Abstract
The Na+/H+ exchanger isoform 3 (NHE3) facilitates Na+ absorption and H+ secretion and is expressed in the intestine, proximal tubule, and thick ascending limb of the kidney. While the function of NHE3 for Na+ and (re)absorption has been defined using conventional NHE3 knockout mice (NHE3−/−), the recent generation of conditional NHE3 knockout mice started to give critical new insight into the role of this protein by allowing for temporal and spatial control of NHE3 expression. For example, in contrast to NHE3−/− mice, knockout of NHE3 in the S1 and S2 segments of the proximal tubule or along the entire tubule/collecting duct does not cause any lethality. Nonabsorbable NHE3 inhibitors have been developed, and preclinical as well as clinical trials indicate possible pharmacological use in fluid overload, hypertension, chronic kidney disease, hyperphosphatemia, and constipation. Some of the therapeutic considerations seem to be directly related to the pharmacodynamic properties of these drugs; however, little is known about the effects of these nonabsorbable NHE3 inhibitors on intestinal phosphate transport and the mechanisms so far remain elusive. This review focuses on novel findings of NHE3 in the intestine and the kidney as well as novel drug developments targeting NHE3.
na+/h+ exchangers (NHE) mediate the electroneutral exchange of extracellular Na+ for cytosolic H+ across biological membranes. The major functions of NHE include, but are not limited to, transepithelial ion transport, intracellular pH, and cell volume regulation (15). The NHE family comprises nine related gene products (NHE1–9). The major focus of this review is NHE3, which mediates Na+ absorption in the intestine and Na+ reabsorption in the kidney (a model is shown in Fig. 1). In the intestine, NHE3 is localized to the small intestine and colon (7, 21), and in the kidney, NHE3 is localized to the proximal tubule (PT) and thick ascending limb (5, 13). Because of its role in Na+ (re)absorption, NHE3 plays a key role in extracellular volume homeostasis and regulation of blood pressure. NHE3 knockout (NHE3−/−) mice exhibit hypovolemia, hypotension, mild metabolic acidosis, and diarrhea; thereby demonstrating that NHE3 is the major transporter of Na+ in both kidney and intestine (32). These mice have since been used to study many aspects of Na+-fluid homeostasis; however, the challenge is that the contributing roles of the kidney versus intestine cannot easily be differentiated. In recent years, investigators have generated tissue-specific NHE3 knockout mice (13, 27), which provide critical new insights into the role of this protein by allowing for temporal and spatial control of NHE3 expression. In this review, we will highlight recent key findings about the role of NHE3 in the kidney and intestine. In addition, we will discuss novel pharmacological developments targeting NHE3 that aim to reduce Na+ absorption and fluid overload in conditions such as hypertension, chronic kidney disease (CKD), hyperphosphatemia, and constipation.
Fig. 1.
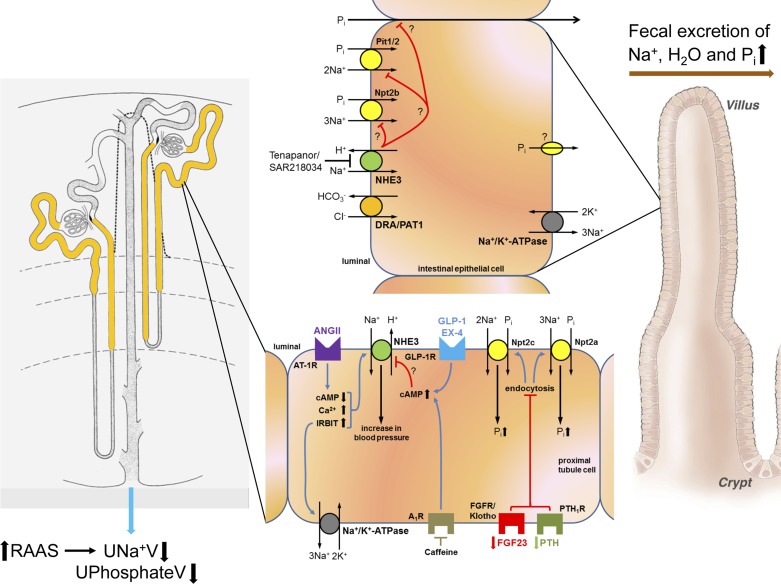
Model of intestinal and renal Na+/H+ exchanger isoform 3 (NHE3) and potential compensating mechanisms involved as a consequence of NHE3 inhibitors. Left: expression of NHE3 along the nephron is shown in yellow. Right: expression of NHE3 along villi of the intestine where NHE3 mediates electroneutral NaCl absorption in conjunction with the Cl−/ exchange (DRA, downregulated in adenoma; PAT1, putative anion transporter). Glucagon-like peptide 1 (GLP-1) and exendin-4 (EX-4) activate Gs protein-coupled GLP-1 receptors (GLP-1R). While there is evidence that phosphorylation of NHE3 is affected by these agonists, data in NHE3loxloxPax8Cre mice indicate that NHE3 is not the protein mediating the diuretic and natriuretic effect. Adenosine A1 receptors (A1R) are Gi protein-coupled receptors. Caffeine, a nonselective adenosine receptor antagonist, blocks the inhibitory action of A1R resulting in increased cAMP levels, which could consequently inhibit NHE3. A1R are required for the diuresis and natriuresis in response to caffeine; however, new data in NHE3loxloxPax8Cre mice provide no evidence that NHE3 is causing these responses because NHE3loxloxPax8Cre mice show comparable renal effects as control mice. Blood pressure effects in the proximal tubule in response to ANG II are mediated by NHE3 and were shown to involve cAMP, Ca2+, and IRBIT (inositol 1,4,5-trisphosphate receptor binding protein released with inositol 1,4,5-trisphosphate). Intestinal NHE3 blockade with Tenapanor/SAR218034 causes increased fecal Na+, H2O, and inorganic phosphate (Pi). It is still unknown if NHE3 blockade inhibits intestinal Pi transport via Na+/Pi cotransporter Npt2b, Na+-dependent Pi transporters Pit1 and/or Pit2, and/or paracellular Pi transport. It is also unknown if NHE3 inhibition affects the basolateral cellular exit route of Pi, which has so far not been identified. Possibly as a consequence of reduced intestinal Pi uptake resulting in increased fecal Pi excretion, fibroblast factor 23 (FGF23) is decreased to maintain homeostasis. Because parathyroid hormone (PTH, so far not determined) also regulates Pi transport, we suspect hormone levels to be decreased. Reduced PTH and FGF23 levels inhibit internalization of Npt2a and Npt2c from the brush-border membrane, consequently increasing proximal tubule Pi uptake and reducing urinary Pi excretion. The inhibition of intestinal Na+ uptake may also result in activation of the renin-angiotensin system (RAAS) with subsequent increased renal Na+ reabsorption and reduced urinary Na+ excretion. For simplicity, not all signaling pathways are shown and some receptors may have luminal and basolateral localizations. See text for additional details.
Recent Findings on Role of Renal NHE3 in Blood Pressure Regulation
Up to 60% of hypertensive patients are sensitive to dietary NaCl, possibly a result from increased Na+ reabsorption or retention due to abnormal Na+ handling by the kidneys. Therefore, many studies have focused on the role of Na+ transporters, such as NHE3, in blood pressure regulation (for review see Ref. 46). Hormones, like angiotensin II (ANG II), have been shown to increase expression of NHE3 in the PT and thus may play an important role in ANG II-dependent hypertension (15, 32). Consistent with this, NHE3−/− mice, which have lower blood pressure compared with wild-type (WT) mice (25, 29, 42), show attenuated acute and chronic pressor effects in response to ANG II when plasma ANG II levels are increased via osmotic minipumps (29). Whereas an angiotensin-converting enzyme (ACE) inhibitor reduced blood pressure in WT mice, NHE3−/− mice died as a consequence of this treatment (29). This effect is possibly attributable to the blockade of compensatory responses in NHE3−/− mice, which include the activation of the renin-angiotensin-aldosterone system (RAAS), and are required to maintain blood pressure when intestine and kidney are prone to Na+ wasting (29). Importantly, the contribution of the intestine versus the kidney in these studies could not be differentiated because whole body NHE3−/− mice were studied. In another publication from the same group, the authors aimed to tackle this problem by using NHE3−/− mice where the intestinal phenotype was rescued by expression of rat NHE3 (tgNHE3−/−) (28). By using NHE3−/− mice with transgenic rescue of intestinal NHE3, the authors tested whether loss of NHE3 in the kidney attenuates ANG II-induced hypertension (28). The authors concluded that renal NHE3 is required for maintaining normal blood pressure and for fully developing ANG II‐induced hypertension. However, this conclusion must be interpreted with caution because the intestinal phenotype in this model is only partially rescued when gut weight and cecal fluid accumulation were compared with controls, and 20% of the pups had moderate to severe diarrhea associated with mortality during the first few weeks after birth (28). Studies in tubule-specific and/or proximal tubule NHE3 knockout mice will hopefully confirm this observation.
Interestingly, both the RAAS and renal NHE3 have been implicated in the circadian regulation of blood pressure. Under physiological conditions, blood pressure surges in the early morning, followed by a plateau during the daytime, and then dips during sleep. The normal blood pressure drop during nighttime, defined as a >10% decrease of the diurnal blood pressure values (termed dippers), is absent in some hypertensive patients (termed nondippers) and is associated with an increased risk of damage to the brain, kidneys, heart, and blood vessels compared with dippers (38). Several studies have indicated that NHE3 is regulated by circadian clock genes in the PT of the rat (41) and the thick ascending limb of the mouse (33). Two circadian clock genes, CLOCK and Per1, were both detected in the promotor region of NHE3 (43). Blocking the entry of Per1 into the nucleus decreased membrane and intracellular protein levels of NHE3 in the renal cortex of mice (43). However, the overall contribution of NHE3 for the circadian regulation of blood pressure still needs to be better studied considering that NHE3−/− and tgNHE3−/− mice show normal circadian blood pressure variation (34). This suggests that the nocturnal dip in blood pressure occurs independently of NHE3, and therefore one could speculate that nocturnal blood pressure dipping is not dependent on renal regulation of Na+ and H2O.
Regulation of Renal NHE3
Renal nerve activity is another regulator of PT Na+ reabsorption (4). A recent study found that renal nerve stimulation induces NHE3-mediated PT Na+ reabsorption via intrarenal RAAS activation and triggering the AT1 receptor (36). Stimulation of renal nerves did not alter total cortical NHE3 abundance but decreased the ratio of NHE3 phosphorylated at serine 552 versus total NHE3 (36). In contrast, studies in rats indicated that norepinephrine released from sympathetic nerve terminals activates α1-adrenoceptors to increase the apical abundance of NHE3, consequently increasing Na+ reabsorption in the PT (20). While some of these effects have been speculated to require ANG II type 1 receptor (AT1)/Gi protein-coupled signaling pathways, mostly because cAMP formation and protein kinase A activity were reduced (36), other studies imply the involvement of a Ca2+-signaling pathway in response to ANG II (Fig. 1). IRBIT (inositol 1,4,5-trisphosphate receptor binding protein released with inositol 1,4,5-trisphosphate) is abundantly expressed in the PT and, in response to ANG II, binds and activates NHE3 (16, 18). The ANG II-induced NHE3 activation possibly relates to the PDZ1 domain of NHE regulatory factor 1 (NHERF1) because the assembly of a NHE3-IRBIT-NHERF1 complex was found to increase NHE3 exocytosis and activity (19). In addition to the luminal effect of IRBIT increasing Na+ reabsorption via NHE3-IRBIT-NHERF1, IRBIT colocalizes with the Na+-K+-ATPase to the basolateral membrane where they are coexpressed in 350- and 650-kDa macromolecular complexes (17). Therefore, IRBIT might provide a novel link in the regulation of Na+-K+-ATPase by ANG II and may mediate transepithelial Na+ transport. This stimulation was dependent on changes in intracellular Ca2+ and Ca2+/calmodulin-dependent protein kinase II (16, 18). Calcineurin, a Ca2+/calmodulin-stimulated protein phosphatase, is also targeted by the Ca2+-binding protein calcineurin homologous protein (CHP) (12). CHP-1 interacts with NHE3 and establishes the set point for NHE3 activity via intracellular pH (3). Renal silencing of CHP-1 via renal subcapsular infusion of small interfering RNA (siRNA) in mice significantly reduced NHE3 protein expression (by 50%). Importantly, CHP-1 siRNA-treated mice had a 30% reduced systolic blood pressure compared with scrambled siRNA-treated mice (11).
Development of Novel Kidney-Specific NHE3 Knockout Mice
To understand the role of PT NHE3 without the confounding defects in the intestine, two novel kidney-specific NHE3 knockout models have been developed by using Cre-loxP recombination. The first model used the Na+-glucose cotransporter 2 Cre system (Sglt2Cre) targeting segments 1 and 2 (S1 and S2) of the PT (27). Studies in NHE3lox/-Sglt2Cre mice demonstrated reduced and H2O reabsorption as well as mild metabolic acidosis (27). Of note, this model has a 50% NHE3 reduction in the entire body because of the existence of one NHE3 knockout and one floxed allele. The second model uses the Pax8Cre system, which targets the entire tubule system and the collecting duct (13).
Several studies have used the NHE3loxloxPax8Cre mice to investigate kidney-specific roles of NHE3 (13, 14). Activation of the Gs protein-coupled glucagon-like 1 (GLP-1) receptors either via GLP-1 or the GLP-1 receptor agonist Exendin-4 (EX-4) can induce diuresis and natriuresis (10, 37). The diuretic and natriuretic effect of EX-4 has been associated with increased phosphorylation of NHE3 at serine residues 552 and 605 in the rat and mouse kidney, which possibly inhibits NHE3 activity (Fig. 1) without affecting total NHE3 abundance (10, 37). In contrast to these findings, a recent study in tubule-specific NHE3loxloxPax8Cre mice identified in metabolic cage and clearance experiments that NHE3 is not required for the acute EX-4-induced diuresis and natriuresis (14). Taken together, the role of cAMP and NHE3 for the diuresis/natriuresis observed in response to EX-4 is ambiguous and additional studies are needed to determine which mechanism(s) contribute to these renal effects.
Studies in NHE3loxloxPax8Cre mice showed that, possibly as a consequence of reduced reabsorption in the PT, urinary pH was significantly higher compared with control mice (13, 35). We tested the hypothesis if blocking the Gi protein-coupled adenosine A1 receptor with the nonselective adenosine receptor antagonist caffeine changes NHE3 localization and phosphorylation, consequently causing diuresis and natriuresis. We found in control mice that caffeine had no effect on NHE3 localization and phosphorylation (13). NHE3loxloxPax8Cre mice responded to caffeine with a diuresis and natriuresis that was comparable to control mice, indicating that tubular NHE3 is not the primary mechanism how caffeine induces its renal effects (13). Preliminary data studying Na+ homeostasis in NHE3loxloxPax8Cre mice identified a higher urinary Na+-to-creatinine ratio when fed a control diet (9, 35). In contrast to NHE3−/− mice, which die of severe dehydration as a consequence of hypovolemia and body weight loss when exposed to zero NaCl intake (25), NHE3loxloxPax8Cre mice do not lose weight or show lethality in response to NaCl restriction (9). Further studies are needed to better define the contribution of renal NHE3 for Na+ homeostasis and blood pressure regulation.
Recent Findings on Role of Intestinal NHE3
Diarrheal diseases are a global public health issue and are caused by altered intestinal transport of electrolytes and water. Under normal conditions, electroneutral NaCl absorption occurs along the entire length of the intestinal tract. However, when Na+ absorption is inhibited and electrogenic Cl− secretion is stimulated, luminal fluid accumulation and diarrhea ensues. Given the key role of NHE3 in the intestine for Na+ absorption and volume regulation, it is not surprising that NHE3−/− mice exhibit severe diarrhea, enlargement of the intestinal tract, and a high mortality rate beginning just after weaning, with only ∼30% surviving into adulthood (8, 42). In fact, most diarrheal diseases are associated with inhibition of NHE3. For example, both human patients and animal models of inflammatory bowel disease have demonstrated impaired NHE3-mediated Na+ absorption (15). It is thought that proinflammatory cytokines can directly repress NHE3 promoter activity (1) and enterotoxins (e.g., cholera toxin) can lead to increased cAMP, cGMP, or intracellular Ca2+, which inhibit NHE3 activity (15, 45). Vice versa, too much NaCl absorption from the gut reduces fecal water content and can lead to constipation.
To date there are no published studies using intestine-specific NHE3 knockout animals that we are aware of. Our group wanted to develop a model where the contributions of intestinal NHE3 could be differentiated independently from renal NHE3. Thus we generated an intestinal-specific NHE3 knockout mouse using noninducible VillinCre. We found that conditional noninducible intestinal-specific NHE3 knockout mice have an almost 0% survival rate into adulthood (T. Rieg, unpublished observations). The majority of intestinal-specific NHE3 knockout mice die within the first few days, and we observed only one mouse that survived ~2 wk (out of >50 offspring). This may indicate that in NHE3−/− mice surviving into adulthood, the detrimental effects of a lack of NHE3 in the intestine might be rescued by lack of NHE3 in the kidney or that the defects in the intestine are solely responsible for the lethality. Inducible VillinCre NHE3 knockout mice should circumvent these problems; however, reduced expression of NHE3 in the kidney needs careful consideration, given the fact that villin is also expressed in the kidney. Interestingly, some mutants of the original NHE3−/− strain also do not survive beyond weaning, whereas the survival of others were indistinguishable from WT mice (26). The reason for this remains elusive but might relate to differences in the intestinal microbiome, genetic background, and/or the existence of locus/loci that modify the genotype. Interestingly, NHE3 may be more than just a transporter; several groups have suggested the NHE3 may also function as a gene modifier. For example, Kiela et al. (23) described that NHE3−/− mice have a compromised innate immune response that leaves them more susceptible to colitis. In addition, it might be possible that NHE3 indirectly interacts with junctional proteins and plays a role in epithelial barrier function. Therefore, it seems plausible that the high mortality rate in our intestinal-specific NHE3 knockout mice might be due to a dysfunctional mucosal immune system and perhaps a compromised mucosal barrier. More studies are needed to understand these mechanisms.
Pharmacological Targeting of NHE3
Based on the physiological role of NHE3, pharmacological inhibition of intestinal NHE3 is an interesting treatment strategy for hypertension and constipation. Not only would it allow for reduced total body Na+ and fluid overload, but it would do so independent of kidney function. Patients with fluid overload, hypertension, CKD, and constipation could all benefit from decreased Na+ absorption from the gut. Currently, there are two small-molecule, orally nonabsorbable NHE3 inhibitors being evaluated: SAR218034 and tenapanor (AZD1722/RDX5791), both of which exhibit minimal cellular permeability and low or unquantifiable plasma concentrations (31, 44).
Treatment with SAR218034 decreased systolic blood pressure and increased fecal water content in two spontaneously hypertensive rat models. Although plasma Na+ did not change during treatment, inhibition of NHE3 led to increased fecal Na+ excretion and reduced urinary Na+ excretion (31). Based on the homeostatic concept originally described by Claude Bernard in the 19th century, the reduction of urinary Na+ excretion is inevitable under steady-state conditions and may be the consequence of a reduced filtered load or increased fractional Na+ reabsorption in an effort to maintain Na+ balance. In support of this feedback hypothesis, when spontaneously hypertensive rats were treated long term (14 wk) with SAR218034, animals exhibited increased plasma renin activity and aldosterone levels (31), consistent with RAAS activation. Furthermore, when hypertensive rats were treated with a combination of SAR218034 and an ACE inhibitor, there were additive effects on reducing blood pressure (31). However, it still needs to be determined whether long-term inhibition of intestinal NHE3 exacerbates the negative effects observed with chronic RAAS activation.
Treatment with tenapanor dose dependently increased fecal Na+ excretion, fecal water content, and intestinal transit rate in healthy human volunteers (22, 44) without affecting urinary or fecal K+ excretion (44). Comparable to SAR218034, tenapanor treatment (1, 3, or 10 mg/kg daily) in rats increased plasma aldosterone levels by 6-, 11- and 23-fold, respectively, compared with baseline (44). In a rat 5/6th nephrectomy model with increased extracellular fluid volume (ECFV) and hypertension (imposed via a 4% NaCl diet), tenapanor treatment reduced ECFV, hypertension, albuminuria, renal hypertrophy, as well as left ventricular hypertrophy (44). In terms of blood pressure and left ventricular hypertrophy, similar findings were reported in obese spontaneously hypertensive rats treated with SAR218034 (30). In contrast, patients with CKD stage 5 treated with hemodialysis did not show a significant effect of tenapanor on interdialytic weight gain versus placebo over a 4 wk treatment period despite increased fecal Na+ and weight (6).
Importantly, in addition to the effects of NHE3 inhibition on Na+ uptake, these inhibitors also affect inorganic phosphate (Pi) uptake in an unexplained mechanism that clearly differs from Pi binders (which react in the intestine with Pi and form complexes that cannot be absorbed). Intestinal Pi uptake is paracellular, dependent on passive diffusion, and Na+ dependent, requiring Na+-K+-ATPase activity. The latter occurs through Na+/Pi cotransporters, namely Npt2b, Pit1, and Pit2 (39). Intestinal Npt2b was found to contribute ~50% to the total acute Pi absorption and 90% of Na+-dependent absorption (40). Tenapanor not only increased fecal Na+ but also fecal Pi accompanied by a decrease in urinary Pi (22, 24). It is still unclear if suppression of parathyroid hormone and fibroblast growth factor 23 (FGF-23) contribute to the reduced renal Pi excretion under baseline conditions. In a rat model of CKD (5/6th nephrectomy) and vascular calcification, tenapanor treatment reduced soft tissue and ectopic vascular calcifications. In addition, hyperphosphatemia and FGF-23 levels were reduced compared with vehicle treatment (24). The mechanisms by which NHE3 inhibitors affect Pi uptake in the intestine, possibly via Npt2b and/or Pit1/2 and/or paracellular transport, remain to be determined. Intestinal Npt2b knockout mice might possibly help to answer the question for this problem. Alternatively, intestinal NHE3 inhibition could affect pH of the intestinal content thereby inhibiting Pi uptake (2).
Side Effects of NHE3 Inhibitors
Intestinal inhibition of NHE3 seems generally well tolerated. As what would be expected from blocking the physiological role of NHE3 in the intestine (which would ultimately reflect the observations in NHE3−/− mice), the most common side effect observed by inhibiting NHE3 was diarrhea, as evidenced from studies in rats and humans where tenapanor significantly increased stool form scores (6, 44). In addition, compensatory NHE3 upregulation occurs in the ileum and jejunum in response to long-term SAR218034 treatment (31). So far it is unclear whether patients treated with NHE3 inhibitors require regular monitoring of acid-base homeostasis. In addition, the existence of intestinal drug-drug interactions has so far not been evaluated.
Perspectives and Significance
The availability of novel tissues-specific NHE3 knockout mouse models and selective nonabsorbable NHE3 inhibitors offer so far unprecedented insight into the renal- and intestinal-specific roles of NHE3. The novel models will also be instrumental in confirming the selectivity of NHE3 inhibitors. In addition, intestinal-specific NHE3 knockout mice can possibly give insight into the mechanisms how Pi transport is affected. In summary, NHE3 inhibition might provide novel therapeutic approaches for the treatment of hypertension, fluid overload, CKD, hyperphosphatemia, and constipation.
GRANTS
This work was supported by an APS STRIDE Undergraduate Summer Research Fellowship 1R25HL115473-01 (to S. de la Mora Chavez), a Bastyr University Faculty Research Seed Grant (to J. Dominguez Rieg), National Institute of Diabetes and Digestive and Kidney Diseases Grant 1R01DK110621-01 (T. Rieg), the O’Brien Center for Acute Kidney Injury Research Grant P30DK079337 (to T. Rieg), Diabetes Endocrinology Research Center P30DK063491 (to T. Rieg), American Heart Association 15BGIA22410018 (to T. Rieg), and Satellite Healthcare (a not-for-profit renal care provider) (to T. Rieg).
DISCLOSURES
No conflicts of interest (financial or otherwise) are delcared by the author(s).
AUTHOR CONTRIBUTIONS
J.A.D.R. and T.R. conception and design of research; A.D.R. and T.R. performed experiments; J.A.D.R. and T.R. analyzed data; J.A.D.R. and T.R. interpreted results of experiments; J.A.D.R. and T.R. prepared figures; J.A.D.R. and T.R. drafted manuscript; J.A.D.R., S.d.l.M.C., and T.R. edited and revised manuscript; J.A.D.R., S.d.l.M.C., and T.R. approved final version of manuscript.
REFERENCES
- 1.Amin MR, Malakooti J, Sandoval R, Dudeja PK, Ramaswamy K. IFN-γ and TNF-α regulate human NHE3 gene expression by modulating the Sp family transcription factors in human intestinal epithelial cell line C2BBe1. Am J Physiol Cell Physiol 291: C887–C896, 2006. doi: 10.1152/ajpcell.00630.2005. [DOI] [PubMed] [Google Scholar]
- 2.Amstutz M, Mohrmann M, Gmaj P, Murer H. Effect of pH on phosphate transport in rat renal brush border membrane vesicles. Am J Physiol Renal Fluid Electrolyte Physiol 248: F705–F710, 1985. [DOI] [PubMed] [Google Scholar]
- 3.Babich V, Vadnagara K, Di Sole F. The biophysical and molecular basis of intracellular pH sensing by Na+/H+ exchanger-3. FASEB J 27: 4646–4658, 2013. doi: 10.1096/fj.12-225466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell-Reuss E, Trevino DL, Gottschalk CW. Effect of renal sympathetic nerve stimulation on proximal water and sodium reabsorption. J Clin Invest 57: 1104–1107, 1976. doi: 10.1172/JCI108355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biemesderfer D, Rutherford PA, Nagy T, Pizzonia JH, Abu-Alfa AK, Aronson PS. Monoclonal antibodies for high-resolution localization of NHE3 in adult and neonatal rat kidney. Am J Physiol Renal Fluid 273: F289–F299, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Block GA, Rosenbaum DP, Leonsson-Zachrisson M, Stefansson BV, Rydén-Bergsten T, Greasley PJ, Johansson SA, Knutsson M, Carlsson BC. Effect of tenapanor on interdialytic weight gain in patients on hemodialysis. Clin J Am Soc Nephrol 11: 1597–1605, 2016. doi: 10.2215/CJN.09050815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bookstein C, DePaoli AM, Xie Y, Niu P, Musch MW, Rao MC, Chang EB. