Abstract
The major subunit isoform of GABAA receptors is α1β2γ2. The subunits are thought to surround an ion pore with the counterclockwise arrangement α1γ2β2α1β2 as seen from the outside of the neuron. These receptors have two agonist sites and one high affinity drug binding site specific for benzodiazepines. Recently, this receptor was postulated to assume alternative subunit stoichiometries and arrangements resulting in only one agonist site and one or even two sites for benzodiazepines. In order to force a defined subunit arrangement we expressed a combination of triple and dual concatenated subunits. Here we report that these unconventional receptors express only small current amplitudes in Xenopus oocytes. We determined agonist properties and modulation by diazepam of two of these receptors that resulted in currents large enough for a characterization, that is, β2-α1-γ2/α1-γ2 and β2-α1-γ2/β2-γ2. The first pentamer predicted to have two benzodiazepine binding sites shows similar response to diazepam as the standard receptor. As expected for both receptors with a single predicted agonist site the concentration response curves for GABA were characterized by a Hill coefficient < 1. β2-α1-γ2/β2-γ2 displayed a mM apparent GABA affinity for channel opening instead of the expected μM affinity. Based on their subunit and binding site stoichiometry, that contradicts all previous observations, their unusual functional properties and their very low expression levels in oocytes, we consider it unlikely that these unconventional receptors are expressed in neurons to an appreciable extent.
Introduction
GABAA receptors are the major inhibitory neurotransmitter receptors in mammalian brain [1–3]. The major isoform of this receptor is composed of α1, β2 and γ2 subunits. The consensus subunit stoichiometry is 2α1, 2β2 and 1γ2 [4,5], arranged α1γ2β2α1β2 counterclockwise as seen from the synaptic cleft [6–9]. α1β2γ2 receptors have one benzodiazepine binding site and two GABA sites [10], located at the α1/γ2 and β2/α1 subunit interfaces, respectively in a homologous position [3]. The presence of more than one agonist site for GABA is also predicted from their GABA concentration dependence that are characterized by a Hill coefficient of >1 (e.g. [8]). These α1β2γ2 receptors, unlike many other isoform containing δ or ε subunits [11–15], are thought to have a well-defined subunit arrangement. Subunit stoichiometry and arrangement are important as they govern functional properties [16]. Recently, unconventional subunit arrangements of α1β2γ2 receptors have been proposed [17]. Some of these receptors do not conform to the above stoichiometry as they contain 2γ2 subunits. Furthermore, these receptors are predicted to have one agonist site only and some of them two benzodiazepine sites. Functional properties of these novel receptors, such as EC50 and corresponding Hill coefficient for the agonist GABA and modulation by diazepam were not analyzed. We set out to determine these properties. While receptors conforming the conventional subunit arrangement could easily expressed in Xenopus oocytes, the receptors with unconventional arrangement form rather inefficiently, making their analysis difficult. Nevertheless, we describe the properties of some of them, but think that if these receptors are formed at all, they represent only a very minor part of α1β2γ2 GABAA receptors.
Materials and Methods
Expression of GABAA receptors in xenopus oocytes
Capped cRNAs were synthesized (Ambion, Austin, TX, USA) from the linearized plasmids with a cytomegalovirus promotor (pCMV vectors) containing the different subunits, respectively. A poly-A tail of about 400 residues was added to each transcript using yeast poly-A polymerase (United States Biologicals, Cleveland, OH, USA). The concentration of the cRNA was quantified on a formaldehyde gel using Radiant Red stain (Bio-Rad) for visualization of the RNA. Known concentrations of RNA ladder (Invitrogen) were loaded as standard on the same gel. cRNAs were precipitated in ethanol/isoamylalcohol 19:1, the dried pellet dissolved in water and stored at −80°C. cRNA mixtures were prepared from these stock solutions and stored at −80°C.
