Ophthalmol. 1953; 50: 557–571. [DOI] [PubMed] [Google Scholar]
- 4. Grant WM. Clinical measurements of aqueous outflow. AMA Arch Ophthalmol. 1951; 46: 113–131. [DOI] [PubMed] [Google Scholar]
- 5. Dastiridou AI,, Tsironi EE,, Tsilimbaris MK,, et al. Ocular rigidity, outflow facility, ocular pulse amplitude, and pulsatile ocular blood flow in open-angle glaucoma: a manometric study. Invest Ophthalmol Vis Sci. 2013; 54: 4571–4577. [DOI] [PubMed] [Google Scholar]
- 6. Wheeler NC,, Lee DA,, Cheng Q,, Ross WF,, Hadjiaghai L. Reproducibility of intraocular pressure and outflow facility measured by pneumatic tonography and Schiotz tonography. J Ocul Pharmacol Ther. 1998; 14: 5–13. [DOI] [PubMed] [Google Scholar]
- 7. Cameron D,, Finlay ET,, Jackson CR. Tonometry and tonography in the diagnosis of chronic simple glaucoma. Br J Ophthalmol. 1971; 55: 738–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goldmann H. The aqueous veins and the Poiseuille law [in German]. Ophthalmologica. 1949; 118: 496–519. [DOI] [PubMed] [Google Scholar]
- 9. Grant WM. Tonographic method for measuring the facility and rate of aqueous flow in human eyes. Arch Ophthalmol. 1950; 44: 204–214. [DOI] [PubMed] [Google Scholar]
- 10. Langham ME,, Leydhecker W,, Krieglstein G,, Waller W. Pneumatonographic studies on normal and glaucomatus eyes. Adv Ophthalmol. 1976; 32: 108–133. [PubMed] [Google Scholar]
- 11. Friedenwald JS. Contribution to the theory and practice of tonometry. Am J Ophthalmol. 1937; 20: 985–1024. [Google Scholar]
- 12. Feghali JG,, Azar DT,, Kaufman PL. Comparative aqueous outflow facility measurements by pneumatonography and Schiotz tonography. Invest Ophthalmol Vis Sci. 1986; 27: 1776–1780. [PubMed] [Google Scholar]
- 13. Lim KS,, Nau CB,, O'Byrne MM,, et al. Mechanism of action of bimatoprost, latanoprost, and travoprost in healthy subjects: a crossover study. Ophthalmology. 2008; 115: 790–795.e794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Linner E. Further studies of the episcleral venous pressure in glaucoma. Am J Ophthalmol. 1956; 41: 646–651. [PubMed] [Google Scholar]
- 15. Selvadurai D,, Hodge D,, Sit AJ. Aqueous humor outflow facility by tonography does not change with body position. Invest Ophthalmol Vis Sci. 2010; 51: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 16. Friedenwald JS. Tonometer calibration: an attempt to remove discrepancies found in the 1954 calibration scale for Schiotz tonometers. Trans Am Acad Ophthalmol Otolaryngol. 1957; 61: 108–122. [PubMed] [Google Scholar]
- 17. Bland JM,, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986; 1: 307–310. [PubMed] [Google Scholar]
- 18. Friedenwald JS. Clinical significance of ocular rigidity in relation to the tonometric measurement. Trans Am Acad Ophthalmol Otolaryngol. 1949; 53: 262–264. [PubMed] [Google Scholar]
- 19. Grant WM. Clinical tonography. Trans Am Acad Ophthalmol Otolaryngol. 1951; 55: 774–781. [PubMed] [Google Scholar]
- 20. Ytteborg J. The role of intraocular blood volume in rigidity measurements on human eyes. Acta Ophthalmol (Copenh). 1960; 38: 410–436. [DOI] [PubMed] [Google Scholar]
- 21. Ytteborg J. Influence of bulbar compression on rigidity coefficient of human eyes, in vivo and encleated. Acta Ophthalmol (Copenh). 1960; 38: 562–577. [DOI] [PubMed] [Google Scholar]
- 22. Eisenlohr JE,, Langham ME,, Maumenee AE. Manometric studies of the pressure-volume relationship in living and enucleated eyes of individual human subjects. Br J Ophthalmol. 1962; 46: 536–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pallikaris IG,, Kymionis GD,, Ginis HS,, Kounis GA,, Tsilimbaris MK. Ocular rigidity in living human eyes. Invest Ophthalmol Vis Sci. 2005; 46: 409–414. [DOI] [PubMed] [Google Scholar]
- 24. Detorakis ET,, Pallikaris IG. Ocular rigidity: biomechanical role, in vivo measurements and clinical significance. Clin Exp Ophthalmol. 2013; 41: 73–81. [DOI] [PubMed] [Google Scholar]
