Abstract
Background/Aims
Acerola (Malpighia emarginata DC.) is a fruit that is known to contain high amounts of ascorbic acid (AA) and various phytochemicals. We have previously reported that AA deficiency leads to ultraviolet B (UVB)-induced skin pigmentation in senescence marker protein 30 (SMP30)/gluconolactonase (GNL) knockout (KO) hairless mice. The present study was undertaken to investigate the effects of acerola juice (AJ) intake on the skin of UVB-irradiated SMP30/GNL KO mice.
Research design/Principal findings
Five-week old hairless mice were given drinking water containing physiologically sufficient AA (1.5 g/L) [AA (+)], no AA [AA (-)] or 1.67% acerola juice [AJ]. All mice were exposed to UVB irradiation for 6 weeks. UVB irradiation was performed three times per week. The dorsal skin color and stratum corneum water content were measured every weekly, and finally, the AA contents of the skin was determined. The skin AA and stratum corneum water content was similar between the AA (+) and AJ groups. The L* value of the AA (+) group was significantly decreased by UVB irradiation, whereas AJ intake suppressed the decrease in the L* value throughout the experiment. Moreover, in the AJ group, there was a significant decrease in the expression level of dopachrome tautomerase, an enzyme that is involved in melanin biosynthesis.
Conclusion
These results indicate that AJ intake is effective in suppressing UVB-induced skin pigmentation by inhibiting melanogenesis-related genes.
Introduction
Senescence marker protein-30 (SMP30) was originally identified as a 34-kDa protein whose expression in the liver decreases with aging in an androgen-independent manner [1]. Previously, we found that SMP30 acts as a gluconolactonase (GNL) (EC 3.1.1.17) and is responsible for the conversion of L-gulonic acid into L-gulono-γ-lactone during the penultimate step of ascorbic acid (AA) synthesis [2]. Additionally, we demonstrated that SMP30/GNL knockout (KO) mice are unable to synthesize AA in vivo and develop scurvy when fed an AA-deficient diet [2]. Because humans cannot synthesize AA due to numerous mutations in the gene for L-gulono-γ-lactone oxidase, which is the terminal enzyme of the AA synthetic pathway, SMP30/GNL KO mice have been utilized to investigate the role of AA in vivo [3]. Thus far, we have reported that a complete lack of AA leads to an increase in superoxide generation in the brain [4, 5], an increase in protein oxidation in the liver [6, 7], the development of pulmonary emphysema [8–10], and decreases in the noradrenaline and adrenaline contents of the adrenal glands [11]. In this manner, we have revealed the physiological functions of AA by utilizing SMP30/GNL KO mice as an animal model. Furthermore, to clarify the pivotal role of AA in the skin, we developed and established SMP30/GNL KO hairless mice by mating SMP30/GNL KO mice with hairless mice [12]. The characteristic features of the SMP30/GNL KO hairless mice are as follows: an inability to synthesize AA, hairlessness, and the ability to undergo normal melanogenesis. We have reported that AA-deficient SMP30/GNL KO hairless mice with chronic UVB irradiation exhibit suppressed epidermal hyperplasia and develop excessive skin pigmentation [12]. Because SMP30/GNL KO hairless mice derive AA primarily through dietary intake, SMP30/GNL KO hairless mice can be valuable for evaluating not only AA but also the functional foods that promote AA in the skin.
Acerola (Malpighia emarginata DC.) is a fruit that is found throughout Central America and within the northern part of South America. Because acerola has high concentrations of AA in the edible part, it is considered to be one of the best sources of AA [13]. Additionally, acerola contains numerous functional phytochemicals, such as carotenoids and polyphenols [14–16]. Previously, we tested for various polyphenols in acerola and found that cyanidin-3-α-O-rhamnoside, pelargonidin-3-α-O-rhamnoside, quercetin-3-α-O-rhamnoside, kaempferol glycosides, astilbin, and proanthocyanidin are present [14, 15, 17]. In addition to polyphenols, we have reported on the contents of various nutritional components such as glucose, fructose, malic acid and several amino acids in the edible part of acerola [17]. The contents of glucose, fructose, malic acid, total polyphenols and total amino acids in acerola was 15 to 16, 14 to 16, 4 to 5, 1.2 to 1.5, and 1.8 to 2.1 g/kg edible portion, respectively [17]. It has been demonstrated that acerola intake exerts protective effects against DNA damage generated by FeSO4 [18], inhibits lung tumorigenesis induced by nicotine-derived nitrosamine ketone [19], regulates blood glucose levels and exerts anti-inflammatory effects in obese mice [20, 21]. Similarly, we have demonstrated a beneficial effect of acerola in humans; i.e., the intake of acerola juice (AJ) affects the absorption of AA in plasma and minimizes its excretion via urine in healthy Japanese subjects, which suggests improvement of the bioavailability of AA in humans [22]. In the skin, we have previously reported that the intake of acerola polyphenol extracts prevents UVB-induced skin pigmentation in brownish guinea pigs, which possess moderate numbers of melanocytes and melanosomes in the epidermis [23]. Because acerola contains not only a large amount of AA but also beneficial ingredients as noted above, it is important to elucidate the effect of whole juice intake in UVB-irradiated skin. Therefore, in this study, we focused on the effects of AJ intake on UVB-induced skin damage in SMP30/GNL KO hairless mice.
Materials and Methods
Preparation of acerola juice
AJ was prepared from concentrated mature clear juice, which was purchased from Nichirei do Brazil (Recife, Brazil). The concentrated clear juice was diluted with a hydrochloric acid solution and then filtered (No. 5C, Toyo Advantec Co., Tokyo, Japan). The prepared AJ was immediately frozen and stored at—80°C until use.
Animals
The animal experiments were performed in accordance with the animal care and use protocol approved by the Institutional Animal Care and Use Committee of Tokyo Metropolitan Institute of Gerontology (TMIG) and in accordance with the Guidelines for the Care and Use of Laboratory Animals of TMIG.
SMP30/GNL KO hairless mice were established as previously described [12]. Briefly, SMP30/GNL KO hairless mice were generated by mating SMP30/GNL KO mice (C57BL/6 background) with hairless mice (Hos:HR-1, Hoshino Laboratory Animals, Ibaraki, Japan). A minimum of three generations of this strain were interbred for the present study. After weaning at 5 weeks old, male SMP30/GNL KO hairless mice were divided into the following three groups: AA deficient [AA (-)], AA supplemented [AA (+)], and AJ supplemented [AJ]. The AA (+) group had free access to water containing 1.5 g/L AA and 10 μM EDTA, whereas the AA (-) group was given water without AA. The AJ group was given 1.67% acerola juice diluted with the same drinking water that was given to the AA (-) group. The diluted AJ contained 1.5 g/L AA. The concentration of AA in the drinking water was sufficient to maintain normal AA levels in many tissues [24, 25]. Throughout the experiment, the drinking water was changed every 3 or 4 days. All mice were fed an AA-deficient diet (CL-2, CLEA Japan, Tokyo, Japan), and a 12-h light/dark cycle was maintained in a controlled environment.