Animal experiments were carried out in strict accordance to the Swiss ethical guidelines, and have been approved by the local committee of the Canton Bern Kantonstierarzt, Kantonaler Veterinärdienst Bern (BE85/15). Surgery of female adult Xenopus laevis was done under anesthesia (0.2% tricaine solution). Oocytes were prepared, injected and defolliculated as described previously [18]. cRNA coding for each dual and triple subunit concatemer was injected either alone or in different combinations in oocytes. Oocytes were injected with 50 nl RNA solution containing RNA. In the case of non-concatenated α1β2γ2 receptors, cRNA coding for α1, β2 and γ2 subunits were injected at a ratio of 0.5:0.5:2.5 fMol/oocyte. In case of concatenated receptors, oocytes were injected with cRNA coding for dual and triple subunits at 1.25 fMol each. Injected oocytes were incubated in modified Barth’s solution at 18°C for at least 24 h before the measurements.
Functional characterization of the GABAA receptors
We used a two-electrode voltage clamp amplifier in combination with a XY-recorder (90% response time 0.1 s) or digitized at 100 Hz using a PowerLab 2/20 using the computer programs Chart (ADInstruments–Europe, Oxford, England). Electrodes were filled with 3 M KCl and had resistances of 0.5–0.8 MΩ. Tests with a model oocyte were performed to ensure linearity in the larger current range. The response was linear up to 15 μA. The holding membrane potential was −80 mV. The perfusion medium contained 90 mM NaCl, 1 mM KCl, 1 mM MgCl2, 1 mM CaCl2, and 5 mM Na-HEPES (pH 7.4). Concentration response curves for the compounds were fitted with the equation I(c) = Imax/[1 + (EC50/c)n], where c is the concentration of the compound, EC50 the concentration eliciting half-maximal current amplitude, Imax is the maximal current amplitude, I the current amplitude, and n is the Hill coefficient. Maximal current amplitudes (Imax) were obtained from the fits of the concentration-response curves. For all receptors studied, modulation was measured at a GABA concentration eliciting 1–3% of the maximal GABA current amplitude. GABA was applied twice alone, and 30 s application in combination with the different compounds. The duration of washout periods was 3 min in between agonist or agonist/drug aplications to prevent receptor desensitization. At the beginning of the experiments, GABA applications were repeated when the elicited current amplitude altered by >5%. Potentiation was calculated by the following equation: (IModulator + GABA/IGABA− 1) * 100%. The perfusion solution was applied through a glass capillary with an inner diameter of 1.35 mm, the mouth of which was placed about 0.4 mm from the surface of the oocyte. This allowed fast changes in agonist concentration around the oocyte. The rate of change was estimated 70% in less than 0.5 s. The perfusion system was cleaned between drug applications by washing with DMSO to avoid contamination.
All data are from as a minimum of three different oocytes from at least two different batches of oocytes. Data represent mean ± SEM or SD as indicated.
