Abstract
Background
The prognosis of primary CNS lymphoma (PCNSL) recurring after methotrexate is poor (objective response rates [ORR] = 26%–53%; 1-year overall survival [OS] = 35%–57%). Salvage PCNSL chemotherapies have been based on the use of different agents to avoid cross-resistance; however, methotrexate is the most active agent in PCNSL, and methotrexate re-challenge may be an effective strategy for recurrent disease. We report our experience with methotrexate re-challenge in PCNSL.
Methods
We reviewed 39 patients with histologically confirmed PCNSL who responded to methotrexate at initial diagnosis, experienced disease relapse and received methotrexate re-challenge.
Results
At the time of re-challenge, median age was 66 and median Karnofsky Performance Score (KPS) was 70. Median time from initial diagnosis was 26m. Twenty-six patients were at first relapse and 13 at second or later relapse. At re-challenge, methotrexate was given in combination with other agents to 33 patients and as a single agent to six. The objective response rate was 85%, with a complete response in 29 (75%) patients, partial response in four (10%) and disease progression in six (15%). At median follow-up of 26m, the median progression-free survival was 16m; 1-year OS was 79% (95%CI 63–89) and median OS was 41m. KPS was a prognostic factor for progression free survival (p=0.04).
Conclusion
In this population selected by previous methotrexate response, methotrexate re-challenge was a safe and effective strategy, indicating chemosensitivity was retained. Efficacy compared favorably to other salvage treatments suggesting methotrexate re-challenge should be considered in recurrent PCNSL patients who previously responded to methotrexate.
Keywords: Primary CNS lymphoma, recurrence, methotrexate
Introduction
Primary central nervous system lymphoma (PCNSL) is a relatively rare non-Hodgkin lymphoma arising within the brain, cerebral spinal fluid (CSF), spinal cord or eyes. In more than 90% of patients, the histology corresponds to a diffuse large B-cell lymphoma (DLBCL). Although traditional DLBCL chemotherapy regimens such as cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) and variations are ineffective in PCNSL, the use of high-dose methotrexate (MTX) based regimens, with or without radiotherapy, resulted in significantly improved efficacy, achieving response rates (RR) as high as 70%–90%, and median overall survival (OS) of 40–70 months. Unfortunately, relapses remain frequent, mostly occurring within the first two years after initial response, with late relapses also occasionally reported [1–4].
Salvage treatments for PCNSL have been poorly characterized in the literature, and available studies have reported variable outcomes with objective response rates (ORR) of 26%–91% and 1y OS of 35%–71%. Traditionally, salvage chemotherapies have been based on the use of agents different from MTX in order to avoid cross-resistance [5–12]. However, because MTX is by far the most active agent in PCNSL, MTX re-challenge has been proposed as a possible salvage strategy for recurrent disease,[13] although to date such practice has not been widely adopted, as exemplified by salvage treatment patterns observed in a large recent trial in newly diagnosed PCNSL [14]. In this study, we report our experience with MTX re-challenge in recurrent PCNSL.
Patients and Methods
This retrospective study was approved by the Memorial Sloan-Kettering Cancer Center (MSKCC) Institutional Review Board. The MSKCC Department of Neurology database was used to identify patients with PCNSL who responded to MTX and then received MTX-based chemotherapy as a salvage treatment for recurrent disease between March 1998 and October 2010.
Additional inclusion criteria consisted of age ≥ 18, histological confirmation of PCNSL, radiographic evidence of brain involvement at recurrence, and absence of systemic involvement by lymphoma. All patients had pathology confirmed at MSKCC. Patients were included irrespective of the number of prior recurrences or therapies. Kaplan-Meier survival distributions were estimated to assess OS and progression-free survival (PFS). The OS was calculated from the date of initiation of MTX-re-challenge until death or last follow-up. PFS was calculated from date of initiation of MTX re-challenge to date of tumor progression or death. Potential prognostic factors were evaluated by a Cox proportional hazard model. Toxicity was assessed utilizing the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Responses were determined utilizing the International Primary CNS Lymphoma Group (IPCG) response criteria.[15]
Results
Patient characteristics
Thirty-nine patients with PCNSL were identified; 20 (51%) were women (Table 1). All patients had a histological diagnosis of PCNSL confirmed by pathology review at MSKCC. A total of 38 patients (95%) had a DLBCL, and in one patient (2.5%) the lymphoma type could not be determined.