- 25. Prijot E,, Weekers R. Contribution to the study of the rigidity of the normal human eye [in French]. Ophthalmologica. 1959; 138: 1–9. [DOI] [PubMed] [Google Scholar]
- 26. Prijot E. Contribution to the study of tonometry and tonography in ophthalmology [in French]. Doc Ophthalmol. 1961; 15: 1–225. [DOI] [PubMed] [Google Scholar]
- 27. Ytteborg J. The effect of intraocular pressure on rigidity coefficient in the human eye. Acta Ophthalmol (Copenh). 1960; 38: 548–561. [DOI] [PubMed] [Google Scholar]
- 28. Ytteborg J. Further investigations of factors influencing size of rigidity coefficient. Acta Ophthalmol (Copenh). 1960; 38: 643–657. [DOI] [PubMed] [Google Scholar]
- 29. Karyotakis NG,, Ginis HS,, Dastiridou AI,, Tsilimbaris MK,, Pallikaris IG. Manometric measurement of the outflow facility in the living human eye and its dependence on intraocular pressure. Acta Ophthalmol (Copenh). 2015; 93: e343–e348. [DOI] [PubMed] [Google Scholar]
- 30. Dastiridou AI,, Ginis HS,, De Brouwere D,, Tsilimbaris MK,, Pallikaris IG. Ocular rigidity, ocular pulse amplitude, and pulsatile ocular blood flow: the effect of intraocular pressure. Invest Ophthalmol Vis Sci. 2009; 50: 5718–5722. [DOI] [PubMed] [Google Scholar]
- 31. Linner E,, Rickenbach C,, Werner H. Comparative measurements of the pressure in the aqueous veins and the conjunctival veins using different methods. Acta Ophthalmol (Copenh). 1950; 28: 469–478. [DOI] [PubMed] [Google Scholar]
- 32. Armaly MF,, Salamoun SG. Schiotz and applanation tonometry. Arch Ophthalmol. 1963; 70: 603–609. [DOI] [PubMed] [Google Scholar]
- 33. Galin MA,, Mc IJ,, Magruder GB. Influence of position on intraocular pressure. Am J Ophthalmol. 1963; 55: 720–723. [PubMed] [Google Scholar]
- 34. Schwartz JT,, Dell'Osso GG. Comparison of Goldmann and Schiotz tonometry in a community. Arch Ophthalmol. 1966; 75: 788–795. [DOI] [PubMed] [Google Scholar]
- 35. Langham ME,, McCarthy E. A rapid pneumatic applanation tonometer: comparative findings and evaluation. Arch Ophthalmol. 1968; 79: 389–399. [DOI] [PubMed] [Google Scholar]
- 36. Roberts W,, Rogers JW. Postural effects on pressure and ocular rigidity measurements. Am J Ophthalmol. 1964; 57: 111–118. [DOI] [PubMed] [Google Scholar]
- 37. Hetland-Eriksen J. On tonometry—5: the pressure of glaucomatous eyes measured in the sitting and the lying positions by means of the Goldmann applanation tonometer. Acta Ophthalmol (Copenh). 1966; 44: 515–521. [DOI] [PubMed] [Google Scholar]
- 38. Hetland-Eriksen J. On tonometry—6: comparative tonometry with the Goldmann applanation tonometer and the Schiotz tonometer in the lying position. Acta Ophthalmol (Copenh). 1966; 44: 522–538. [DOI] [PubMed] [Google Scholar]
- 39. Anderson DR,, Grant WM. Re-evaluation of the Schiotz tonometer calibration. Invest Ophthalmol. 1970; 9: 430–446. [PubMed] [Google Scholar]
- 40. Liu H,, Fan S,, Gulati V,, et al. Aqueous humor dynamics during the day and night in healthy mature volunteers. Arch Ophthalmol. 2011; 129: 269–275. [DOI] [PubMed] [Google Scholar]
- 41. Toris CB,, Yablonski ME,, Wang YL,, Camras CB. Aqueous humor dynamics in the aging human eye. Am J Ophthalmol. 1999; 127: 407–412. [DOI] [PubMed] [Google Scholar]
- 42. Becker B. Tonography in the diagnosis of simple (open angle) glaucoma. Trans Am Acad Ophthalmol Otolaryngol. 1961; 65: 156–162. [PubMed] [Google Scholar]
- 43. Nau CB,, Malihi M,, McLaren JW,, Hodge DO,, Sit AJ. Circadian variation of aqueous humor dynamics in older healthy adults. Invest Ophthalmol Vis Sci. 2013; 54: 7623–7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sit AJ,, Nau CB,, McLaren JW,, Johnson DH,, Hodge D. Circadian variation of aqueous dynamics in young healthy adults. Invest Ophthalmol Vis Sci. 2008; 49: 1473–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]