Results and Discussion
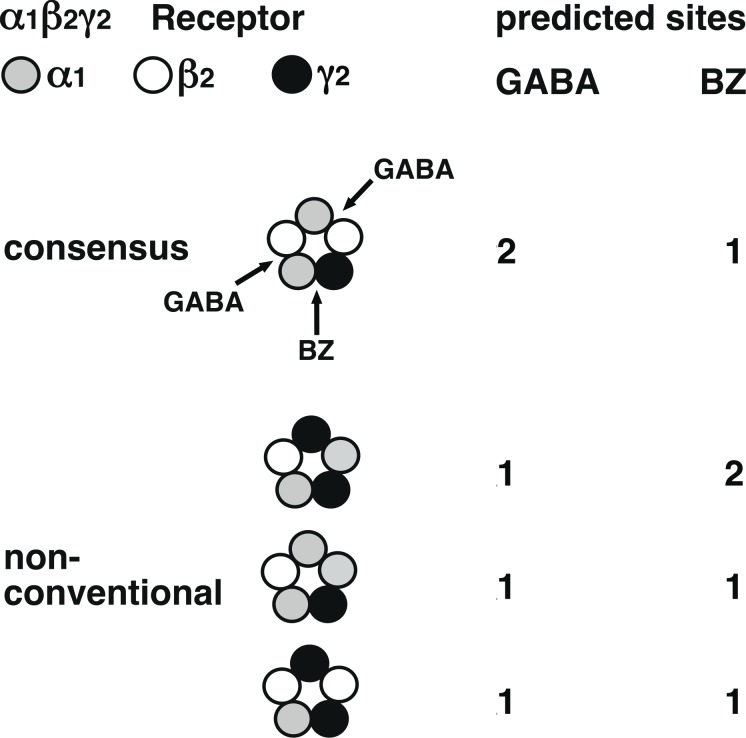
The subunit arrangement of the α1β2γ2 GABAA receptor has been derived from subunit interface studies [6] and from work with concatenated subunits [7–9]. Fig 1 shows a this arrangement characterized by the presence of two β2/α1 subunit interfaces each harboring agonist sites for GABA and one α1/γ2 subunit interface harboring a benzodiazepine binding site. This is in agreement with the observation that receptors purified from bovine brain have more than one GABA site per benzodiazepine binding site [10]. GABA concentration response curves obtained for α1β2γ2 GABAA receptors also indicate the presence of more than one agonist site (e.g. [19]), as their Hill coefficient is > 1. In addition to the consensus receptor, Fig 1 shows three unconventional receptors [17] with one predicted agonist site each, two of them with one, one of them with two predicted benzodiazepine binding sites. All these unconventional receptors do not conform to the well-established subunit stoichiometry of 2:2:1 for α1, β2 and γ2 subunits [4,5]. We attempted to characterize the functional properties of these unconventional receptors.
Fig 1. Consensus subunit arrangement of α1β2γ2 GABAA receptors and three newly proposed receptors with unconventional subunit arrangement.
Number of predicted agonist sites for GABA and modulatory sites for benzodiazepines (BZ) is shown for each receptor form. On the top left is the color code for the subunits.
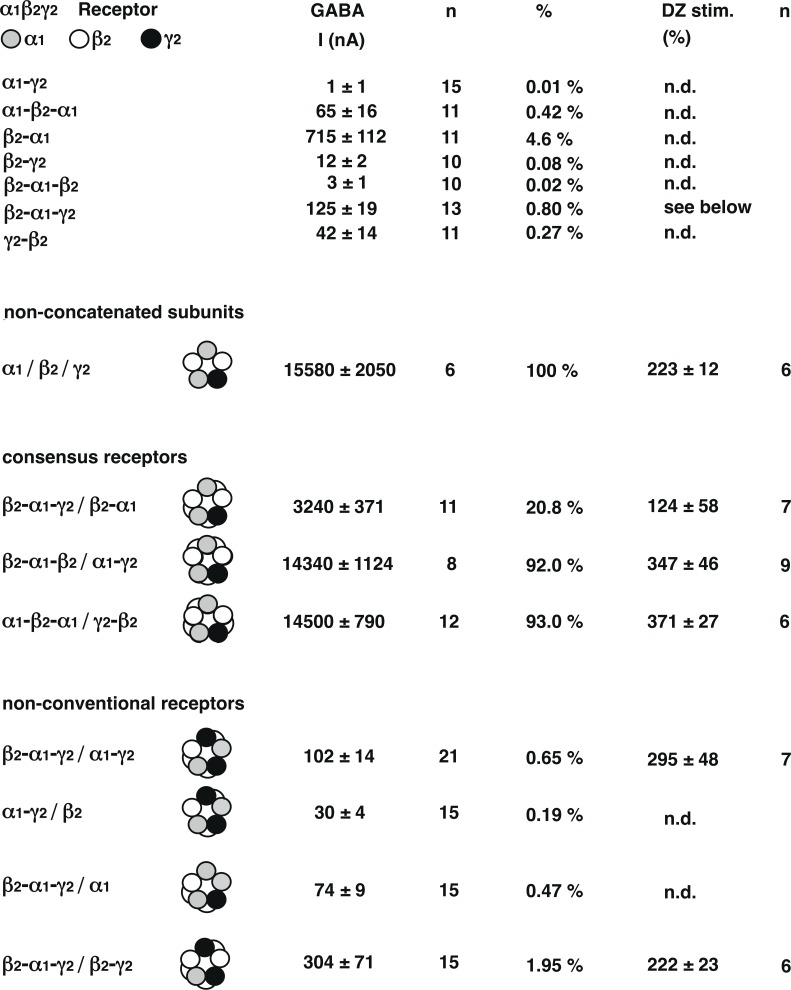
Non-concatenated α1β2γ2 GABAA receptors were expressed in Xenopus oocytes. Current amplitudes elicited by 1 mM GABA were determined as about 15 μA. This current amplitude was compared with those resulting from three concatenated dual subunit constructs and three triple subunit constructs used later as part of pentameric receptors (Fig 2). While