Table 1.
Patient Characteristics at the Time of Methotrexate Re-challenge
| N= 39 | N | % or range |
|---|---|---|
| Sex | ||
| Women | 20 | 51% |
| Men | 19 | 49% |
| Median age | 66 years | 41–82 |
| Median KPS | 70 | 50–100 |
| Median time between initial diagnosis and MTX re-challenge |
26 months | 8.7–178 |
| Positive CSF | 4 | 10% |
| Ocular involvement | 7 | 18% |
| Number of relapses prior to MTX re- challenge |
||
| One | 26 | 66% |
| Two or more | 13 | 34% |
| Prior WBRT | 16 | 41% |
| Initial methotrexate treatment | ||
| MPV | 37 | 95% |
| with high-dose cytarabine | 22/37 | |
| with rituximab | 9/37 | |
| Single-agent MTX (5g/m2) | 1 | 2.5% |
| MTX, rituximab, temozolomide | 1 | 2.5% |
| Other prior treatments in patients with >2 previous relapses |
13 | |
| Rituximab-temozolomide | 9 | 68% |
| Rituximab-IT MTX | 1 | 8% |
| Temozolomide | 1 | 8% |
| Rituximab-temozolomide-thiotepa | 1 | 8% |
| Ocular radiotherapy | 1 | 8% |
KPS: Karnofsky Performance score; MTX: Methotrexate; CSF: Cerebro-spinal fluid; WBRT: Whole-brain radiation therapy; MPV: Methotrexate, procarbazine, vincristine; IT: intrathecal
At the start of MTX re-challenge, the median age was 66 years (range 41–82 years) and the median Karnofsky Performance score (KPS) was 70 (range 50–100). The median time from initial diagnosis to disease progression leading to MTX re-challenge was 26 months. Twenty-six patients (66%) received MTX again at first relapse and 13 (34%) at second or later relapse after failing other chemotherapy regimens. A total of 16 patients (41%) had received whole brain radiotherapy (WBRT) prior to MTX re-challenge. Thirty-seven patients had received prior MTX in combination with procarbazine and vincristine. In nine of those patients, rituximab was added, and 22 also received high-dose cytarabine at consolidation. Single agent MTX was used in one patient and the remaining patient received MTX, rituximab and temozolomide as part of a CALGB protocol. One patient underwent high-dose chemotherapy with autologous stem cell transplant protocol (HDC-ASCT) as consolidation treatment.
Prior to initiation of MTX re-challenge, all patients underwent re-staging with MRI, ophthalmological evaluation, CSF cytology and body PET/CT. All patients had parenchymal brain lesions; seven patients (17%) also had ocular involvement, and four had a positive CSF cytology. Three patients received radiation to the orbits at the time of recurrence for progressive visual loss prior to MTX re-challenge therapy. No other patients received radiation therapy as part of MTX re-challenge.
Methotrexate Re-challenge Treatment
At re-challenge, MTX was given in combination with other agents to 33 patients (85%) (Table 2). A combination of rituximab, MTX, vincristine and procarbazine (R-MVP) was used in 17 patients, MVP was used in nine, single agent MTX in six, MTX, carmustine (BCNU) and etoposide in four, MTX and temozolomide in two, and MTX and etoposide in one. In 34 patients, the MTX dose was 3.5g/m2 infused over 2 hours; in the remainder, doses varying from 2.5–8g/m2 were used. In twelve patients, a regimen identical to the initial therapy (rituximab, MTX, procarbazine, and vincristine) was used.
Table 2.
