Abstract
Background
Postnatal corticosteroids (PNC) were widely used to treat and prevent bronchopulmonary dysplasia in preterm infants until studies showed increased risk of cerebral palsy and neurodevelopmental impairment. We aimed to describe PNC use in Europe and evaluate the determinants of their use, including neonatal characteristics and adherence to evidence-based practices in neonatal intensive care units (NICUs).
Methods
3917/4096 (95,6%) infants born between 24 and 29 weeks gestational age in 19 regions of 11 European countries of the EPICE cohort we included. We examined neonatal characteristics associated with PNC use. The cohort was divided by tertiles of probability of PNC use determined by logistic regression analysis. We also evaluated the impact of the neonatal unit’s reported adherence to European recommendations for respiratory management and a stated policy of reduced PNC use.
Results
PNC were prescribed for 545/3917 (13.9%) infants (regional range 3.1–49.4%) and for 29.7% of infants in the highest risk tertile (regional range 5.4–72.4%). After adjustment, independent predictors of PNC use were a low gestational age, small for gestational age, male sex, mechanical ventilation, use of non-steroidal anti-inflammatory drugs to treat persistent ductus arteriosus and region. A stated NICU policy reduced PNC use (odds ratio 0.29 [95% CI 0.17; 0.50]).
Conclusion
PNC are frequently used in Europe, but with wide regional variation that was unexplained by neonatal characteristics. Even for infants at highest risk for PNC use, some regions only rarely prescribed PNC. A stated policy of reduced PNC use was associated with observed practice and is recommended.
Introduction
Bronchopulmonary dysplasia (BPD) is a chronic lung disease frequently associated with very preterm birth [1,2] and defined as the need for oxygen at 36 weeks gestational age (GA) [3]. Inflammatory mechanisms in BPD have been described [4]. Therefore, postnatal corticosteroids (PNC) have been used to wean infants off ventilators [5]. In the 1990s, up to 25% of very preterm infants received dexamethasone [6].
Dexamethasone has been found to be effective for decreasing BPD, BPD or mortality, as a composite outcome, and facilitating extubation, but adverse effects include hyperglycemia, hypertension, gastrointestinal bleeding or perforation, and hypertrophic cardiomyopathy. Longer term consequences include a higher risk of cerebral palsy. [7–11]. The evidence for the use of other PNC is weak. There have been only a few trials of hydrocortisone to prevent BPD [12]. Only one study showed a reduction of BPD, but long term outcome wasn’t assessed [13]. Results from an observational study showing reduced white matter injury with betamethasone compared to dexamethasone in antenatal care [14] led to a preference for betamethasone as postnatal steroid therapy for BPD, but no trial results have been published for this indication. In 2002, the American Academy of Pediatrics and Canadian Paediatric Society published recommendations concerning PNC [15], revised in 2012 [16]: the routine use of PNC to prevent or treat BPD should be avoided; because of adverse effects, its use should be limited to exceptional clinical circumstances after parental information and PNC initiation should be delayed at least after 7 days of life.
After the publication of the AAP-recommendations, the use of PNC decreased [6,17,18]. However, the Models for OrganiSing Access to Intensive Care For Very Preterm Babies in Europe (MOSAIC) study showed that PNC was still in use in Europe in 2003, with rates from 4% to 35% among regions [19].
The Effective Perinatal Intensive Care in Europe (EPICE) study enrolled a population-based cohort of births before 32 weeks GA that occurred in 2011 and 2012 in 19 regions in 11 European countries. The study aimed to assess the use of evidence-based medicine for the care of very preterm infants. In this paper, we describe the rate of PNC use in participating EPICE regions and identify determinants of its use, including case-mix characteristics and neonatal intensive care unit (NICU) policies. Our hypothesis is that evidence-based policies can reduce PNC use.
Methods
Study design
The EPICE cohort is a geographically defined study of all very preterm stillbirths and live births from 22 +0 to 31 +6 weeks GA in 19 European regions. Data collection started between March and July 2011 and the inclusion period lasted 12 months, except in France, where it lasted 6 months.
Study population
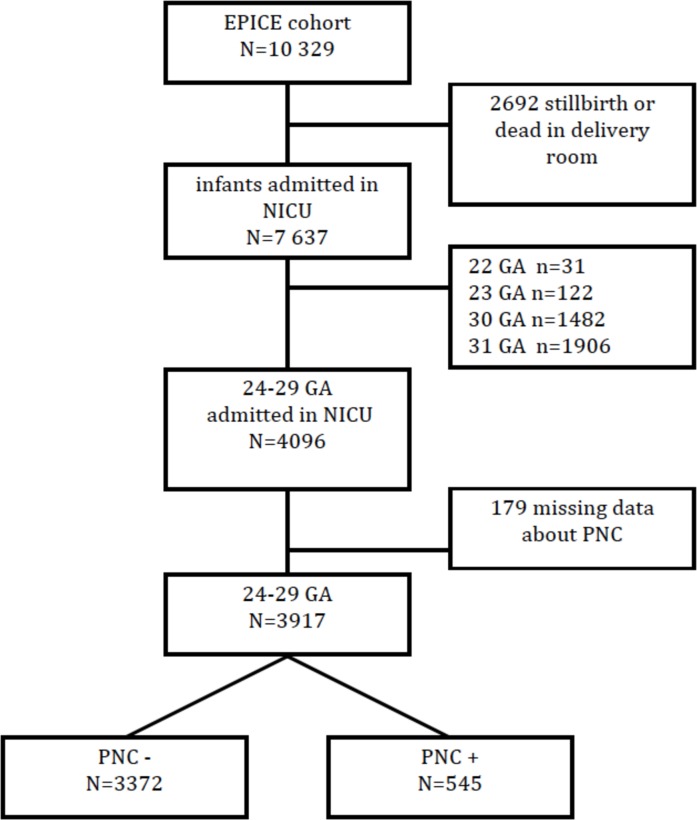
For this study, all infants admitted to a neonatal unit were included. We excluded infants born before 24 weeks GA because of heterogeneous management of this subgroup in participating regions and infants born after 29+6 weeks GA because of reduced risk of BPD [20]. The flow chart is displayed in Fig 1.