UVB irradiation of SMP30/GNL KO hairless mice
UVB irradiation was performed as previously described [12, 26]. SMP30/GNL KO hairless mice were irradiated with 94 mJ/cm2 of UVB three times per week for 6 weeks under three UVB lamps (GL20SE, Sankyo, Kanagawa, Japan). The irradiance of the UVB lamps was measured using a UV radiometer (UV-340, Custom, Tokyo, Japan). The UVB lamps emit a continuous light spectrum between 280–380 nm with a peak emission at 306 nm. At the end of the six-week experimental period, the mice were euthanized by cervical dislocation, and their skin was subsequently collected and stored at -80°C until use.
Measurements of dorsal skin color and stratum corneum water content
During the experiment, the dorsal skin color and stratum corneum water content were measured every week for 6 weeks. The degree of dorsal skin color was quantified using a portable reflectance colorimeter in the CIE-L*, a*, b* system (Minolta CR-200 colorimeter, Konica Minolta, Tokyo, Japan) by measuring the reflected object color. The results are expressed as an L* value, which is a measurement of skin lightness on a continuous black to white scale, where 100 is completely white and 0 is completely black. The stratum corneum water content, as assessed indirectly by high-frequency superficial skin conductance, was measured using a Skicon-200 (IBS Co., Hamamatsu, Japan).
Measurement of AA and DHA contents in the skin
The skin levels of AA and dehydroascorbic acid (DHA), which is an oxidized form of AA, were measured using HPLC and electrochemical detection (ECD), as previously reported [12, 27]. Briefly, the skin was homogenized with 14 volumes of ice-cold 5.4% metaphosphoric acid (MPA) containing 1 mM EDTA (MPA/EDTA) solution and centrifuged at 21,000 x g for 10 min at 4°C. The total AA (AA + DHA) and AA contents in the centrifugal supernatants were determined individually as follows, and the DHA content was calculated by subtracting the AA content from the total AA content. To determine of the total AA, the centrifugal supernatants were reduced with Tris(2-carboxyethyl)phosphine hydrochloride (TCEP) for 2 h on ice. After the reduction, the reaction mixture was diluted with 5% MPA/EDTA and analyzed for total AA by HPLC coupled with ECD. For the determination of AA, the non-reduced centrifugal supernatants were diluted with 5% MPA/EDTA and analyzed by HPLC coupled with ECD. Separation was achieved on an Atlantis dC18 5-μm column (4.6 × 150 mm) combined with an Atlantis dC18 5-μm guard column (4.6 × 20 mm) from Nihon Waters (Tokyo, Japan). The mobile phase consisted of 50 mM phosphate buffer (pH 2.8), 540 μM EDTA and 2% methanol. The flow rate was 1.3 mL/min. The temperatures for the column and Waters 2465 electrochemical detector were set at 30°C, and the temperature for the autosampler thermostat was set at 4°C. The sample was injected into the HPLC coupled with ECD for the AA analysis. Electrical signals were recorded using an electrochemical detector with a glassy carbon electrode at +0.6 V. All electrical signal data from the electrochemical detector were collected using Waters Empower2 software (Nihon Waters, Tokyo, Japan).
Histology
Dorsal skin tissues were fixed in a 10% formalin neutral buffer solution (Wako Pure Chemicals, Osaka, Japan), embedded in paraffin, and sectioned on a microtome at a thickness of 3 μm. For the hematoxylin and eosin (HE) staining, the sections were deparaffinized with xylene, rehydrated with a graded series of ethanol, and then subjected to HE staining. The epidermal thicknesses were determined using the Multi Gauge, version 3.0 (Fuji Photo Film, Tokyo, Japan).
Quantitative real-time RT-PCR analysis
Total RNA was extracted from the skin tissues using TRIzol reagent (Invitrogen, Karlsruhe, Germany) according to the manufacturer’s protocol. The total RNA (1 μg) was reverse transcribed at 37°C using PrimeScript™ RT Enzyme (Takara, Shiga, Japan). Real-time RT-PCR was performed using a real-time PCR detection system (Takara Bio, Madison, WI, USA), and expression of the following genes was detected in the AA (-), AA (+), and AJ groups: tyrosinase (Tyr), tyrosinase related protein-1 (Tyrp1), dopachrome tautomerase (Dct), Tnf-α, endothelin 1 (Edn1), and cyclin D1 (Ccnd1). The final mixture for RT-PCR consisted of 1× SYBR Premix Ex Taq Mix (Takara), 0.4 μM of primers (forward and reverse), and cDNA. The primer sequences are provided in Table 1. The PCR amplification consisted of 40 cycles (95°C for 5 s and 60°C for 30 s) after an initial denaturation step (95°C for 10 s). The mRNA expression levels were evaluated relative to the level of ribosomal protein, large, P1 (Rplp1), and the mRNA levels of the AA (+) group were designated as 1.0.
Table 1. Primer sets used for qPCR analysis.
| Target gene | Sequence | |
|---|---|---|
| Tyrosinase (Tyr) | Forward | 5'-CAAGTACAGGGATCGGCCAAC-3' |
| Reverse | 5'-GGTGCATTGGCTTCTGGGTAA-3' | |
| Tyrosinase-related protein 1 (Tyrp1) | Forward | 5'-TGATGCGGTCTTTGACGAATG-3' |
| Reverse | 5'-GTTGGTAACTGGAGGCCAGAATG-3' | |
| Dopachrome tautomerase (Dct) | Forward | 5'-AACCGCAGAGCAACTTGGCTAC-3' |
| Reverse | 5'-CTCCCAGGATTCCAATGACCAC-3' | |
| Tnf-α | Forward | 5'-TTGTTGCCTCCTCTTTTGCT-3' |
| Reverse | 5'-TGGTCACCAAATCAGCGTTA-3' | |
| Endothelin 1 (Edn1) | Forward | 5'-GTGTTCCCTAGCCTGTCTGC-3' |
| Reverse | 5'-TGGAATCTCCTGGCTCTCTG-3' | |
| Cyclin D (Ccnd1) | Forward | 5'-ATTTGCACACCTCTGGCTCT-3' |
| Reverse | 5'-TCACCTCTTCCCTCACATCC-3' | |
| Ribosomal protein, large, P1 (Rplp1) | Forward | 5’-TCCGAGCTCGCTTGCATCTA-3’ |
| Reverse | 5’-CAGATGAGGCTCCCAATGTTGA-3’ |
Statistical analysis
The probability of significant differences between the experimental groups was determined by one-way ANOVA followed by Tukey’s HSD post hoc comparisons. Dunnett's test was also used to assess the effects of UVB on the L* value. KaleidaGraph software was used (Synergy Software, Reading, PA, USA) to perform the one-way ANOVAs. Differences were considered significant at P values of <0.05.