α1-γ2, β2-γ2, γ2-β2, α1-β2-α1 and β2-α1-β2 all resulted in the relative expression of less than 0.5% of the above current amplitude, β2-α1 and β2-α1-γ2 resulted in 4.6% and 0.8%, respectively. This extent of current expression from single concatenated subunits was not observed in previous work [7]. The reason for the discrepancy is not clear.
Fig 2. Dual and triple concatenated constructs used for the construction of pentameric receptors were expressed in Xenopus oocytes.
Currents were standardized to the amplitude elicited in oocytes expressing non-concatenated α1, β2 and γ2 subunits (non-concatenated receptors). The same parameters are shown for receptors with consensus subunit arrangement (consensus receptors) that were built in three different ways and for three receptors with non-consensus subunit arrangement (non-consensus receptors), one of them built in two different ways. For some receptors, stimulation by 1 μM diazepam is also shown.
Non-concatenated receptors were also compared with three concatenated receptors with the consensus subunit arrangement and with four concatenated receptors with unusual subunit arrangement (Fig 2). Data on β2-α1-γ2/β2-α1 should be taken with care as the individual concatenated subunits result in current expression. All receptors with unusual subunit arrangement formed very inefficiently with expression levels well below 1%, with the exception of β2-α1-γ2/β2-γ2 with 1.95% (Fig 2). For two of these receptors, namely β2-α1-γ2/α1 and α1-γ2/β2, currents were too small for a further analysis. The observation that β2-α1-γ2/α1 fails to express has been made before [7]. In control experiments, β2-α1-γ2/α1 was expressed at a RNA ratio of 1:2. The current amplitude elicited by 1 mM GABA amounted to 108 ± 13 nA (mean ± SEM, n = 5). This is not significantly different from the same receptors expressed at an 1:1 ratio (Fig 2).
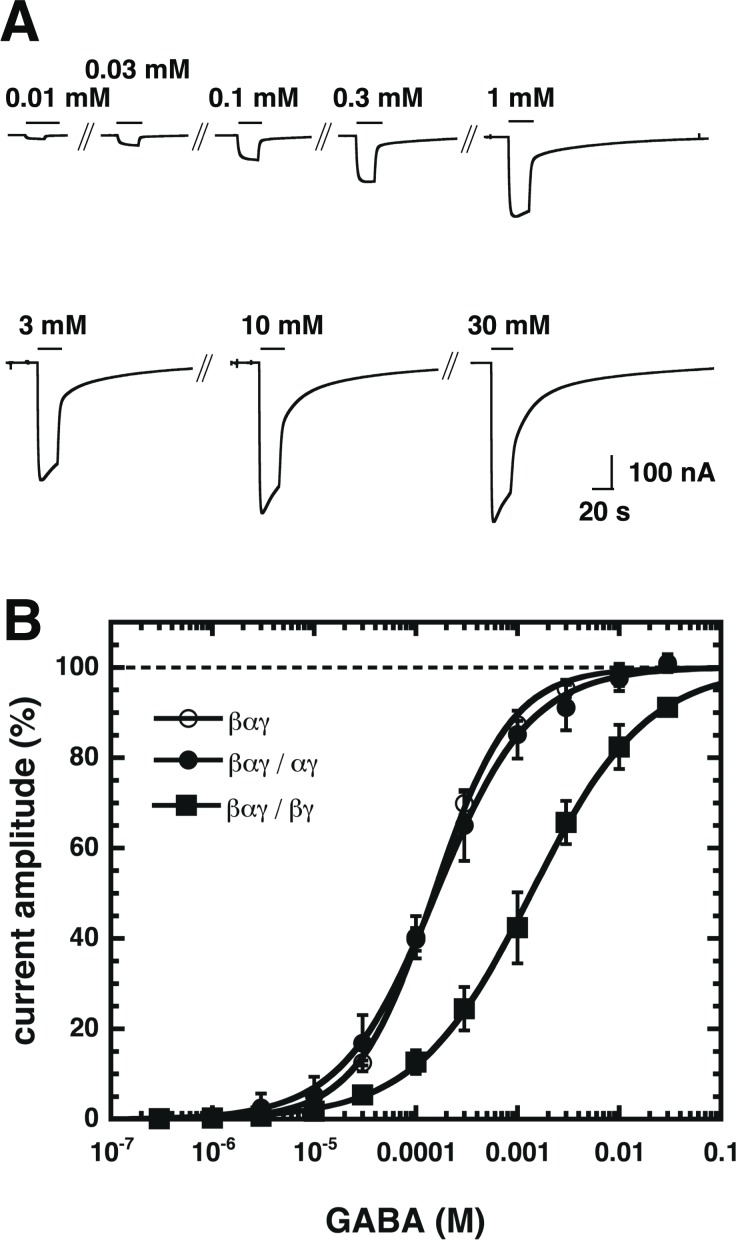
Agonist concentration-response curves were created for the non-conventional β2-α1-γ2/α1-γ2 and β2-α1-γ2/β2-γ2 receptors and for the triple construct β2-α1-γ2. Both pentameric receptors are predicted to have only one agonist site (Fig 1). Fig 3A shows original current traces for β2-α1-γ2/β2-γ2. After wash-out of GABA the current decreased in a bi-phasic manner. This unusual kinetic behavior was not observed with β2-α1-γ2 and β2-α1-γ2/α1-γ2. Fig 3B shows averaged concentration-response curves for β2-α1-γ2, β2-α1-γ2/α1-γ2 and β2-α1-γ2/β2-γ2 receptors. EC50s and Hill coefficients characterizing the corresponding curves are shown in Table 1. Parameters describing the concentration response curve of β2-α1-γ2/α1-γ2 indicate efficient isomerization to the open state of the singly ligated receptor. The concentration dependence of β2-α1-γ2/β2-γ2 receptors differs strongly from receptors built from non-concatenated α1, β2 and γ2 subunits with an EC50 of about 40 μM and a Hill coefficient of about 1.4 [8]. This indicates that little if any β2-α1-γ2/β2-γ2 receptors are built from non-concatenated α1, β2 and γ2 subunits.
Fig 3. Concentration-response curves for β2-α1-γ2, β2-α1-γ2/α1-γ2 and β2-α1-γ2/β2-γ2 receptors.
Receptors were exposed to subsequently higher concentrations of GABA and the elicited current amplitude was determined. Individual curves were first normalized to the fitted maximal current amplitude and subsequently averaged. Data are expressed as mean ± S.D., n = 3–5 from two batches of oocytes. A) Original current traces recorded in an oocyte expressing β2-α1-γ2/β2-γ2. B) Averaged Concentration-response curves for β2-α1-γ2, β2-α1-γ2/α1-γ2 and β2-α1-γ2/β2-γ2.
Table 1. Properties of GABA concentration response curves.
| receptor | EC50 (μM) | Hill coeff. | n |
|---|---|---|---|
| β2-α1-γ2 | 142 ± 18 | 1.14 ± 0.03 | 3 |
| β2-α1-γ2/α1-γ2 | 163 ± 35 | 0.97 ± 0.23 | 5 |
| β2-α1-γ2/β2-γ2 | 1420 ± 530 | 0.78 ± 0.02 | 3 |
EC50s are given as mean ± SD.
The fact that amplitude and agonist dependent properties of currents resulting from β2-α1-γ2/α1-γ2 are similar to the ones resulting from β2-α1-γ2 could suggest that two copies of β2-α1-γ2 form a pentameric receptor with one β2 hanging out, such that the pentamer would be β2-α1-γ2/α1-γ2. However, the Hill coefficient characterizing the concentration response curve for GABA at β2-α1-γ2 indicates that at least in some receptors the γ2 subunit must hang out, to allow formation of a second agonist site.
Modulation of the currents by 1 μM diazepam was determined. As reported before [8] concatenated receptors with consensus subunit arrangement showed a larger stimulation than non-concatenated receptors (Fig 2). Presumably, injection of α1, β2 and γ2 subunits even at a ratio of 1:1:5 results in diazepam responsive α1β2γ2 receptors and diazepam non-responsive α1β2 receptors. As expected, receptors composed of concatenated β2-α1 and β2-α1-γ2 showed reduced extent of modulation, as the current may partly be produced by the individual concatenated constructs, specifically by β2-α1.
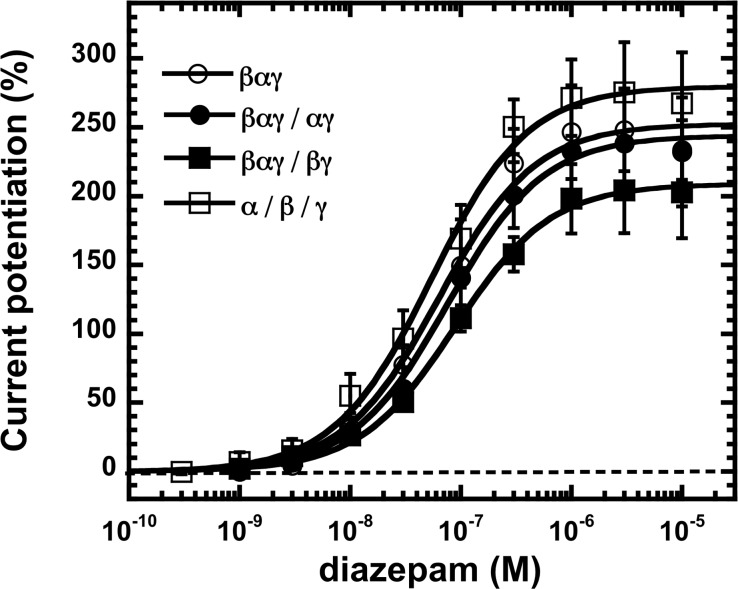
Effects of diazepam at the non-conventional β2-α1-γ2/α1-γ2, α1-γ2/β2 and β2-α1-γ2/β2-γ2 receptors were also determined. The first two receptors are predicted to have two sites for benzodiazepines. 1 μM diazepam by itself did not elicit any currents in β2-α1-γ2/α1-γ2 receptors (n = 5) and α1-γ2/β2 receptors (n = 3). Modulation of GABA current by the same concentration of diazepam in β2-α1-γ2/α1-γ2 receptors was comparable to that in concatenated consensus receptors, in spite of the predicted presence of two benzodiazepine binding sites in a receptor. In β2-α1-γ2/β2-γ2 receptors extent of modulation was slightly lower (Fig 2). Fig 4 shows averaged diazepam concentration-response curves from oocytes expressing β2-α1-γ2, β2-α1-γ2/α1-γ2, β2-α1-γ2/β2-γ2 or α1/β2/γ2 receptors. The curves were characterized by EC50s of 65 ± 9 nM (mean ± SD, n = 3), 77 ± 20 nM (mean ± SD, n = 3), 88 ± 18 nM (mean ± SD, n = 3) and 59 ± 13 nM (mean ± SD, n = 3), respectively. Maximal current stimulation was similar for all constructs varying between 209 ± 31% for β2-α1-γ2/ β2-γ2 and 285 ± 29% for α1/β2/γ2. The fact that the curve for β2-α1-γ2/α1-γ2 looks similar to that for α1/β2/γ2 [8] indicates that the two predicted sites show no cooperativity or additivity of modulatory effects.
Fig 4. Stimulation of β2-α1-γ2, β2-α1-γ2/α1-γ2, β2-α1-γ2/β2-γ2 and α1/β2/γ2 receptors by diazepam.
Receptors were exposed to subsequently higher concentrations of diazepam in combination with an EC2 GABA concentration and the elicited current amplitude was determined. Individual curves were first normalized to the fitted maximal current amplitude and subsequently averaged. Data are expressed as mean ± S.D., n = 3 from two batches of oocytes.
Concerning receptor subunit stoichiometry, the subset of GABAA receptors containing binding site for benzodiazepines, mainly α1βxγ2, α2βxγ2, α3βxγ2 and α5βxγ2, purified from bovine brain using a benzodiazepine affinity column [10] show a stoichiometry of agonist sites to benzodiazepine binding sites of 1.2–2.4. The stoichiometry of non-conventional GABAA receptors β2α1γ2α1α1, β2α1γ2α1γ2 and β2α1γ2β2γ2 would be predicted to be 1:1, 0.5:1 and 1:1, respectively [17] and that of conventional GABAA receptors 2:1. The subunit stoichiometries of non-conventional GABAA receptors clearly do not agree to the reported stoichiometry.
In summary, the non-conventional α1β2γ2 GABAA receptors are not in agreement with literature data on subunit and binding site stoichiometry and functional properties. They show low expression levels in Xenopus oocytes. Functional properties, e.g very low GABA affinity for β2-α1-γ2/β2-γ2 channel gating and low Hill coefficient for β2-α1-γ2/α1-γ2 differ from those of non-concatenated α1β2γ2 GABAA receptors expressed in various expression systems. This together makes it very unlikely that non-conventional GABAA receptors of the subunit arrangement β2α1γ2α1α1, β2α1γ2α1γ2 and β2α1γ2β2γ2 are being formed to any appreciable extent. Non-concatenated α1, β2 and γ2 subunits as previously concluded most likely assemble to β2α1γ2β2α1 GABAA receptors.
Acknowledgments
We thank Dr. V. Niggli for useful comments on the manuscript.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This work was supported by the Swiss National Science Foundation, grant 315230_156929/1 (http://www.snf.ch). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994; 17: 569–602. 10.1146/annurev.ne.17.030194.003033 [DOI] [PubMed] [Google Scholar]
- 2.Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008; 60: 243–260. 10.1124/pr.108.00505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sigel E, Steinmann ME. Structure, function, and modulation of GABAA receptors. J Biol Chem. 2012; 287: 40224–40231. 10.1074/jbc.R112.386664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang Y, Wang R, Barot S, Weiss DS. Stoichiometry of a recombinant GABAA receptor. J Neurosci. 1996; 16: 5415–5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farrar SJ, Whiting PJ, Bonnert TP, McKernan RM. Stoichiometry of a ligand-gated ion channel determined by fluorescence energy transfer. J Biol Chem. 1999; 274: 10100–10104. [DOI] [PubMed] [Google Scholar]
- 6.Tretter V, Ehya N, Fuchs K, Sieghart W. Stoichiometry and assembly of a recombinant GABAA receptor subtype. J Neurosci. 1997; 17: 2728–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumann SW, Baur R, Sigel E. Subunit arrangement of γ-aminobutyric acid type A receptors. J Biol Chem. 2001; 276: 36275–36280. 10.1074/jbc.M105240200 [DOI] [PubMed] [Google Scholar]
- 8.Baumann SW, Baur R, Sigel E. Forced subunit assembly in α1β2γ2 GABAA receptors. Insight into the absolute arrangement. J Biol Chem. 2002; 277: 46020–46025 10.1074/jbc.M207663200 [DOI] [PubMed] [Google Scholar]
- 9.Baur R, Minier F, Sigel E. A GABAA receptor of defined subunit composition and positioning: concatenation of five subunits. FEBS Letters. 2006; 580: 1616–1620. 10.1016/j.febslet.2006.02.002 [DOI] [PubMed] [Google Scholar]
- 10.Sigel E, Barnard EA. A γ-aminobutyric acid/benzodiazepine receptor complex from bovine cerebral cortex. Improved purification with preservation of regulatory sites and their interactions. J Biol Chem. 1984; 259: 7219–7223. [PubMed] [Google Scholar]
- 11.Baur R, Kaur KH, Sigel E. Structure of α6β3δ GABAA receptors and their lack of ethanol sensitivity. J Neurochem. 2009; 111:1172–1181. 10.1111/j.1471-4159.2009.06387.x [DOI] [PubMed] [Google Scholar]
- 12.Kaur KH, Baur R, Sigel E. Unanticipated structural and functional properties of δ subunit-containing GABAA receptors. J Biol Chem. 2009; 284: 7889–7896. 10.1074/jbc.M806484200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baur R, Kaur KH, Sigel E. Diversity of structure and function of α1α6β3δ GABAA receptors: comparison with α1β3δ and α6β3δ receptors. J Biol Chem. 2010; 285: 17398–17405. 10.1074/jbc.M110.108670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bollan KA, Baur R, Hales TG, Sigel E, Connolly CN. The promiscuous role of the ε subunit in GABAA receptor biogenesis. Mol Cell Neurosci. 2008; 37: 610–621. 10.1016/j.mcn.2007.12.011 [DOI] [PubMed] [Google Scholar]
- 15.Wongsamitkul N, Baur R, Sigel E. Towards understanding functional properties and subunit arrangement of α4β2δ GABAA receptors. J Biol Chem. 2016; 291: 18474–18483. 10.1074/jbc.M116.738906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minier F, Sigel E. Positioning of the α-subunit isoforms confers a functional signature to γ-aminobutyric acid type A receptors. Proc Natl Acad Sci USA. 2004; 101: 7769–7774. 10.1073/pnas.0400220101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Botzolakis EJ, Gurba KN, Lagrange AH, Feng HJ, Stanic AK, Hu N, et al. Comparison of γ-Aminobutyric Acid, Type A (GABAA), Receptor αβγ and αβδβExpression Using Flow Cytometry and Electrophysiology: Evidence for alternative subunit stoichiometries and arrangements. J Biol Chem. 2016; 291: 20440–20461. 10.1074/jbc.M115.698860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sigel E and Minier F. Educational paper: The Xenopus oocyte: System for the study of functional expression and modulation of proteins. Mol Nutr Food Res. 2005; 49: 228–234. 10.1002/mnfr.200400104 [DOI] [PubMed] [Google Scholar]
- 19.Teissére JA, Czajkowski C. A (beta)-strand in the (gamma)2 subunit lines the benzodiazepine binding site of the GABA A receptor: structural rearrangements detected during channel gating. J Neurosci. 2001; 21: 4977–4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.