Methotrexate Re-challenge Treatment
| MTX dose | N | % |
|---|---|---|
| 3.5 g/m2 | 34 | 87 |
| Other doses (2.5–8.0 g/m2) | 5 | 13 |
| Regimen utilized | ||
| MPV + rituximab | 17 | 44 |
| MPV | 9 | 23 |
| Single agent methotrexate | 6 | 15 |
| MTX, BCNU, etoposide | 4 | 10 |
| MTX, temozolomide | 2 | 5 |
| MTX, etoposide | 1 | 3 |
| Consolidation treatment after MTX re- challenge |
||
| Reduced WBRT | 3 | 8 |
| Ocular RT | 1 | 3 |
| HDC-ASCT | 4 | 10 |
| Rituximab maintenance | 2 | 6 |
MTX: Methotrexate; MPV: Methotrexate, procarbazine, vincristine; BCNU: carmustine; WBRT: Whole-brain radiation therapy; RT: Radiation therapy; HDC-ASCT: High-dose chemotherapy with autologous stem-cell transplant.
Toxicity
The MTX re-challenge treatment was generally well tolerated (Table 3). Grade 3/4 toxicities at re-challenge included pulmonary toxicity in two patients and reversible nephrotoxicity in four; hematotoxicity varied according to the combination used. Only one patient discontinued treatment due to toxicity (renal failure). No MTX-related acute neurotoxicity was documented, although formal neuropsychological evaluation was not consistently available to determine rates of chronic neurotoxicity.
Table 3.
Toxicities Observed During Methotrexate Re-Challenge
| Grade 3 or 4 toxicity (CTCAE v4) | N |
|---|---|
| Infection | 5 |
| Neuropathy | 10 |
| Thrombocytopenia | 5 |
| Neutropenia | 7 |
| ALT | 6 |
| AST | 4 |
| Renal toxicity | 4 |
| Pulmonary toxicity | 2 |
| Leukoencephalopathy | 2 |
| Fatigue | 6 |
| Hyponatremia | 1 |
| Deep venous thrombosis | 1 |
Response, Progression-Free Survival and Overall Survival
A complete response (CR) was achieved in 29 (75%) patients, a partial response (PR) in four (10%) and progression of disease in six (15%); the ORR was 85%. Among patients who achieved a CR, four underwent high dose chemotherapy with thiotepa, busulfan, and cyclophosphamide followed by autologous stem-cell transplantation (HDC-ASCT), three received reduced dose WBRT, one received ocular RT and two continued on rituximab maintenance. Among the four patients who achieved PR, two remain alive in a sustained PR after 23 and 61 months, and two others progressed.
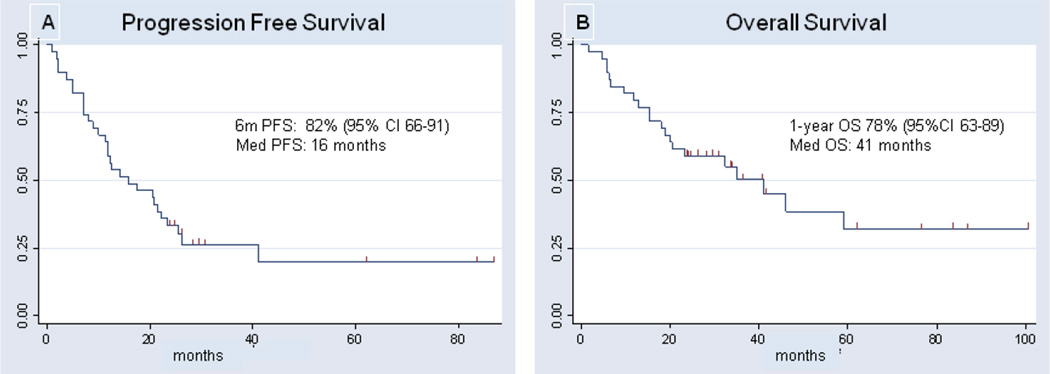
The median PFS was 16 months (Fig. 1A), the 6m-PFS was 82% (95% CI 66–91) and 1y PFS was 57% (95% CI 40–71). The median OS was 41 months and the 1year OS was 78% (95% CI 63–89) (Fig. 1B). The median follow up of survivors was 26 months.
Figure 1.
Kaplan-Meyer estimates of progression free survival (A) and overall survival (B) for patients treated with MTX re-challenge for recurrent PCNSL.
On univariate analysis, KPS at the time of MTX re-challenge was a prognostic factor for PFS (p=0.04), with a trend in predicting OS (p=0.074). There were no statistically significant differences in PFS or OS according to MSKCC RPA class, gender, age, time from initial diagnosis (continuous variable and above versus below median), previous exposure to WBRT, use of an MTX combination similar to a previously used regimen (versus a different regimen), or number of previous relapses (one versus more than one).
Discussion
We report a cohort of PCNSL patients who recurred despite an initial response to MTX-based chemotherapy, and who were re-challenged with a salvage MTX-based regimen. In comparison to other available treatments for recurrent PCNSL, these patients achieved favorable outcomes, with an ORR of 85% and median OS of 41 months, suggesting that chemosensitivity to MTX was retained in spite of previous exposure to the drug. Importantly, an acceptable toxicity profile, with no significant cumulative side effects, was observed.
WBRT is often considered for salvage treatment of PCNSL in patients who have not been previously irradiated [16, 17]. However, WBRT may be associated with a high risk of neurotoxicity, especially in the setting of additional tumor-burden associated with recurrent disease, which by itself can also affect neurocognitive function. Therefore, developing salvage chemotherapy options remains of high interest. Only a few studies have reported outcomes with salvage chemotherapy for recurrent PCNSL (Table 4), mostly focusing on determining activity of single agents such as topotecan, temozolomide and rituximab [8, 9, 18, 19]. Interpreting these reports is difficult because of small sample sizes, and the studied populations have included variable proportions of patients with progressive disease primarily refractory to MTX, and patients with disease relapse developing after initial response to MTX. As a whole, such studies have found only modest efficacy, highlighting the need to develop new options for both progressive and recurrent disease.
Table 4.
Studies on Treatment for Relapsed or Progressive Primary CNS Lymphoma
| N | ORR | 1y- OS | |
|---|---|---|---|
| Prospective studies | |||
| Topotecan [9] | 27 | 33% | 35% |
| Temozolomide [18] | 23 | 26% | 38% |
| Rituximab [8] | 12 | 36% | Na |
| Temozolomide, rituximab [4] | 16 | 14% | 71% |
| Ara-C, VP-16 + HDCASCT (patients under age 65 only) [20] |
43 | 47% | 60% |
| Pemetrexed [12] | 11 | 55% | 45% |
| Retrospective studies | |||
| Ara-C, VP-16, ifosfamide [6] | 16 | 37% | 41% |
| Procarbazine, CCNU, vincristine [11] | 7 | 86% | 57% |
| Temozolomide, rituximab [10] | 15 | 53% | 55% |
| Temozolomide [19] | 17 | 59% | 35% |
| WBRT [16] | 27 | 74% | 50% |
| WBRT [17] | 48 | 77% | 54% |
| MTX re-challenge [13] | 22 | 91% | Na |
| MTX re-challenge (this study) | 39 | 85% | 79% |
Ara-C: high-dose cytarabine; VP-16: Etoposide; MTX: Methotrexate; HDC-ASCT: High-dose chemotherapy with autologous stem-cell transplant; CCNU: Lomustine; WBRT: Whole-brain radiation therapy; Na: not available; ORR: Objective response rates; 1y-OS: One-year overall survival.
A single retrospective study has examined the role of MTX re-challenge in recurrent PCNSL [13]. That study evaluated 22 patients who had failed single agent MTX, including two patients with isolated ocular involvement. Patients were re-treated with single agent MTX, with doses varying from 3–8 g/m2. A favorable toxicity profile was observed, and the ORR was 91%. However, the OS of those patients from the time of MTX re-challenge was not provided, and therefore it is difficult to establish the efficacy of the regimen in comparison to other salvage strategies. Our results confirm the observed activity in a larger and more heavily pre-treated population, including patients with multiply relapsed disease and patients who had failed prior WBRT.