Fig 1. Flow chart of infants who met inclusion/exclusion criteria for the study.
PNC, postnatal corticosteroids; NICU, neonatal intensive care unit; GA: gestational age.
Data collection
Data on infants were collected using a standardised and pretested questionnaire from obstetrical and neonatal medical records. Data on the characteristics and practices of NICUs providing care for very preterm infants in participating regions were collected by use of a structured questionnaire sent to NICU administrative heads by mail or e-mail. Questions were formulated so that they could be answered in the same way by different staff members and several people within the NICU could discuss responses and complete the questionnaire. Data were collected on the structural characteristics of NICUs (level of specialization), their activity levels in 2011, policies, protocols and practices related to selected medical interventions, ethical decisions, decision-making processes and existence of healthcare quality monitoring systems.
NICUs with at least 10 very preterm admissions per year and their associated maternity units were included in the unit studies. These parameters were fixed before the study’s onset.
Ethics approval was obtained in each study region from regional and/or hospital ethics committees, as required by national legislation. The European study was also approved by the French Advisory Committee on Use of Health Data in Medical Research and the French National Commission for Data Protection and Liberties.
Study outcome
The outcome in this study was the specific use of systemic PNC to treat BPD, including the type of steroid used, the initial dose, the age at initiation, and the length of treatment. When systemic steroids for this indication were given before 36 weeks GA, we considered they were used for infants with respiratory disease at high risk of BPD.
Neonatal characteristics
GA was based on ultrasound measures and information on last menstrual period. Small for gestational age (SGA) was defined by a birthweight <10th percentile by using references generated from all live births in the EPICE cohort. We distinguished babies with a birthweight between the 10th and 25th percentile as they have been found to be at higher risk of mortality and respiratory morbidity [21]. Birthweight, sex, type of pregnancy (singleton or multiple), mode of delivery, Apgar score at 5 min of life, surfactant therapy, mechanical ventilation, and use of non-steroidal anti-inflammatory drugs (NSAIDs) to treat persistent ductus arteriosus (PDA) were recorded. Antenatal steroids were scored as used if at least one dose had been given. Preterm premature rupture of membranes (PROM) was defined as a rupture of the membranes lasting longer than 12 hours before labor onset. Preeclampsia or eclampsia or Hemolysis, Elevated Liver enzyme Low Platelet (HELLP) syndrome was summarized as one variable.
NICU policies on evidence-based practices
Using questions from the NICU questionnaire, we generated 2 variables to reflect each unit’s adherence to evidence-based practices. The first definition was based on the 2010 European guidelines for managing respiratory distress syndrome in very preterm infants [22]. We classified the NICU as having an evidence-based approach if the NICU reported that they administered surfactant to all newborns born before 26 weeks GA and to all newborns requiring intubation in the delivery room, as recommended. The second definition relied on declared policies concerning PNC prescription. We considered the NICU as having an evidence-based approach if it reported a “PNC restricted use policy”. The NICU was classified as evidence-based if it answered “no (or exceptional circumstances)” to both questions “In the unit, are systemic postnatal corticosteroids given to infants less than 32 weeks GA to prevent BPD” or “to treat BPD”. We analysed the effect of both approaches on PNC use separately.
Statistical analysis
We used descriptive statistics to portray PNC prescription by region. We analysed the association between perinatal characteristics and PNC prescription by Student t or Mann-Whitney U test for quantitative variables and chi-square or Fisher exact test for qualitative variables. Variables with p < 0.2 on univariate analysis were included in a stepwise logistic regression analysis. The selected variables were consistent with the literature as risk factors for BPD [1,2]. For each infant, we then estimated the probability of receiving PNC by the logistic regression model, stratifying the sample by tertiles of probability of receiving PNC, as a risk profile, and selected the infants in the highest tertile (highest risk profile) for analysing PNC prescription. This subgroup included infants with the most severe respiratory condition. Finally, we analyzed the association between reported NICU policy for evidence-based practices and PNC prescription with a two-level hierarchical linear mixed model, with patients as the first level and the NICU as the second level. This last analysis involved the whole population and infants in the highest probability tertile. Results are expressed as crude and adjusted odd ratios (ORs) with 95% confidence intervals (95% CIs). All analyses were done using SAS 9.3 (SAS Inst. Inc., Cary, NC, USA).