Results
AA concentration in acerola juice
To adjust the AA concentration in the drinking water to 1.5 g/L, we first determined the total AA concentration in the AJ. As illustrated in Table 2, the total AA, AA, and DHA concentrations were 89.5 ± 1.5, 85.9 ± 2.2, and 3.6 ± 0.7 g/L, respectively. Because DHA can be reduced to AA by several enzymes, including glutaredoxin, protein disulfide isomerase, omega class glutathione transferase, 3α-hydroxysteroid dehydrogenase, and thioredoxin reductase [28], we used the total AA concentration in the AJ for the preparation of the drinking water for the AJ group. That is, the AJ group was given 1.67% AJ, which contained 1.5 g/L total AA.
Table 2. Total AA, AA, and DHA concentrations in acerola juice.
| Total AA (g/L) | AA (g/L) | DHA (g/L) | |
|---|---|---|---|
| Acerola juice | 89.5 ± 1.5 | 85.9 ± 2.2 | 3.6 ± 0.7 |
The data are presented as the mean ± SEM of three acerola juice samples.
Body weight in skin
To investigate the influence of AJ intake on the growth of the SMP30/GNL KO hairless mice, we measured the body weight changes of the three groups during the experiment. The body weights of the mice did not vary significantly among the three groups from the start date to the fifth week of the experimental period, but the body weight of the AA (-) group was significantly lower than that of the AJ group at 6 weeks (Table 3).
Table 3. Body weight change.
| AA (-) (g) | AA (+) (g) | AJ (g) | |
|---|---|---|---|
| Start date | 16.7 ± 0.8 | 16.7 ± 0.8 | 16.5 ± 1.2 |
| 1 w | 18.2 ± 1.3 | 16.3 ± 0.7 | 18.0 ± 0.8 |
| 2 w | 20.1 ± 0.9 | 18.0 ± 0.9 | 18.8 ± 0.7 |
| 3 w | 21.3 ± 0.8 | 19.4 ± 0.9 | 19.9 ± 0.7 |
| 4 w | 21.7 ± 0.8 | 20.7 ± 0.9 | 21.0 ± 0.5 |
| 5 w | 19.2 ± 0.9 | 20.3 ± 1.1 | 20.2 ± 0.6 |
| 6 w | 18.6 ± 1.0 | 21.3 ± 0.9 | 22.0 ± 0.6* |
The values are expressed as the means ± the SEMs of five animals. ANOVA analysis F2, 12 = 4.6, P < 0.05 for 6 weeks. Asterisk indicate significant comparisons (*P < 0.05) between the AA (-) and AJ groups by Tukey’s HSD post hoc comparisons.
AA content in skin
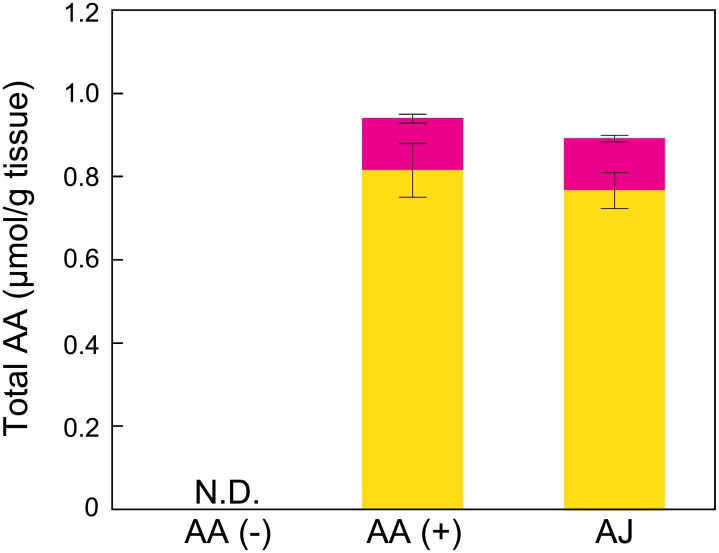
At the end of the experiment (6 weeks), the AA and DHA content in the skin of the AA (-) group was below the level of detection (Fig 1). In contrast, the AA and DHA content was not significantly different between the AA (+) and AJ groups. The amounts of water consumption in the AA (-), AA (+), and AJ groups were 4.9 ± 0.4, 4.1 ± 0.3, and 4.7 ± 0.3 mL/day/mouse, respectively. The consumption of total AA in the AA (+) and AJ groups were 6.2 ± 0.5, and 7.1 ± 0.5 mg/day/mouse, respectively. There was no significant difference in the amount of water ingested during the experiment. Moreover, there was no significant difference in the consumption of total AA between the AA (+) and AJ groups.
Fig 1. AA and DHA contents.
The AA content (yellow bar) was determined by HPLC coupled with ECD, as described in the Materials and Methods section. The total AA (total bar height) content was determined by the reduction of DHA with TCEP. The DHA content (red bar) was calculated by subtracting the AA content from the total AA content determined in a different chromatographic run. N.D. indicates not detected. The values are expressed as the means ± the SEMs of five animals. ANOVA analysis: F2, 12 = 104.3, P < 0.001 for the AA content and F2, 12 = 90.9, P < 0.001 for the DHA content. The error bars represent the SEMs for the DHA and AA. AA, ascorbic acid; AJ, acerola juice.
Histological skin analysis
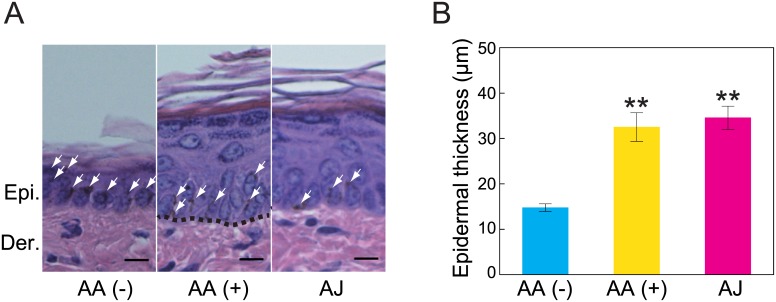
To investigate the effects of AJ intake on the histologically evaluated changes in the skin, we performed HE staining and measured the thicknesses of the epidermal skins of all the mice. Representative histological HE skin staining is provided in Fig 2A. In normal skin, chronic UVB irradiation induces epidermal hyperplasia, which is important for protecting the basal keratinocytes from subsequent DNA damage from UVB [29, 30]. As expected, epidermal hyperplasia was observed in the AA (+) and AJ groups (Fig 2A). The epidermal thicknesses were not significantly different between the AA (+) and AJ groups (Fig 2B). However, the AA (-) group did not manifest epidermal hyperplasia. Massive skin pigmentation was observed in the epidermis in the AA (-) group, but only slight pigmentation was observed in the AA (+) and AJ groups (Fig 2A). The epidermal thickness in the AA (-) group was significantly decreased compared with those of the other two groups (Fig 2B).
