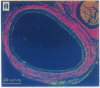
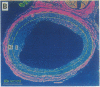
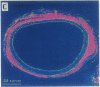
Abstract
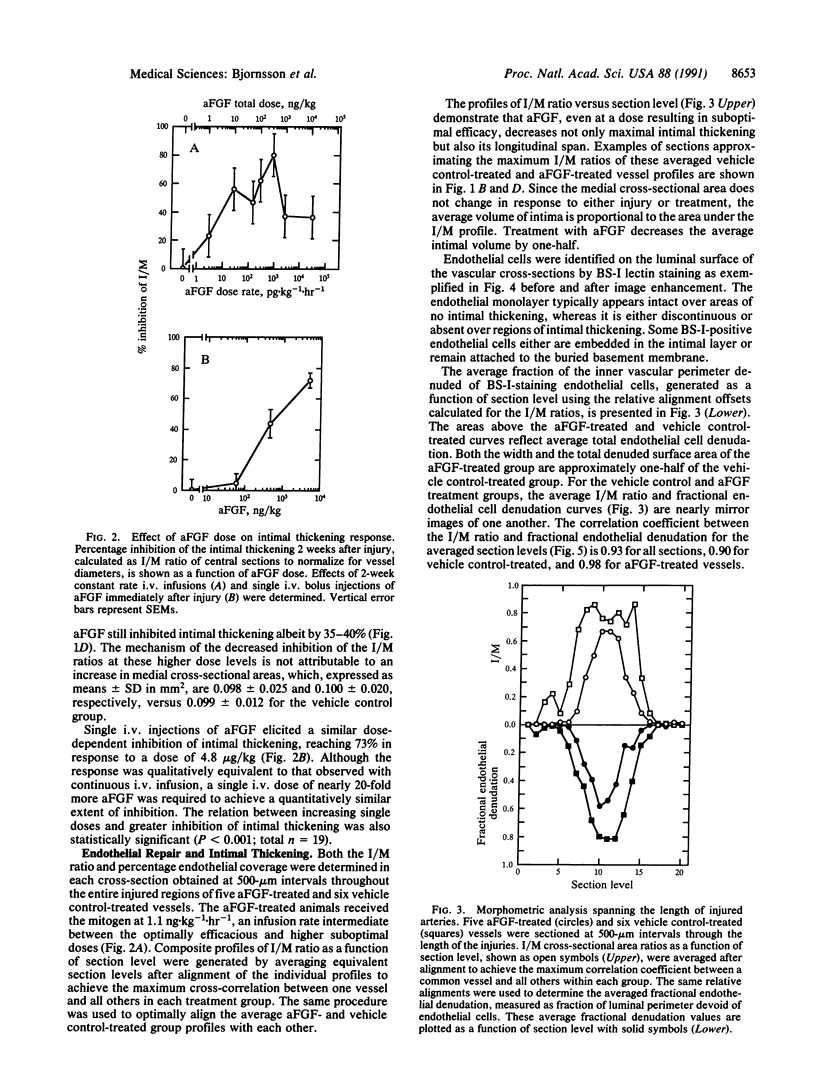
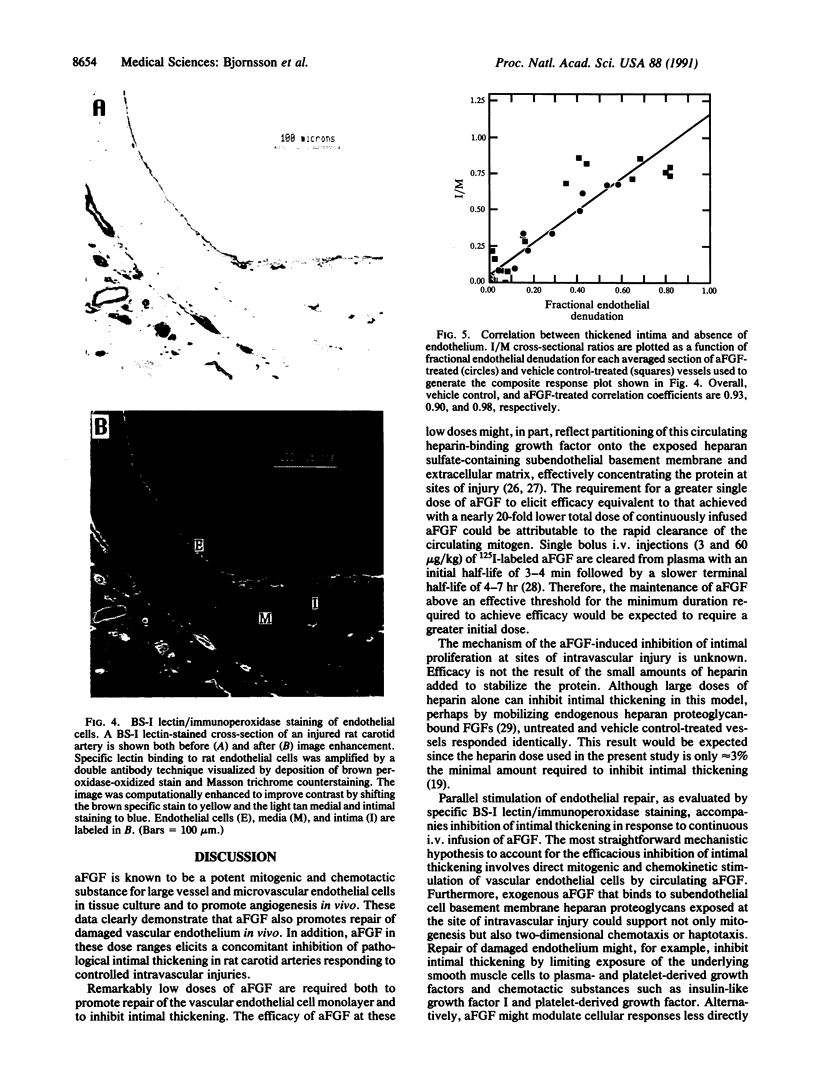
Intravascular injury to arteries can result in thickening of the intimal smooth muscle layer adjacent to the lumen by migration and proliferation of cells from the underlying medial smooth muscle layer accompanied by deposition of extracellular matrix. This pathological response, which decreases lumen diameter, might, in part, be the result of the access of smooth muscle cells to plasma and platelet-derived growth factors as a consequence of denudation of the overlying confluent monolayer of vascular endothelial cells. Injured rat carotid arteries were treated by i.v. administration of acidic fibroblast growth factor, a heparin-binding protein that is chemotactic and mitogenic for vascular endothelial cells. The growth factor treatment resulted in dose-dependent inhibition of intimal thickening with parallel promotion of endothelial regeneration over the injured area. Therefore, acidic fibroblast growth factor might be efficacious in the prevention of restenosis caused by intimal thickening following angioplasty in humans.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alroy J., Goyal V., Skutelsky E. Lectin histochemistry of mammalian endothelium. Histochemistry. 1987;86(6):603–607. doi: 10.1007/BF00489554. [DOI] [PubMed] [Google Scholar]
- Baird A., Ling N. Fibroblast growth factors are present in the extracellular matrix produced by endothelial cells in vitro: implications for a role of heparinase-like enzymes in the neovascular response. Biochem Biophys Res Commun. 1987 Jan 30;142(2):428–435. doi: 10.1016/0006-291x(87)90292-0. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Collazzo R. E., Karnovsky M. J. A morphologic and permeability study of luminal smooth muscle cells after arterial injury in the rat. Lab Invest. 1978 Aug;39(2):141–150. [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Mechanisms of stenosis after arterial injury. Lab Invest. 1983 Aug;49(2):208–215. [PubMed] [Google Scholar]
- Clowes A. W., Schwartz S. M. Significance of quiescent smooth muscle migration in the injured rat carotid artery. Circ Res. 1985 Jan;56(1):139–145. doi: 10.1161/01.res.56.1.139. [DOI] [PubMed] [Google Scholar]
- Conn G., Bayne M. L., Soderman D. D., Kwok P. W., Sullivan K. A., Palisi T. M., Hope D. A., Thomas K. A. Amino acid and cDNA sequences of a vascular endothelial cell mitogen that is homologous to platelet-derived growth factor. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2628–2632. doi: 10.1073/pnas.87.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryjski M., Mikat E., Bjornsson T. D. Inhibition of intimal hyperplasia after arterial injury by heparins and heparinoid. J Vasc Surg. 1988 Nov;8(5):623–633. doi: 10.1067/mva.1988.avs0080623. [DOI] [PubMed] [Google Scholar]