Results
Use of PNC and variation across regions
Among newborns fulfilling inclusion criteria, 179/4096, or 4.3% had missing data on PNC prescription. The characteristics of the final sample retained for the analysis (3917 newborns) are described in Table 1. Infants with missing data had lower gestational ages (mean: 26.7 versus 27.1) and more often SGA (19% versus 9%) (p<0.05) (data not shown).
Table 1. Perinatal population characteristics.
| Characteristics | Infants 24–29 weeks’ GA admitted to an NICU n = 3917 | |
|---|---|---|
| Gestational age (weeks). mean (SD) | 27.1 (1.6) | |
| Birth weight (g). mean (SD) | 1105 (278) | |
| Gestational age | ||
| 24–25 weeks | 748 | (19.1) |
| 26–27 weeks | 1276 | (32.6) |
| 28–29 weeks | 1896 | (48.4) |
| Male gender | 2139 | (54.6) |
| Multiples | 1173 | (29.9) |
| Birth weight percentile | ||
| SGA <10th | 363 | (9.3) |
| 10-25th | 614 | (15.7) |
| >25th | 2940 | (75.1) |
| Preeclampsia/eclampsia/HELLP | 558/3871 | (14.4) |
| PROM | 981/3859 | (25.4) |
| Caesarean section | 2551/3892 | (65.5) |
| Inborn | 3456/3911 | (88.4) |
| Apgar <7 at 5 min | 837/3644 | (23.0) |
| Antenatal steroids | 3465/3884 | (89.2) |
| Surfactant | 2983/3910 | (76.3) |
| Mechanical ventilation | 3030/3907 | (77.6) |
| NSAIDs to treat PDA | 1110/3899 | (28.5) |
| PNC | 545 | (13.9) |
Data are no. (%) unless indicated and for 3917 infants unless indicated.
GA, gestational age; HELLP: Hemolysis Elevated Liver enzyme Low Platelet NICU, neonatal intensive care unit; PNC, postnatal steroids; SGA, small for gestational age; PROM, premature rupture of membrane; NSAIDs, non-steroidal anti-inflammatory drugs; PDA, persistant ductus arteriosus
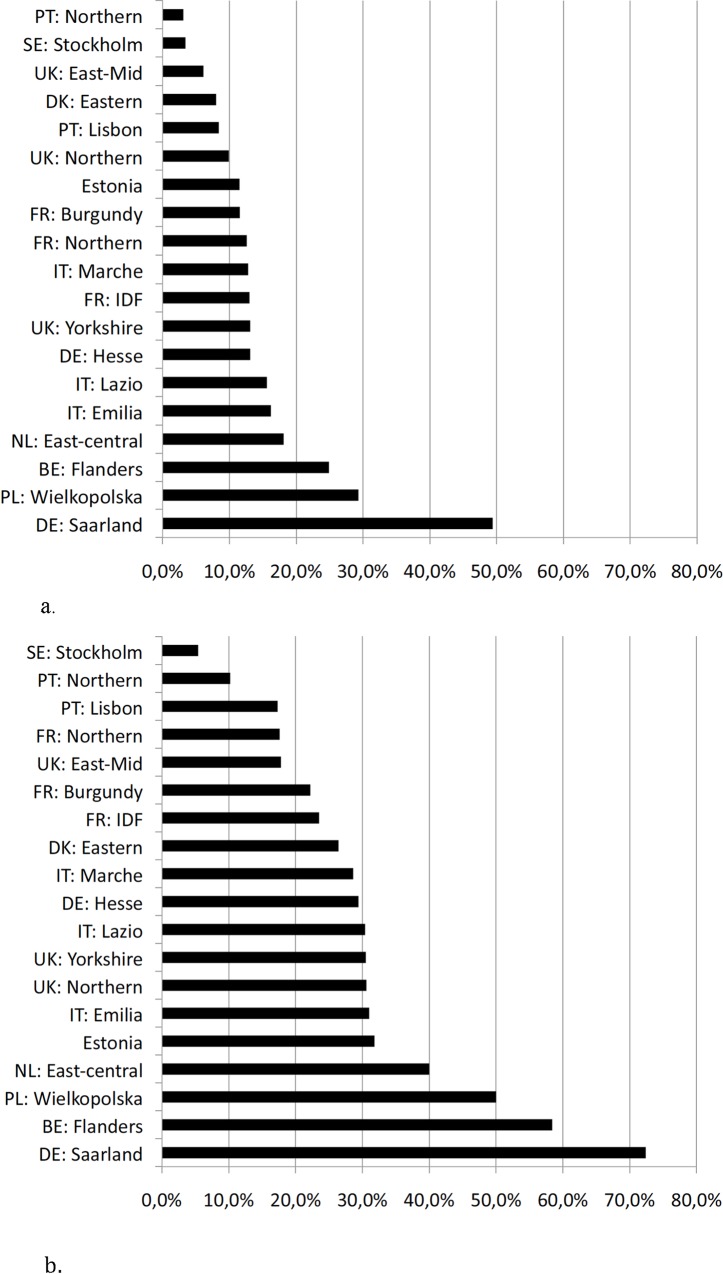
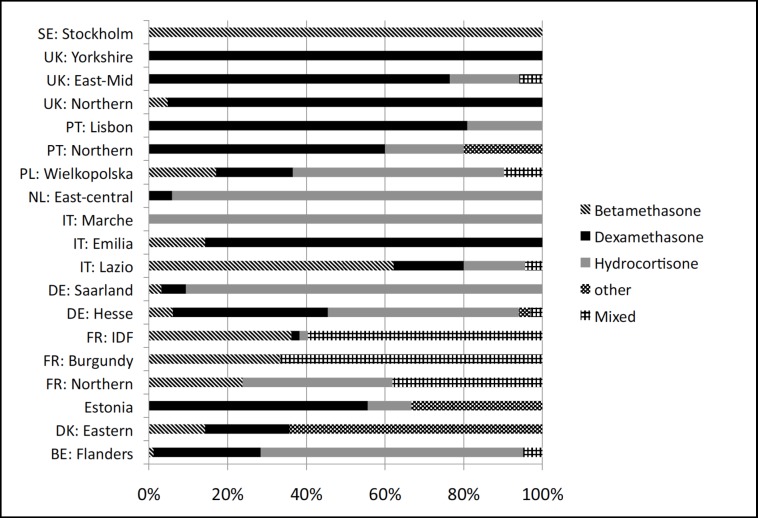
In total, 545/3917 (13,9%) infants between 24 and 29 weeks GA received systemic PNC to treat BPD (Fig 1). Rates varied significantly among regions, from 3.1% (5/162 in northern Portugal) to 49.4% (39/79 in Saarland, Germany) (p<0.001) (Fig 2A). Dexamethasone, hydrocortisone and betamethasone were used in 195/545 (35,8%), 184/545 (33,8%) and 76/545(13,9%) of cases, respectively, and treatment was mixed for 59/545 (10,8%) of newborns (Table 2). Twenty six percent of infants (N = 50/195) received dexamethasone within the first 2 weeks of life. Twenty eight percent of infants received dexamethasone for more than 20 days (40 of 143 infants receiving dexamethasone, with data on treatment duration available). The type of drug used within regions varied significantly (p<0.001) (Fig 3).
Fig 2.
a) Rate of Postnatal Corticosteroid (PNC) use by region. b) Rate of PNC use by region for infants in the highest risk tertile. PT: Portugal, PL: Poland, UK: United Kingdom, SE: Sweden, DK: Denmark, FR: France, IDF: Ile de France, DE: Germany, IT: Italy, NL: The Netherlands, BE: Belgium,
Table 2. PNC use for infants at 24–29 GA (N = 545).
| Dexamethasone (35,8%) | Hydrocortisone (33,8%) | Betamethasone (13,9%) | |
|---|---|---|---|
| Age at initiation (d) | 24 (15–37) | 12 (6–20) | 21 (15–29) |
| Initial dose (mg/kg/d) | 0.2 (0.15–0.5) | 4.0 (1.7–5) | 0.2 (0.1–0.3) |
| Treatment duration (d) | 10 (6–20) | 21 (11–29) | 7 (4–12) |
| GA at birth (weeks) mean (sd) | 25.6 (1.4) | 26.0 (1.6) | 26.5 (1.6) |
Data are median (Q1-Q3) unless indicated.
Fig 3. Type of corticosteroids used to treat bronchopulmonary dysplasia (BPD), by region.
PT: Portugal, PL: Poland, UK: United Kingdom, SE: Sweden, DK: Denmark, FR: France, IDF: Ile de France, DE: Germany, IT: Italy, NL: The Netherlands, BE: Belgium,
Neonatal determinants of PNC use
Low GA, SGA, male sex, mechanical ventilation and NSAIDs to treat PDA were significantly associated with PNC use after multivariate analysis (Table 3). Apgar score < 7 and surfactant treatment were no longer significant after adjustment.
Table 3. Use of PNC by neonatal characteristics.