Fig 2. The effect of acerola juice intake on epidermal morphology and thickness.
(A) High magnifications of the epidermis from the skins of the AA (-), AA (+) and AJ groups are shown. The white arrows indicate the skin pigment. (B) The epidermal thicknesses in the HE-stained sections were measured as the distance between the top of the basement membrane and the bottom of the stratum corneum in five fields randomly selected from each animal. The values are expressed as the means ± the SEMs of five animals. ANOVA analysis: F2, 12 = 20.6, P < 0.001. The asterisk indicates a significant difference (**P < 0.01) compared with the AA (-) group by Tukey’s HSD post hoc comparisons. AA, ascorbic acid; AJ, acerola juice; Der., dermis; Epi., epidermis. Bar = 10 μm.
Stratum corneum water content
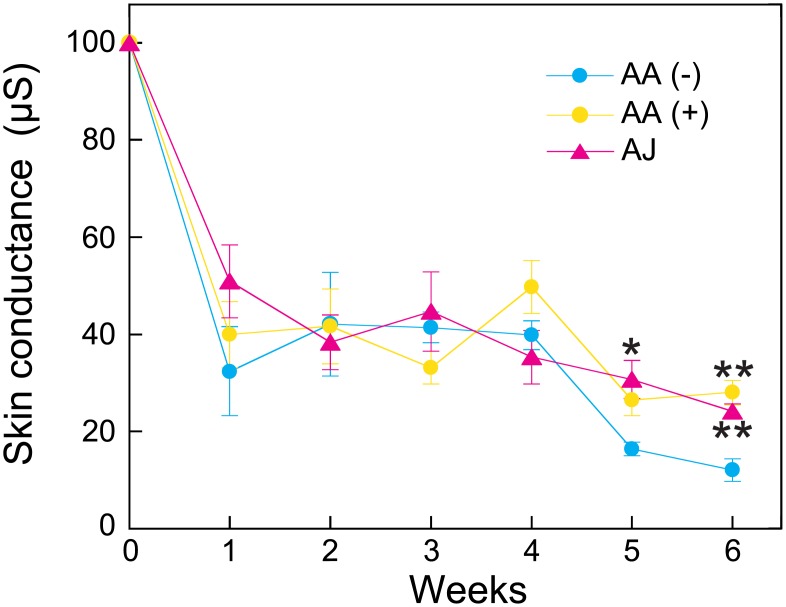
Because chronic UVB irradiation has been known to cause skin barrier dysfunction as well as decreases in the water content of the stratum corneum [31], we measured the water content of the stratum cornea of the three groups during the experiment. As illustrated in Fig 3, the stratum corneum water content decreased gradually in all groups after the initiation of UVB irradiation. The stratum corneum water content was not significantly different between the AA (+) and AJ groups during the experiment. However, the water content of the AJ group was significantly higher than that of the AA (-) group at 5 and 6 weeks. Moreover, the water content of the AA (+) group was significantly higher than that of the AA (-) group only at 6 weeks.
Fig 3. The effect of acerola juice intake on the stratum corneum water content.
The stratum corneum water content was measured every week for 6 weeks as described in the Materials and Methods. The values are expressed as the means ± the SEMs of five animals. ANOVA analysis: F2, 12 = 4.6, P < 0.05 at 5 weeks and F2, 12 = 25.2, P < 0.001 at 6 weeks. The asterisks indicate significant differences (*P < 0.05, **P < 0.01) compared with the AA (-) group by Tukey’s HSD post hoc comparisons. AA, ascorbic acid; AJ, acerola juice.
UVB-induced skin pigmentation
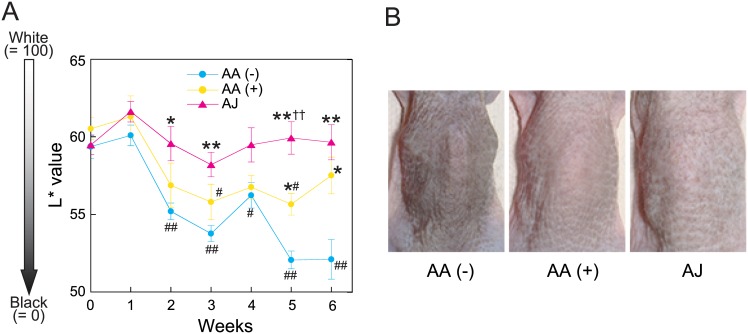
We next assessed the effects of AJ intake on the UVB-induced skin pigmentation of the SMP30/GNL KO hairless mice every week using a colorimeter. The L* values decreased gradually in the AA (-) and AA (+) group after the initiation of UVB irradiation (Fig 4A). Compared with the start day in the same group, significant differences were observed after 2 weeks of irradiation in the AA (-) group, whereas such differences were only observed at 3 and 5 weeks in the AA (+) group. Surprisingly, the L* value of the AJ group did not change significantly during the experiment compared with the value on the start day. The L* value of the AJ group was significantly higher than that of the AA (-) group after 2 weeks of irradiation, except at week 4. In contrast, the L* value of AA (+) group was significantly higher than that of the AA (-) group at weeks 5 and 6. Comparison between the AJ and AA (+) groups revealed that the L* value of the AJ group was higher throughout the experiment and significantly higher than that of the AA (+) group at 5 weeks. Fig 4B provides representative photographs of the dorsal skin colors at the end of the experiments. There was a visible reduction in the pigmentation of the skin that had been treated with AJ.
Fig 4. The effects of acerola juice intake on L* value and back skin colors.
(A) The time course of the L* value (a measure of skin lightness). (B) Representative photographs of UVB-irradiated areas at 6 weeks. The values are expressed as the means ± the SEMs of five animals. ANOVA analysis: F6, 28 = 17.4, P < 0.0001 for the AA (-) group, F6, 28 = 4.4, P < 0.01 for the AA (+) group, and F6, 28 = 1.1, P = 0.39 for the AJ group. Sharps indicate significant differences (#P < 0.05, ##P < 0.01) compared with the start date by Dunnett's test for post hoc comparisons. ANOVA analysis: F2, 12 = 4.1, P < 0.05 at 2 weeks, F2, 12 = 6.7, P < 0.01 at 3 weeks, F2, 12 = 3.8, P = 0.053 at 4 weeks, F2, 12 = 24.2, P < 0.0001 at 5 weeks, and F2, 12 = 10.5, P < 0.01 at 6 weeks. The asterisks indicate significant differences (*P < 0.05, **P < 0.01) compared with the AA (-) group by Tukey’s HSD post hoc comparisons. The double daggers indicate significant differences (††P < 0.01) compared with the AA (+) group by Tukey’s HSD post hoc comparisons. AA, ascorbic acid; AJ, acerola juice.