- Finch P. W., Rubin J. S., Miki T., Ron D., Aaronson S. A. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989 Aug 18;245(4919):752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- Fishman J. A., Ryan G. B., Karnovsky M. J. Endothelial regeneration in the rat carotid artery and the significance of endothelial denudation in the pathogenesis of myointimal thickening. Lab Invest. 1975 Mar;32(3):339–351. [PubMed] [Google Scholar]
- Gay C. G., Winkles J. A. Heparin-binding growth factor-1 stimulation of human endothelial cells induces platelet-derived growth factor A-chain gene expression. J Biol Chem. 1990 Feb 25;265(6):3284–3292. [PubMed] [Google Scholar]
- Guyton J. R., Rosenberg R. D., Clowes A. W., Karnovsky M. J. Inhibition of rat arterial smooth muscle cell proliferation by heparin. In vivo studies with anticoagulant and nonanticoagulant heparin. Circ Res. 1980 May;46(5):625–634. doi: 10.1161/01.res.46.5.625. [DOI] [PubMed] [Google Scholar]
- Herbert J. M., Laplace M. C., Maffrand J. P. Effect of heparin on the angiogenic potency of basic and acidic fibroblast growth factors in the rabbit cornea assay. Int J Tissue React. 1988;10(3):133–139. [PubMed] [Google Scholar]
- Ishikawa F., Miyazono K., Hellman U., Drexler H., Wernstedt C., Hagiwara K., Usuki K., Takaku F., Risau W., Heldin C. H. Identification of angiogenic activity and the cloning and expression of platelet-derived endothelial cell growth factor. Nature. 1989 Apr 13;338(6216):557–562. doi: 10.1038/338557a0. [DOI] [PubMed] [Google Scholar]
- Keck P. J., Hauser S. D., Krivi G., Sanzo K., Warren T., Feder J., Connolly D. T. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989 Dec 8;246(4935):1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M. The fibroblast growth factor family: structural and biological properties. Prog Growth Factor Res. 1989;1(4):207–235. doi: 10.1016/0955-2235(89)90012-4. [DOI] [PubMed] [Google Scholar]
- Konkle B. A., Ginsburg D. The addition of endothelial cell growth factor and heparin to human umbilical vein endothelial cell cultures decreases plasminogen activator inhibitor-1 expression. J Clin Invest. 1988 Aug;82(2):579–585. doi: 10.1172/JCI113635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D. W., Cachianes G., Kuang W. J., Goeddel D. V., Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989 Dec 8;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Lindner V., Lappi D. A., Baird A., Majack R. A., Reidy M. A. Role of basic fibroblast growth factor in vascular lesion formation. Circ Res. 1991 Jan;68(1):106–113. doi: 10.1161/01.res.68.1.106. [DOI] [PubMed] [Google Scholar]
- Lindner V., Majack R. A., Reidy M. A. Basic fibroblast growth factor stimulates endothelial regrowth and proliferation in denuded arteries. J Clin Invest. 1990 Jun;85(6):2004–2008. doi: 10.1172/JCI114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb R. R., Alderman E. M., Fett J. W. Induction of angiogenesis by bovine brain derived class 1 heparin-binding growth factor. Biochemistry. 1985 Sep 10;24(19):4969–4973. doi: 10.1021/bi00340a001. [DOI] [PubMed] [Google Scholar]
- McBride W., Lange R. A., Hillis L. D. Restenosis after successful coronary angioplasty. Pathophysiology and prevention. N Engl J Med. 1988 Jun 30;318(26):1734–1737. doi: 10.1056/NEJM198806303182606. [DOI] [PubMed] [Google Scholar]
- Munro J. M., Cotran R. S. The pathogenesis of atherosclerosis: atherogenesis and inflammation. Lab Invest. 1988 Mar;58(3):249–261. [PubMed] [Google Scholar]
- Ortega S., Schaeffer M. T., Soderman D., DiSalvo J., Linemeyer D. L., Gimenez-Gallego G., Thomas K. A. Conversion of cysteine to serine residues alters the activity, stability, and heparin dependence of acidic fibroblast growth factor. J Biol Chem. 1991 Mar 25;266(9):5842–5846. [PubMed] [Google Scholar]
- Rappaport R. S., Ronchetti-Blume M., Vogel R. L., Hung P. P. Heparin potentiates endothelial cell growth factor stimulation of plasminogen activator synthesis by diploid human lung fibroblasts. Thromb Haemost. 1988 Jun 16;59(3):514–522. [PubMed] [Google Scholar]
- Reidy M. A., Clowes A. W., Schwartz S. M. Endothelial regeneration. V. Inhibition of endothelial regrowth in arteries of rat and rabbit. Lab Invest. 1983 Nov;49(5):569–575. [PubMed] [Google Scholar]
- Rosengart T. K., Kupferschmid J. P., Ferrans V. J., Casscells W., Maciag T., Clark R. E. Heparin-binding growth factor-I (endothelial cell growth factor) binds to endothelium in vivo. J Vasc Surg. 1988 Feb;7(2):311–317. [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Haudenschild C. C., Eddy E. M. Endothelial regneration. I. Quantitative analysis of initial stages of endothelial regeneration in rat aortic intima. Lab Invest. 1978 May;38(5):568–580. [PubMed] [Google Scholar]
- Terranova V. P., DiFlorio R., Lyall R. M., Hic S., Friesel R., Maciag T. Human endothelial cells are chemotactic to endothelial cell growth factor and heparin. J Cell Biol. 1985 Dec;101(6):2330–2334. doi: 10.1083/jcb.101.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. A., Rios-Candelore M., Giménez-Gallego G., DiSalvo J., Bennett C., Rodkey J., Fitzpatrick S. Pure brain-derived acidic fibroblast growth factor is a potent angiogenic vascular endothelial cell mitogen with sequence homology to interleukin 1. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6409–6413. doi: 10.1073/pnas.82.19.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkles J. A., Friesel R., Burgess W. H., Howk R., Mehlman T., Weinstein R., Maciag T. Human vascular smooth muscle cells both express and respond to heparin-binding growth factor I (endothelial cell growth factor). Proc Natl Acad Sci U S A. 1987 Oct;84(20):7124–7128. doi: 10.1073/pnas.84.20.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]