In spite of high complete response rates to MTX re-challenge, patients with recurrent PCNSL remain at high risk of relapse. In some of our patients, the MTX re-challenge was used as a salvage induction chemotherapy in preparation for additional consolidation treatments such as HDC-ASCT and reduced dose WBRT, which likely contributed to prolonged disease control and survival. Interesting results have been observed in recurrent PCNSL patients treated with salvage cytarabine and etoposide (CYVE), followed by a myeloablative regimen consisting of cyclophosphamide, thiotepa and busulfan, and stem-cell rescue [20] However, the CYVE regimen was highly toxic, with frequent and fatal hematologic toxicities in 7% of patients. Our results suggest that MTX re-challenge may be a suitable alternative to CYVE in patients with relapsed disease as a pre-transplant induction chemotherapy. Likewise, the cytoreduction afforded by the MTX re-challenge allowed for the use of consolidation reduced-dose WBRT (23.4 Gy) in some patients, a treatment strategy currently under investigation for newly diagnosed disease [3, 21, 22]. This may constitute an interesting consolidation alternative in patients with recurrent PCNSL who are not transplant candidates.
The current study is inherently limited by its retrospective nature, varying MTX regimens employed, lack of prospective neuropsychological evaluations, and the fact that results only apply to those patients who have responded previously to MTX, who nonetheless account for the vast majority of PCNSL patients. Although confirmation in the prospective setting is warranted, this remains the largest study of PCNSL patients treated with MTX-based therapy at recurrence, and our findings suggest that induction MTX re-challenge should be considered in all patients with recurrent PCNSL who previously responded to MTX.
Supplementary Material
Acknowledgments
Funding/Support: none
We thank Judith Lampron for her editorial assistance.
Footnotes
Disclosures: Dr. Pentsova has no conflict of interest.
Dr. DeAngelis has no conflict of interest.
Dr. Omuro has no conflict of interest.
Presented in part at the American Society of Clinical Oncology, 2011 (poster discussion).
References
- 1.Pels H, Juergens A, Glasmacher A, et al. Early relapses in primary CNS lymphoma after response to polychemotherapy without intraventricular treatment: results of a phase II study. J Neurooncol. 2009;91:299–305. doi: 10.1007/s11060-008-9712-4. [DOI] [PubMed] [Google Scholar]
- 2.DeAngelis LM, Seiferheld W, Schold SC, et al. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol. 2002;20:4643–4648. doi: 10.1200/JCO.2002.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Shah GD, Yahalom J, Correa DD, et al. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2007;25:4730–4735. doi: 10.1200/JCO.2007.12.5062. [DOI] [PubMed] [Google Scholar]
- 4.Nayak L, Hedvat C, Rosenblum MK, Abrey LE, DeAngelis LM. Late relapse in primary central nervous system lymphoma: clonal persistence. Neuro Oncol. 2011;13:525–529. doi: 10.1093/neuonc/nor014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nayak L, Abrey LE, Drappatz J, et al. Multicenter phase II study of rituximab and temozolomide in recurrent primary central nervous system lymphoma. Leuk Lymphoma. 2013;54:58–61. doi: 10.3109/10428194.2012.698736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arellano-Rodrigo E, Lopez-Guillermo A, Bessell EM, et al. Salvage treatment with etoposide (VP-16), ifosfamide and cytarabine (Ara-C) for patients with recurrent primary central nervous system lymphoma. Eur J Haematol. 2003;70:219–224. doi: 10.1034/j.1600-0609.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 7.Mappa S, Marturano E, Licata G, et al. Salvage chemoimmunotherapy with rituximab, ifosfamide and etoposide (R-IE regimen) in patients with primary CNS lymphoma relapsed or refractory to high-dose methotrexate-based chemotherapy. Hematol Oncol. 2012 doi: 10.1002/hon.2037. [DOI] [PubMed] [Google Scholar]
- 8.Batchelor TT, Grossman SA, Mikkelsen T, et al. Rituximab monotherapy for patients with recurrent primary CNS lymphoma. Neurology. 2011;76:929–930. doi: 10.1212/WNL.0b013e31820f2d94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer L, Thiel E, Klasen HA, et al. Prospective trial on topotecan salvage therapy in primary CNS lymphoma. Ann Oncol. 2006;17:1141–1145. doi: 10.1093/annonc/mdl070. [DOI] [PubMed] [Google Scholar]
- 10.Enting RH, Demopoulos A, DeAngelis LM, Abrey LE. Salvage therapy for primary CNS lymphoma with a combination of rituximab and temozolomide. Neurology. 2004;63:901–903. doi: 10.1212/01.wnl.0000137050.43114.42. [DOI] [PubMed] [Google Scholar]
- 11.Herrlinger U, Brugger W, Bamberg M, et al. PCV salvage chemotherapy for recurrent primary CNS lymphoma. Neurology. 2000;54:1707–1708. doi: 10.1212/wnl.54.8.1707. [DOI] [PubMed] [Google Scholar]
- 12.Raizer JJ, Rademaker A, Evens AM, et al. Pemetrexed in the treatment of relapsed/refractory primary central nervous system lymphoma. Cancer. 2012;118:3743–3748. doi: 10.1002/cncr.26709. [DOI] [PubMed] [Google Scholar]
- 13.Plotkin SR, Betensky RA, Hochberg FH, et al. Treatment of relapsed central nervous system lymphoma with high-dose methotrexate. Clin Cancer Res. 2004;10:5643–5646. doi: 10.1158/1078-0432.CCR-04-0159. [DOI] [PubMed] [Google Scholar]
- 14.Thiel E, Korfel A, Martus P, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010;11:1036–1047. doi: 10.1016/S1470-2045(10)70229-1. [DOI] [PubMed] [Google Scholar]
- 15.Abrey LE, Batchelor TT, Ferreri AJ, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23:5034–5043. doi: 10.1200/JCO.2005.13.524. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen PL, Chakravarti A, Finkelstein DM, et al. Results of whole-brain radiation as salvage of methotrexate failure for immunocompetent patients with primary CNS lymphoma. J Clin Oncol. 2005;23:1507–1513. doi: 10.1200/JCO.2005.01.161. [DOI] [PubMed] [Google Scholar]
- 17.Hottinger AF, DeAngelis LM, Yahalom J, Abrey LE. Salvage whole brain radiotherapy for recurrent or refractory primary CNS lymphoma. Neurology. 2007;69:1178–1182. doi: 10.1212/01.wnl.0000276986.19602.c1. [DOI] [PubMed] [Google Scholar]
- 18.Reni M, Zaja F, Mason W, et al. Temozolomide as salvage treatment in primary brain lymphomas. Br J Cancer. 2007;96:864–867. doi: 10.1038/sj.bjc.6603660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makino K, Nakamura H, Hide T, Kuratsu J. Salvage treatment with temozolomide in refractory or relapsed primary central nervous system lymphoma and assessment of the MGMT status. J Neurooncol. 2012;106:155–160. doi: 10.1007/s11060-011-0652-z. [DOI] [PubMed] [Google Scholar]
- 20.Soussain C, Hoang-Xuan K, Taillandier L, et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Societe Francaise de Greffe de Moelle Osseuse-Therapie Cellulaire. J Clin Oncol. 2008;26:2512–2518. doi: 10.1200/JCO.2007.13.5533. [DOI] [PubMed] [Google Scholar]
- 21.Morris PG, Abrey LE, Reiner AS, et al. Methotrexate area under the curve as a prognostic factor in primary central nervous system lymphoma treated with immunochemoradiotherapy. Leuk Lymphoma. 2011;52:1891–1897. doi: 10.3109/10428194.2011.585527. [DOI] [PubMed] [Google Scholar]
- 22.Morris P, Correa DD, Yahalom J, et al. Rituximab, methotrexate, procarbazine and vincristine followed by consolidation reduced-dose whole brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: Final results and long-term outcome. J Clin Oncol. 2013 doi: 10.1200/JCO.2013.50.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.