| PNC | P value | crude | Adj | ||||
|---|---|---|---|---|---|---|---|
| n/N | % | OR | 95% CI | OR* | 95% CI | ||
| GA | |||||||
| 24–25 | 242/748 | 32.4 | <0.0001 | 8.7 | 6.7–11.2 | 5.6 | 4.1–7.5 |
| 26–27 | 204/1276 | 16.0 | <0.0001 | 3.4 | 2.7–4.4 | 2.5 | 1.9–3.3 |
| 28–29 | 99/1893 | 5.2 | - | - | - | - | |
| Birthweight percentile | |||||||
| <10th | 79/363 | 21.8 | 2.0 | 1.5–2.6 | 2.1 | 1.5–3.0 | |
| 10-25th | 110/614 | 17.9 | 1.6 | 1.3–2.0 | 1.4 | 1.1–1.9 | |
| >25th | 356/2940 | 12.1 | <0.0001 | - | - | - | - |
| Sex | |||||||
| Female | 210/1778 | 11.8 | 0.001 | - | - | - | - |
| Male | 335/2139 | 15.7 | 1.4 | 1.2–1.7 | 1.4 | 1.1–1.8 | |
| Multiple pregnancy | |||||||
| Singleton | 392/2744 | 14.3 | 0.30 | - | - | - | - |
| Multiple | 153/1173 | 13.0 | 0.9 | 0.7–1.1 | 0.9 | 0.7–1.2 | |
| PROM | |||||||
| No | 413/2878 | 14.4 | 0.11 | - | - | - | - |
| Yes | 121/981 | 12.3 | 0.8 | 0.7–1.0 | 0.9 | 0.7–1.1 | |
| Preeclampsia/eclampsia/HELLP | |||||||
| No | 448/3313 | 13.5 | 0.16 | - | - | - | - |
| Yes | 88/558 | 15.8 | 1.2 | 0.9–1.5 | 1.1 | 0.8–1.6 | |
| Caesarean | |||||||
| No | 208/1341 | 15.5 | 0.03 | - | - | - | - |
| Yes | 332/2551 | 13.0 | 0.8 | 0.7–1.0 | 1.1 | 0.9–1.4 | |
| Inborn | |||||||
| No | 68/455 | 14.9 | 1.10 | 0.8–1.4 | 0.7 | 0.5–1.0 | |
| Yes | 476/3456 | 13.8 | 0.5 | - | - | - | |
| Apgar score <7 | |||||||
| No | 343/2807 | 12.2 | <0.0001 | - | - | - | - |
| Yes | 150/837 | 17.9 | 1.6 | 1.3–1.9 | 0.9 | 0.7–1.2 | |
| Treatments | |||||||
| Antenatal steroids | |||||||
| No | 62/419 | 14.8 | 0.62 | - | - | - | - |
| Yes | 482/3465 | 13.9 | 0.9 | 0.70–1.2 | 1.1 | 0.8–1.5 | |
| Surfactant | |||||||
| No | 36/927 | 3.9 | <0.0001 | - | - | - | - |
| Yes | 509/2983 | 17.1 | 5.1 | 3.6–7.2 | 1.3 | 0.8–1.9 | |
| Mechanical ventilation | |||||||
| No | 10/877 | 1.1 | <0.0001 | - | - | - | - |
| Yes | 534/3030 | 17.6 | 18.5 | 9.9–34.8 | 7.7 | 3.8–15.8 | |
| NSAIDs to treat PDA | |||||||
| No | 273/2789 | 9.8 | <0.0001 | - | - | - | - |
| Yes | 271/1110 | 24.4 | 3.0 | 2.5–3.6 | 1.8 | 1.5–2.3 | |
* adjusted on gestational age
SGA, sex, PROM, preeclampsia/eclampsia/HELLP:, caesarean section, apgar score, surfactant, mechanical ventilation, NSAIDs to treat PDA
OR: odds ratio; 95% CI: 95% confidence interval. n: number receiving PNC N: total in group
Regional variation after case-mix adjustment
We used the five neonatal factors significantly associated with PNC use to calculate the probability of each infant receiving PNC. This probability could not be calculated for 28 infants because of missing data. We carried out subgroup analysis on infants with the most severe disease, defined as the tertile with highest probability of receiving PNC. The predicted probability of receiving PNC ranged from 17% to 66% in this group. In this subgroup, 392/1320 (29,7%) of infants received PNC to treat BPD and this varied significantly among regions, from 5.4% (2/37 infants) in Stockholm, Sweden to 72.4% (21/29 infants) in Saarland, Germany (p<0.001) (Fig 2B).
A total of 266 NICUs were located in the study regions in 2011, and 135 fulfilled the inclusion criteria. Of these, 134/135 (99,2%) provided completed questionnaires. In our sample, 69/3917 (1.8%) infants were excluded from this analyses because they were hospitalized in neonatal units that did not fulfill the inclusion criteria or did not complete the questionnaire. Twelve percent of neonatal units reported adherence to the European Association of Perinatal Medicine guidelines about surfactant use. Thirty six percent reported a PNC restricted use policy. We found no association between reported adherence to the European Association of Perinatal Medicine guidelines (21) and PNC use; however, infants hospitalized in units that declared having a policy concerning PNC restricted use were significantly less likely to receive PNC (Table 4).
Table 4. Association of NICU practices and PNC use.
| Practice | No. of NICUs | No. Of infants | Infants at 24–29 weeks’ GA | Infants in the highest risk tertile | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | Adj OR* | 95% CI | P value | Adj OR* | 95% CI | P value | |
| European Association of Perinatal Medicine recommendations | 16/132 | 12 | 369/3719 | 10 | 0.91 | 0.4–2.1 | 0.83 | 0.82 | 0.3–2 | 0.65 |
| NICU PNC restricted use policy | 47/132 | 36 | 1069/3706 | 29 | 0.29 | 0.2–0.5 | <0.001 | 0.29 | 0.2–0.5 | <0.001 |
* adjusted on gestational age
SGA, sex, mechanical ventilation, NSAIDs to treat PDA
OR: odds ratio; 95% CI: 95% confidence interval. n: number using NICU Policy N: total in group
Discussion
Our study showed that PNC are still used in Europe, with wide variations across regions. The main neonatal characteristics associated with PNC use were low GA, SGA, male sex, receiving mechanical ventilation, and PDA treatment with NSAIDs. However, these characteristics did not explain the wide variation in PNC prescription between regions, which persisted for infants with the most severe respiratory condition (highest risk tertile). NICUs with a PNC restricted use policy showed significantly lower PNC use.
For a quarter of infants who received dexamethasone, treatment was initiated in the first 2 weeks of life, and for another quarter it lasted for more than 20 days. Based on meta-analysis results and the latest recommendations, this use could be considered inappropriate [23,24]. Both hydrocortisone and betamethasone were commonly used to treat BPD and this could also be considered inappropriate as there was no evidence from trials at the time of the EPICE study suggesting that either is effective for BPD reduction. Since then one randomised controlled trial showed that hydrocortisone was effective for reducing BPD [13]. However there was no long term assessment.
Infants with severe respiratory conditions, dependant on mechanical ventilation after the first week of life, may benefit from PNC concerning BPD. However, our analyses by risk tertiles found that some low risk infants received PNC. Moreover, for infants in the highest-risk tertile, those with the most severe disease, some regions had low rates of PNC use showing that the variability in PNC use could not be explained solely by neonatal case mix and also that some regions used alternative strategies for managing severe respiratory complications.
We then considered evidence-based practices as a potential determinant of PNC use. Evidence-based medicine is the judicious use of current best evidence for making decisions about the care of individual patients [25]. The best evidence is from randomised controlled trials and meta-analyses. However, there is no clear indicator to measure use of evidence-based practices in neonatal medicine. We therefore developed two indicators. The first used the 2010 European Association of Perinatal Medicine (EAPM) guidelines [22] which relied on evidence published up to 2009 and which was released before EPICE, conducted in 2011/2012. In 2010, further studies showed less intubation and shorter mechanical ventilation for infants who received early continuous positive airway pressure [26,27]. Therefore, systematic surfactant became irrelevant, and was no longer recommended in the 2013 EAPM guidelines [24]. In 2011, as infants were included in the EPICE cohort, effective respiratory management evidence was changing. The second indicator was a stated policy of restricted PNC use. Evidence of PNC side effects is strong [8,28] and we considered a reported policy of restricted PNC use as an evidence-based practice [8,15,29]. Our definition selects NICUs with a real will to restrict PNC to exceptional circumstances. According to the current recommandations, dexamethasone should be restricted to infants under mechanical ventilation [9,30].
Our result that a lower probability of receiving PNC after consideration of case-mix was related to the existence of a unit policy of restricted use of PNC is consistent with Kaemps et al., an interventional study that showed that implementing local recommendations for respiratory management practices reduced dexamethasone use to treat BPD [31]. Our findings show an impact of explicit policies on real clinical practice [32] and support the development of clear unit policies for the use of evidence-based interventions or treatments.
While an explicit restricted use policy was a significant determinant of clinical practice, questions remain about the large practice variations observed in our study. This could reflect different interpretations of the evidence, in particular for infants at highest risk of BPD.
Doyle et al updated meta regression reported that in units with high rates of BPD, some children under assisted ventilation might have an increased chance of survival without CP [33]. However these results require confirmation at the individual level which would be possible in a randomized trial of infants at high predicted risk of BPD, as suggested by the authors.
The strengths of our study include its prospective population-based cohort design with few missing data. Data for the type of steroids, initiation date and dose and length of treatment were available. One limitation of our study is that our approach of evidence-based practices relied on a questionnaire and not on individual data. However, the declared PNC restricted use policy was associated with significantly reduced PNC use. Furthermore, despite a low missing data rate, newborns with missing data on PNC use were more immature and had lower birthweight than infants without missing data, which could have implied a selection bias and underestimated the PNC prescription rates. The next step will be to assess if low PNC use is associated with different mortality or survival without BPD rates among EPICE NICUs.
Conclusion
European practices in PNC use for BPD are heterogeneous. Neonatal factors associated with PNC prescription reflected in part disease severity but did not explain the variation in PNC use. Our results suggest that a stated policy of restrictive PNC use could reduce non-evidence–based practices for individual patients. Other potential determinants such as knowledge concerning adverse effects, use of preventive respiratory strategies and saturation targets should be considered.
Acknowledgments
The EPICE research group: BELGIUM: Flanders (E Martens, G Martens, P Van Reempts); DENMARK: Eastern Region (K Boerch, A Hasselager, L Huusom, O Pryds, T Weber); ESTONIA (L Toome, H Varendi); FRANCE: Burgundy, Ile-de France and Northern Region (PY Ancel, B Blondel, A Burguet, PH Jarreau, P Truffert); GERMANY: Hesse (RF Maier, B Misselwitz, S Schmidt), Saarland (L Gortner); ITALY: Emilia Romagna (D Baronciani, G Gargano), Lazio (R Agostino, D DiLallo, F Franco), Marche (V Carnielli), M Cuttini;; NETHERLANDS: Eastern & Central (C Koopman-Esseboom, A van Heijst, J Nijman); POLAND: Wielkopolska (J Gadzinowski, J Mazela); PORTUGAL: Lisbon and Tagus Valley (LM Graça, MC Machado), Northern region (Carina Rodrigues, T Rodrigues), H Barros; SWEDEN: Stockholm (AK Bonamy, M Norman, E Wilson); UK: East Midlands and Yorkshire and Humber (E Boyle, ES Draper, BN Manktelow), Northern Region (AC Fenton, DWA Milligan); INSERM, Paris (J Zeitlin, M Bonet, A Piedvache).