Gene expression
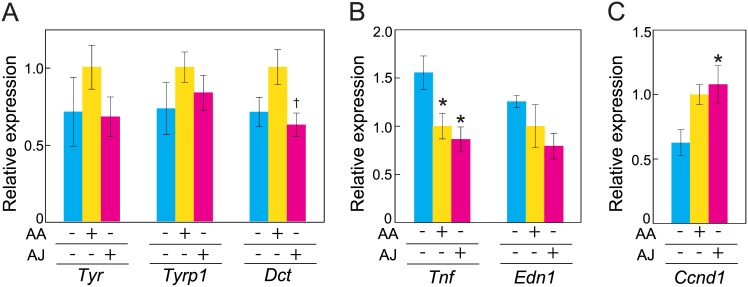
To determine the effects of AJ intake on gene expression, we next examined the mRNA expression levels of melanogenesis-related genes (Tyr, Tyrp1, and Dct), inflammatory cytokines (Tnf-α and Edn1) and a cell cycle-related gene (Ccnd1) in the skins of all groups. The Tyr mRNA expression levels of the AA (-) and AJ groups were 29% and 32% lower, respectively, than that of the AA (+) group, although these differences were not statistically significant (Fig 5A). Similarly, the Tyrp1 mRNA expression levels of the AA (-) and AJ group were 27% and 17% lower, respectively, than that of the AA (+) group, although these differences were not statistically significant (Fig 5A). The Dct mRNA expression levels of the AA (-) and AJ groups were 29% and 37% lower, respectively. Of note, there was a significant difference in the Dct mRNA expression levels between the AA (+) and AJ groups (Fig 5A). As illustrated in Fig 5B, the Tnf-a mRNA expression level of the AA (-) group was significantly higher than those of the AA (+) and AJ groups. In contrast, there was no significant difference in the level of Tnf-α mRNA between the AA (+) and AJ groups. The Edn1 mRNA expression level of the AA (-) group was 26% higher than that of the AA (+) group (Fig 5B). Although there was no significant difference between the AA (+) and AJ groups, the Edn1 mRNA expression level of the AJ group was 21% lower than that of the AA (+) group. Compared with the AA (+) group, the Ccnd1 mRNA expression level of the AA (-) group was relatively lower by 37%, although this difference did not reach significance (p = 0.08; Fig 5C). There was no significant difference between the AA (+) and AJ groups in the Ccnd1 mRNA levels. However, the Ccnd1 mRNA expression level of the AJ group was significantly higher than that that of the AA (-) group.
Fig 5. The effect of acerola juice intake on gene expression in the skin.
(A) Melanogenesis-related enzyme genes Tyr, Tyrp1 and Dct. (B) Cytokine genes Tnf-a and Edn1. (C) Cell cycle progression gene Ccnd1. Quantifications of the mRNA are illustrated relative to the Rplp1 mRNA. The mRNA levels of the AA (+) group were designated as 1.0. The values are expressed as the means ± the SEMs of five animals. ANOVA analysis: F2, 12 = 1.1, P = 0.369 for the Tyr gene, F2, 12 = 1.1, P = 0.372 for the Tyrp1 gene, F2, 12 = 4.1, P < 0.05 for the Dct gene, F2, 12 = 6.3, P < 0.05 for the Tnf-a gene, F2, 12 = 2.3, P = 0.147 for the Edn1 gene, and F2, 12 = 4.7, P < 0.05 for the Ccnd1 gene. The asterisks indicate significant differences (*P < 0.05) compared with the AA (-) group by ANOVA followed by Tukey HSD post hoc comparisons. The daggers indicate significant differences (†P < 0.05) compared with the AA (+) group by ANOVA followed by Tukey’s HSD post hoc comparisons. AA, ascorbic acid; AJ, acerola juice.
Discussion
Acerola is considered a functional fruit due to its strong antioxidant properties and high phenolic contents and is thus consumed worldwide to promote health [14]. Previously, acerola has been demonstrated to exhibit beneficial effects, such as anti-mutagenic activity, an anti-hyperglycemic effect, and an in vivo anti-inflammatory effect [18–21]. Because little is known about the protective effects of AJ intake in UVB-damaged skin, we examined the effects of AJ intake on the skin of SMP30/GNL KO hairless mice. In the present study, we demonstrated that AJ intake suppresses excessive UVB-induced skin pigmentation compared with the same amount of AA intake.
In mammalian species, two main types of melanin are generated, that is, a red-to-yellow pheomelanin and a brown-to-black eumelanin [30]. It has been appreciated that melanin is the major pigment of the skin and determines the skin color. Skin pigmentation is the major photo-protective response of the skin against chronic UV radiation [32]. To elucidate the mechanism of the suppressive effect of AJ intake on skin pigmentation, we investigated the effect of AJ intake on the expression of melanogenesis-related genes. Tyrosinase is an essential enzyme in melanogenesis and catalyzes L-tyrosine to L-DOPA and L-DOPA to dopaquinone. Dopachrome tautomerase catalyzes dopachrome to dihydroxyindole-2-carboxylic acid (DHICA). Tyrosinase-related protein-1 is a DHICA oxidase that catalyzes DHICA to eumelanin [32]. We found that AJ intake significantly decreased the expression level of Dct and tended to decrease the levels of Tyr and Tyrp1. We previously investigated variations in the polyphenol composition of acerola and found that cyanidin-3-α-O-rhamnoside, pelargonidin-3-α-O-rhamnoside, quercetin-3-α-O-rhamnoside, kaempferol glycosides, astilbin and proanthocyanidin are present [14, 15, 17]. Additionally, we revealed that polyphenol extracts from acerola and individual polyphenols (cyanidin-3-α-O-rhamnoside, pelargonidin-3-α-O-rhamnoside and Astilbin) inhibit tyrosinase activity [23]. Previous studies have indicated that polyphenol extracts inhibit melanogenesis through the down-regulations of Tyr, Tyrp1, and Dct [33–35]. Therefore, the suppressive effect of AJ intake on skin pigmentation may have been due to the inhibitory effects of polyphenols on melanogenesis.
Moreover, UVB exposure facilitates the release of pro-inflammatory mediators, including TNF-α and Edn1, from keratinocytes [36–38]. TNF-α stimulates the infiltration of immune cells into the skin, and elastases and collagenases are thereby secreted from the infiltrated immune cells, leading to damage in the skin [39]. Considering that there was no difference in Tnf-a mRNA expression between the AJ and AA (+) groups, it appears that AJ can suppress the inflammation induced by UVB. Edn1 is considered one of the most potent agonists for stimulating melanocytes to accelerate melanogenesis [40, 41]. Because the Edn1 mRNA level in the AJ group was lower than that in the AA (+) group, AJ intake may suppress melanogenesis-related cytokines.
Because keratinocytes differentiate and migrate to the upper layers of the epidermis, melanin pigment is removed from the epidermis along with keratinocytes. We found that the Ccnd1 mRNA expression level of the AJ group was significantly higher than that of the AA (-) group. Cyclin D1 is a critical gene for the progression from the G0/G1 to the S phase of the cell cycle and thus contributes to keratinocyte differentiation [42]. Robles et al. reported that the overexpression of cyclin D1 increases the rate of epidermal proliferation in cyclin D1 transgenic mice [43]. Our results revealed that AJ intake may facilitate the removal of melanized keratinocytes from the epidermis.
Another possibility is the involvement of effects of reactive oxygen species (ROS) on melanogenesis in the skin. UV interacts with oxygen to promote the formation of ROS, such as superoxide radicals, hydrogen peroxide, and highly reactive hydroxyl radicals in the skin [30]. It has been reported that the UV radiation-induced proliferation and melanogenesis of melanocytes are reduced by the topical application of an antioxidant, such as AA or vitamin E, to the skin of hairless mice, which suggests the involvement of ROS in melanogenesis in melanocytes [44]. Mitra et al. also reported that oxidative stress appears to have a role in pheomelanin-mediated melanogenesis [45]. Our previous study demonstrated that polyphenols isolated from the acerola fruit exert superoxide radical scavenging activity using an electron spin resonance spectrometer [14]. Other studies have also indicated that acerola has antioxidant activity using a 1, 1-diphenyl-2-picrylhydrazyl radical scavenging assay [16, 46, 47]. Some studies have reported that the antioxidant activity of acerola juice depends on its polyphenols and AA contents [21, 48]. Because there was no difference in AA content between the AA (+) and AJ groups, as illustrated in Fig 1, various polyphenols from acerola juice may contribute to the reduction of UVB-induced skin pigmentation.
In addition, it has been reported that cortisol and some neuroendocrine mediators such as melatonin and β-endorphin are involved in melanogenesis and UVB-induced skin damage. Cortisol can be produced in the skin by UVB irradiation [49]. It is suggested that production of cortisol maintains skin homeostasis against UVB-induced skin damage [49]. Melatonin is mainly produced in the pineal gland and retina, however, it is also produced in the skin [50–53]. Janjetovic et al. reported that melatonin has strong radical-scavenging activities and has a protective effect against UBV-induced oxidative stress [53]. Moreover β-endorphin can be detected in the components of the skin such as the epidermis, dermis and adnexa [50, 54]. It has been reported that β-endorphin stimulates keratinocyte migration in vitro, induces epidermal and follicular melanogenesis. Additionally, melanocortin receptor 1 is recognized for its role in the regulation of melanin pigmentation [55]. Although the relationship between acerola and these molecules are unclear, acerola may coordinate with these molecules to protect against UVB-induced damage.
We previously reported that AA deficiency suppresses UVB-induced epidermal hyperplasia and increases UVB-induced skin pigmentation [12]. In this study, we confirmed the same results, as illustrated in Figs 2 and 4. Moreover, we found for the first time that AA deficiency decreased the stratum corneum water content and significantly increased the expression level of Tnf-a. These results suggest that AA deficiency facilitates the release of pro-inflammatory mediators induced by UVB radiation and leads to epidermal barrier dysfunction. Moreover, our study also indicates that SMP30/GNL KO hairless mice represent an effective animal model for investigating the effects of functional foods on the skin.
In conclusion, our findings in this study strongly indicate that the intake of AJ compared with AA intake in the same amount significantly prevented skin damage induced by long-term UVB irradiation. Our findings strongly suggest that acerola is not only one of the best sources of AA but is also a functional food for the skin. Further study is needed to elucidate the protective effects on human skin in a human trial or using a reconstructed human skin model.
Acknowledgments
Vitamin C powder was kindly provided by DSM Nutrition Japan.
Abbreviations
- AA
ascorbic acid
- AJ
acerola juice
- Ccnd1
cyclin D1
- Dct
dopachrome tautomerase
- DHA
dehydroascorbic acid
- DHICA
dihydroxyindole-2-carboxylic acid
- ECD
electrochemical detection
- Edn1
endothelin 1
- GNL
gluconolactonase
- KO
knockout
- MPA
metaphosphoric acid
- SMP30
senescence marker protein 30
- TCEP
tris(2-carboxyethyl)phosphine hydrochloride
- Tyr
tyrosinase
- Tyrp1
tyrosinase related protein-1
- UVB
ultraviolet B
Data Availability
All relevant data are within the paper.
Funding Statement
This study is supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant (http://www.mext.go.jp/a_menu/shinkou/hojyo/main5_a5.htm) Number 15H04505 (AI) and 15K15104 (AI). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. EU and TK are current employees of Nichirei Corporation. HA and TH are current employees of Nichirei Foods Corporation. KN is current employee of Nichirei Bioscience Corporation. However, these founders did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fujita T, Uchida K, Maruyama N. Purification of senescence marker protein-30 (SMP30) and its androgen-independent decrease with age in the rat liver. Biochim Biophys Acta. 1992;1116(2):122–8. [DOI] [PubMed] [Google Scholar]
- 2.Kondo Y, Inai Y, Sato Y, Handa S, Kubo S, Shimokado K, et al. Senescence marker protein 30 functions as gluconolactonase in L-ascorbic acid biosynthesis, and its knockout mice are prone to scurvy. Proc Natl Acad Sci U S A. 2006;103(15):5723–8. 10.1073/pnas.0511225103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishikimi M, Yagi K. Molecular basis for the deficiency in humans of gulonolactone oxidase, a key enzyme for ascorbic acid biosynthesis. Am J Clin Nutr. 1991;54(6 Suppl):1203S–8S. [DOI] [PubMed] [Google Scholar]
- 4.Sato Y, Kajiyama S, Amano A, Kondo Y, Sasaki T, Handa S, et al. Hydrogen-rich pure water prevents superoxide formation in brain slices of vitamin C-depleted SMP30/GNL knockout mice. Biochem Biophys Res Commun. 2008;375(3):346–50. 10.1016/j.bbrc.2008.08.020 [DOI] [PubMed] [Google Scholar]
- 5.Kondo Y, Sasaki T, Sato Y, Amano A, Aizawa S, Iwama M, et al. Vitamin C depletion increases superoxide generation in brains of SMP30/GNL knockout mice. Biochem Biophys Res Commun. 2008;377(1):291–6. 10.1016/j.bbrc.2008.09.132 [DOI] [PubMed] [Google Scholar]
- 6.Amano A, Sato Y, Kishimoto Y, Takahashi K, Handa S, Aigaki T, et al. Effects of ascorbic acid deficiency on protein and lipid oxidation in livers from SMP30/GNL knockout mice. J Nutr Sci Vitaminol (Tokyo). 2013;59(6):489–95. [DOI] [PubMed] [Google Scholar]
- 7.Sato Y, Amano A, Kishimoto Y, Takahashi K, Handa S, Maruyama N, et al. Ascorbic acid prevents protein oxidation in livers of senescence marker protein-30/gluconolactonase knockout mice. Geriatr Gerontol Int. 2014;14(4):989–95. Epub 2013/10/15. 10.1111/ggi.12162 [DOI] [PubMed] [Google Scholar]
- 8.Sato T, Seyama K, Sato Y, Mori H, Souma S, Akiyoshi T, et al. Senescence marker protein-30 protects mice lungs from oxidative stress, aging, and smoking. Am J Respir Crit Care Med. 2006;174(5):530–7. 10.1164/rccm.200511-1816OC [DOI] [PubMed] [Google Scholar]
- 9.Koike K, Kondo Y, Sekiya M, Sato Y, Tobino K, Iwakami SI, et al. Complete lack of vitamin C intake generates pulmonary emphysema in senescence marker protein-30 knockout mice. Am J Physiol Lung Cell Mol Physiol. 2010;298(6):L784–92. 10.1152/ajplung.00256.2009 [DOI] [PubMed] [Google Scholar]
- 10.Koike K, Ishigami A, Sato Y, Hirai T, Yuan Y, Kobayashi E, et al. Vitamin C prevents cigarette smoke-induced pulmonary emphysema in mice and provides pulmonary restoration. Am J Respir Cell Mol Biol. 2014;50(2):347–57. 10.1165/rcmb.2013-0121OC [DOI] [PubMed] [Google Scholar]
- 11.Amano A, Tsunoda M, Aigaki T, Maruyama N, Ishigami A. Effect of ascorbic acid deficiency on catecholamine synthesis in adrenal glands of SMP30/GNL knockout mice. Eur J Nutr. 2014;53(1):177–85. 10.1007/s00394-013-0515-9 [DOI] [PubMed] [Google Scholar]
- 12.Sato Y, Arai KY, Nishiyama T, Nomura Y, Kishimoto Y, Aizawa S, et al. Ascorbic acid deficiency leads to epidermal atrophy and UVB-induced skin pigmentation in SMP30/GNL knockout hairless mice. J Invest Dermatol. 2012;132(8):2112–5. 10.1038/jid.2012.105 [DOI] [PubMed] [Google Scholar]
- 13.Vendramini AL, Trugo LC. Chemical composition of acerola fruit (Malpighia punicifolia L.) at three stages of maturity. Food Chemistry. 2000;71(2):195–8. [Google Scholar]
- 14.Hanamura T, Hagiwara T, Kawagishi H. Structural and functional characterization of polyphenols isolated from acerola (Malpighia emarginata DC.) fruit. Biosci Biotechnol Biochem. 2005;69(2):280–6. 10.1271/bbb.69.280 [DOI] [PubMed] [Google Scholar]
- 15.Hanamura T, Aoki H. Toxicological evaluation of polyphenol extract from Acerola (Malpighia emarginata DC.) fruit. J Food Sci. 2008;73(4):T55–61. 10.1111/j.1750-3841.2008.00708.x [DOI] [PubMed] [Google Scholar]
- 16.Nóbrega EM, Oliveira EL, Genovese MI, Correia RTP. The Impact of Hot Air Drying on the Physical-Chemical Characteristics, Bioactive Compounds and Antioxidant Activity of Acerola (Malphigia emarginata) Residue. Journal of Food Processing and Preservation. 2015;39(2):131–41. [Google Scholar]
- 17.Hanamura T, Uchida E, Aoki H. Changes of the composition in acerola (Malpighia emarginata DC.) fruit in relation to cultivar, growing region and maturity. Journal of the Science of Food and Agriculture. 2008;88(10):1813–20. [Google Scholar]
- 18.Horta RN, Kahl VF, Sarmento Mda S, Nunes MF, Porto CR, Andrade VM, et al. Protective effects of acerola juice on genotoxicity induced by iron in vivo. Genet Mol Biol. 2016;39(1):122–8. 10.1590/1678-4685-GMB-2015-0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagamine I, Akiyama T, Kainuma M, Kumagai H, Satoh H, Yamada K, et al. Effect of acerola cherry extract on cell proliferation and activation of ras signal pathway at the promotion stage of lung tumorigenesis in mice. J Nutr Sci Vitaminol (Tokyo). 2002;48(1):69–72. [DOI] [PubMed] [Google Scholar]
- 20.Hanamura T, Mayama C, Aoki H, Hirayama Y, Shimizu M. Antihyperglycemic effect of polyphenols from Acerola (Malpighia emarginata DC.) fruit. Biosci Biotechnol Biochem. 2006;70(8):1813–20. [DOI] [PubMed] [Google Scholar]
- 21.Dias FM, Leffa DD, Daumann F, Marques Sde O, Luciano TF, Possato JC, et al. Acerola (Malpighia emarginata DC.) juice intake protects against alterations to proteins involved in inflammatory and lipolysis pathways in the adipose tissue of obese mice fed a cafeteria diet. Lipids Health Dis. 2014;13:24 10.1186/1476-511X-13-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uchida E, Kondo Y, Amano A, Aizawa S, Hanamura T, Aoki H, et al. Absorption and excretion of ascorbic acid alone and in acerola (Malpighia emarginata) juice: comparison in healthy Japanese subjects. Biol Pharm Bull. 2011;34(11):1744–7. [DOI] [PubMed] [Google Scholar]
- 23.Hanamura T, Uchida E, Aoki H. Skin-lightening effect of a polyphenol extract from Acerola (Malpighia emarginata DC.) fruit on UV-induced pigmentation. Biosci Biotechnol Biochem. 2008;72(12):3211–8. 10.1271/bbb.80421 [DOI] [PubMed] [Google Scholar]
- 24.Iwama M, Shimokado K, Maruyama N, Ishigami A. Time course of vitamin C distribution and absorption after oral administration in SMP30/GNL knockout mice. Nutrition. 2011;27(4):471–8. 10.1016/j.nut.2010.04.010 [DOI] [PubMed] [Google Scholar]
- 25.Kondo Y, Sakuma R, Ichisawa M, Ishihara K, Kubo M, Handa S, et al. Potato chip intake increases ascorbic acid levels and decreases reactive oxygen species in SMP30/GNL knockout mouse tissues. J Agric Food Chem. 2014;62(38):9286–95. 10.1021/jf502587j [DOI] [PubMed] [Google Scholar]
- 26.Naganumaa M, Yagi E, Fukuda M. Delayed induction of pigmented spots on UVB-irradiated hairless mice. J Dermatol Sci. 2001;25(1):29–35. [DOI] [PubMed] [Google Scholar]
- 27.Sato Y, Uchiki T, Iwama M, Kishimoto Y, Takahashi R, Ishigami A. Determination of dehydroascorbic acid in mouse tissues and plasma by using tris(2-carboxyethyl)phosphine hydrochloride as reductant in metaphosphoric acid/ethylenediaminetetraacetic acid solution. Biol Pharm Bull. 2010;33(3):364–9. [DOI] [PubMed] [Google Scholar]
- 28.Linster CL, Van Schaftingen E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J. 2007;274(1):1–22. 10.1111/j.1742-4658.2006.05607.x [DOI] [PubMed] [Google Scholar]
- 29.Seike M, Ikeda M, Morimoto A, Matsumoto M, Kodama H. Increased synthesis of calcitonin gene-related peptide stimulates keratinocyte proliferation in murine UVB-irradiated skin. J Dermatol Sci. 2002;28(2):135–43. [DOI] [PubMed] [Google Scholar]
- 30.D'Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. Int J Mol Sci. 2013;14(6):12222–48. 10.3390/ijms140612222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamada Y, Obayashi M, Ishikawa T, Kiso Y, Ono Y, Yamashita K. Dietary tocotrienol reduces UVB-induced skin damage and sesamin enhances tocotrienol effects in hairless mice. J Nutr Sci Vitaminol (Tokyo). 2008;54(2):117–23. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Weng QY, Fisher DE. UV signaling pathways within the skin. J Invest Dermatol. 2014;134(8):2080–5. Epub 2014/04/25. 10.1038/jid.2014.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee MH, Lin YP, Hsu FL, Zhan GR, Yen KY. Bioactive constituents of Spatholobus suberectus in regulating tyrosinase-related proteins and mRNA in HEMn cells. Phytochemistry. 2006;67(12):1262–70. 10.1016/j.phytochem.2006.05.008 [DOI] [PubMed] [Google Scholar]
- 34.Zi SX, Ma HJ, Li Y, Liu W, Yang QQ, Zhao G, et al. Oligomeric proanthocyanidins from grape seeds effectively inhibit ultraviolet-induced melanogenesis of human melanocytes in vitro. Int J Mol Med. 2009;23(2):197–204. [PubMed] [Google Scholar]
- 35.Kim JH, Baek SH, Kim DH, Choi TY, Yoon TJ, Hwang JS, et al. Downregulation of melanin synthesis by haginin A and its application to in vivo lightening model. J Invest Dermatol. 2008;128(5):1227–35. 10.1038/sj.jid.5701177 [DOI] [PubMed] [Google Scholar]
- 36.Bashir MM, Sharma MR, Werth VP. UVB and proinflammatory cytokines synergistically activate TNF-alpha production in keratinocytes through enhanced gene transcription. J Invest Dermatol. 2009;129(4):994–1001. 10.1038/jid.2008.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strickland I, Rhodes LE, Flanagan BF, Friedmann PS. TNF-alpha and IL-8 are upregulated in the epidermis of normal human skin after UVB exposure: correlation with neutrophil accumulation and E-selectin expression. J Invest Dermatol. 1997;108(5):763–8. [DOI] [PubMed] [Google Scholar]
- 38.Imokawa G, Miyagishi M, Yada Y. Endothelin-1 as a new melanogen: coordinated expression of its gene and the tyrosinase gene in UVB-exposed human epidermis. J Invest Dermatol. 1995;105(1):32–7. [DOI] [PubMed] [Google Scholar]
- 39.Rijken F, Bruijnzeel PL. The pathogenesis of photoaging: the role of neutrophils and neutrophil-derived enzymes. J Investig Dermatol Symp Proc. 2009;14(1):67–72. 10.1038/jidsymp.2009.15 [DOI] [PubMed] [Google Scholar]
- 40.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84(4):1155–228. Epub 2004/09/24. 10.1152/physrev.00044.2003 [DOI] [PubMed] [Google Scholar]
- 41.Murase D, Hachiya A, Kikuchi-Onoe M, Fullenkamp R, Ohuchi A, Kitahara T, et al. Cooperation of endothelin-1 signaling with melanosomes plays a role in developing and/or maintaining human skin hyperpigmentation. Biol Open. 2015;4(10):1213–21. 10.1242/bio.011973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandez-Hernandez R, Rafel M, Fuste NP, Aguayo RS, Casanova JM, Egea J, et al. Cyclin D1 localizes in the cytoplasm of keratinocytes during skin differentiation and regulates cell-matrix adhesion. Cell Cycle. 2013;12(15):2510–7. 10.4161/cc.25590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robles AI, Larcher F, Whalin RB, Murillas R, Richie E, Gimenez-Conti IB, et al. Expression of cyclin D1 in epithelial tissues of transgenic mice results in epidermal hyperproliferation and severe thymic hyperplasia. Proc Natl Acad Sci U S A. 1996;93(15):7634–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quevedo WC Jr., Holstein TJ, Dyckman J, McDonald CJ, Isaacson EL. Inhibition of UVR-induced tanning and immunosuppression by topical applications of vitamins C and E to the skin of hairless (hr/hr) mice. Pigment Cell Res. 2000;13(2):89–98. [DOI] [PubMed] [Google Scholar]
- 45.Mitra D, Luo X, Morgan A, Wang J, Hoang MP, Lo J, et al. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature. 2012;491(7424):449–53. 10.1038/nature11624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sagar S. Blessy K C, Kuna Aparna. Antioxidant Properties of Acerola (Malpighia Emarginata Dc.) and Acerola squash. International Journal of Science and Research 2014;3(7):2176–9. [Google Scholar]
- 47.Lima VLAGD, Melo EA, Pinheiro IO, Guerra NB. Antioxidant capacity of anthocyanins from acerola genotypes. Food Science and Technology (Campinas). 2011;31:86–92. [Google Scholar]
- 48.Leffa DD, da Silva J, Daumann F, Dajori AL, Longaretti LM, Damiani AP, et al. Corrective effects of acerola (Malpighia emarginata DC.) juice intake on biochemical and genotoxical parameters in mice fed on a high-fat diet. Mutat Res. 2014;770:144–52. 10.1016/j.mrfmmm.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 49.Skobowiat C, Sayre RM, Dowdy JC, Slominski AT. Ultraviolet radiation regulates cortisol activity in a waveband-dependent manner in human skin ex vivo. Br J Dermatol. 2013;168(3):595–601. 10.1111/bjd.12096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v, vii,, 1–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Slominski A, Fischer TW, Zmijewski MA, Wortsman J, Semak I, Zbytek B, et al. On the role of melatonin in skin physiology and pathology. Endocrine. 2005;27(2):137–48. 10.1385/ENDO:27:2:137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005;19(2):176–94. 10.1096/fj.04-2079rev [DOI] [PubMed] [Google Scholar]
- 53.Janjetovic Z, Nahmias ZP, Hanna S, Jarrett SG, Kim TK, Reiter RJ, et al. Melatonin and its metabolites ameliorate ultraviolet B-induced damage in human epidermal keratinocytes. J Pineal Res. 2014;57(1):90–102. 10.1111/jpi.12146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tobin DJ, Kauser S. Beta-endorphin: the forgotten hair follicle melanotropin. J Investig Dermatol Symp Proc. 2005;10(3):212–6. 10.1111/j.1087-0024.2005.10108.x [DOI] [PubMed] [Google Scholar]
- 55.Zmijewski MA, Slominski AT. Is Mc1r an important regulator of non-pigmentary responses to UV radiation? Exp Dermatol. 2013;22(12):790–1. 10.1111/exd.12129 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.