Abbreviations
- BPD
bronchopulmonary dysplasia
- GA
gestational age
- HELLP
Hemolysis, Elevated Liver enzyme Low Platelet
- NICU
neonatal intensive car unit
- NSAID
nonsteroidal anti-inflammatory drugs
- PDA
persistent ductus arteriosus
- PNC
postnatal corticosteroids
- PROM
premature rupture of membrane
- SGA
small for gestational age
- WG
weeks’ gestation
Data Availability
We do not have IRB approval to share personal patient data outside of the European consortium. The EPICE project was approved by the French Advisory Committee on Use of Health Data in Medical Research (CCTIRS, N° 13.020 on 24/01/2013) and the French National Commission for Data Protection and Liberties (DR-2013-194, on 10/04/2013). Data will be made available upon request to all researchers who qualify for access to confidential data. The ethical and data protection approvals authorize the project to provide anonymized European data to members of each participating team after approval of each research study by the scientific committee and signature of data access and confidentiality agreements. The data sharing procedures are also regulated by the EPICE Consortium Agreement, which is the legal foundation for the EPICE scientific committee. For requests about the data, please contact Jennifer Zeitlin, Coordinator, EPICE study (Jennifer.zeitlin@inserm.fr).
Funding Statement
The research leading to these results received funding from the European Union's Seventh Framework Programme ([FP7/2007-2013]) under grant agreement n°259882. Additional funding in some countries: France (French Institute of Public Health Research/Institute of Public Health and its partners the French Health Ministry, the National Institute of Health and Medical Research, the National Institute of Cancer, and the National Solidarity Fund for Autonomy; grant ANR-11-EQPX-0038 from the National Research Agency through the French Equipex Program of Investments in the Future; and the PremUp Foundation); Poland (2012-2015 allocation of funds for international projects from the Polish Ministry of Science and Higher Education); Sweden (regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet, and by the Department of Neonatal Medicine, Karolinska University Hospital). UK (funding for The Neonatal Survey from Neonatal Networks for East Midlands and Yorkshire & Humber regions).
References
- 1.Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet. 2006;367: 1421–1431. 10.1016/S0140-6736(06)68615-7 [DOI] [PubMed] [Google Scholar]
- 2.Egreteau L, Pauchard JY, Semama DS, Matis J, Liska A, Romeo B, et al. Chronic oxygen dependency in infants born at less than 32 weeks’ gestation: incidence and risk factors. Pediatrics. 2001;108: E26 [DOI] [PubMed] [Google Scholar]
- 3.Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. 1988;82: 527–532. [PubMed] [Google Scholar]
- 4.Bhandari V. Postnatal inflammation in the pathogenesis of bronchopulmonary dysplasia. Birt Defects Res A Clin Mol Teratol. 2014;100: 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halliday HL. Postnatal steroids: a dilemma for the neonatologist. Acta Paediatr Oslo Nor 1992. 2001;90: 116–118. [DOI] [PubMed] [Google Scholar]
- 6.Yoder BA, Harrison M, Clark RH. Time-related changes in steroid use and bronchopulmonary dysplasia in preterm infants. Pediatrics. 2009;124: 673–679. 10.1542/peds.2008-2793 [DOI] [PubMed] [Google Scholar]
- 7.Romagnoli C, Zecca E, Vento G, De Carolis MP, Papacci P, Tortorolo G. Early postnatal dexamethasone for the prevention of chronic lung disease in high-risk preterm infants. Intensive Care Med. 1999;25: 717–721. [DOI] [PubMed] [Google Scholar]
- 8.Doyle LW, Ehrenkranz RA, Halliday HL. Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2014;5: CD001146. [DOI] [PubMed] [Google Scholar]
- 9.Doyle LW, Ehrenkranz RA, Halliday HL. Late (> 7 days) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2014;5: CD001145. [DOI] [PubMed] [Google Scholar]
- 10.Doyle LW, Ehrenkranz RA, Halliday HL. Dexamethasone treatment in the first week of life for preventing bronchopulmonary dysplasia in preterm infants: a systematic review. Neonatology. 2010;98: 217–224. 10.1159/000286210 [DOI] [PubMed] [Google Scholar]
- 11.Doyle LW, Ehrenkranz RA, Halliday HL. Dexamethasone treatment after the first week of life for bronchopulmonary dysplasia in preterm infants: a systematic review. Neonatology. 2010;98: 289–296. 10.1159/000286212 [DOI] [PubMed] [Google Scholar]
- 12.Doyle LW, Ehrenkranz RA, Halliday HL. Postnatal hydrocortisone for preventing or treating bronchopulmonary dysplasia in preterm infants: a systematic review. Neonatology. 2010;98: 111–117. 10.1159/000279992 [DOI] [PubMed] [Google Scholar]
- 13.Baud O, Maury L, Lebail F, Ramful D, El Moussawi F, Nicaise C, et al. Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): a double-blind, placebo-controlled, multicentre, randomised trial. Lancet Lond Engl. 2016;387: 1827–1836. [DOI] [PubMed] [Google Scholar]
- 14.Baud O, Foix-L’Helias L, Kaminski M, Audibert F, Jarreau PH, Papiernik E, et al. Antenatal glucocorticoid treatment and cystic periventricular leukomalacia in very premature infants. N Engl J Med. 1999;341: 1190–1196. 10.1056/NEJM199910143411604 [DOI] [PubMed] [Google Scholar]
- 15.Committee on Fetus and Newborn. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics. 2002;109: 330–338. [DOI] [PubMed] [Google Scholar]
- 16.Jefferies AL. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Paediatr Child Health. 2012;17: 573–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh MC, Yao Q, Horbar JD, Carpenter JH, Lee SK, Ohlsson A. Changes in the Use of Postnatal Steroids for Bronchopulmonary Dysplasia in 3 Large Neonatal Networks. PEDIATRICS. 2006;118: e1328–e1335. 10.1542/peds.2006-0359 [DOI] [PubMed] [Google Scholar]
- 18.Shinwell ES, Lerner-Geva L, Lusky A, Reichman B. Less postnatal steroids, more bronchopulmonary dysplasia: a population-based study in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2007;92: F30–33. 10.1136/adc.2006.094474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gortner L, Misselwitz B, Milligan D, Zeitlin J, Kollée L, Boerch K, et al. Rates of Bronchopulmonary Dysplasia in Very Preterm Neonates in Europe: Results from the MOSAIC Cohort. Neonatology. 2011;99: 112–117. 10.1159/000313024 [DOI] [PubMed] [Google Scholar]
- 20.Ancel P, Goffinet F, and the EPIPAGE-2 Writing Group. Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in france in 2011: Results of the epipage-2 cohort study. JAMA Pediatr. 2015 [DOI] [PubMed] [Google Scholar]
- 21.Zeitlin J, El Ayoubi M, Jarreau P-H, Draper ES, Blondel B, Künzel W, et al. Impact of fetal growth restriction on mortality and morbidity in a very preterm birth cohort. J Pediatr. 2010;157: 733–739.e1. 10.1016/j.jpeds.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 22.Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants—2010 update. Neonatology. 2010;97: 402–417. 10.1159/000297773 [DOI] [PubMed] [Google Scholar]
- 23.Halliday HL, Ehrenkranz RA, Doyle LW. Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2010; CD001146 10.1002/14651858.CD001146.pub3 [DOI] [PubMed] [Google Scholar]
- 24.Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants—2013 update. Neonatology. 2013;103: 353–368. 10.1159/000349928 [DOI] [PubMed] [Google Scholar]
- 25.Sackett DL, Rosenberg WMC, Gray JAM, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. 1996. Clin Orthop. 2007;455: 3–5. [PubMed] [Google Scholar]
- 26.SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network, Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362: 1970–1979. 10.1056/NEJMoa0911783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandri F, Plavka R, Ancora G, Simeoni U, Stranak Z, Martinelli S, et al. Prophylactic or early selective surfactant combined with nCPAP in very preterm infants. Pediatrics. 2010;125: e1402–1409. 10.1542/peds.2009-2131 [DOI] [PubMed] [Google Scholar]
- 28.Barrington KJ. The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs. BMC Pediatr. 2001;1: 1 10.1186/1471-2431-1-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarreau P-H, Fayon M, Baud O, Autret-Leca E, Danan M, de Verdelhan A, et al. Utilisation de la corticothérapie postnatale chez le nouveau-né prématuré dans la prévention et le traitement de la dysplasie bronchopulmonaire: état des lieux et conduite à tenir. Arch Pédiatrie. 2010;17: 1480–1487. [DOI] [PubMed] [Google Scholar]
- 30.Newborn C on F and. Postnatal Corticosteroids to Prevent or Treat Bronchopulmonary Dysplasia. Pediatrics. 2010;126: 800–808. 10.1542/peds.2010-1534 [DOI] [PubMed] [Google Scholar]
- 31.Kaempf JW, Campbell B, Sklar RS, Arduza C, Gallegos R, Zabari M, et al. Implementing potentially better practices to improve neonatal outcomes after reducing postnatal dexamethasone use in infants born between 501 and 1250 grams. Pediatrics. 2003;111: e534–e541. [PubMed] [Google Scholar]
- 32.Morton SC, Costlow MR, Graff JS, Dubois RW. Standards and Guidelines for Observational Studies: Quality is in the Eye of the Beholder. J Clin Epidemiol. 2015 [DOI] [PubMed] [Google Scholar]
- 33.Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. An Update on the Impact of Postnatal Systemic Corticosteroids on Mortality and Cerebral Palsy in Preterm Infants: Effect Modification by Risk of Bronchopulmonary Dysplasia. J Pediatr. 2014;165: 1258–1260. 10.1016/j.jpeds.2014.07.049 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We do not have IRB approval to share personal patient data outside of the European consortium. The EPICE project was approved by the French Advisory Committee on Use of Health Data in Medical Research (CCTIRS, N° 13.020 on 24/01/2013) and the French National Commission for Data Protection and Liberties (DR-2013-194, on 10/04/2013). Data will be made available upon request to all researchers who qualify for access to confidential data. The ethical and data protection approvals authorize the project to provide anonymized European data to members of each participating team after approval of each research study by the scientific committee and signature of data access and confidentiality agreements. The data sharing procedures are also regulated by the EPICE Consortium Agreement, which is the legal foundation for the EPICE scientific committee. For requests about the data, please contact Jennifer Zeitlin, Coordinator, EPICE study (Jennifer.zeitlin@inserm.fr).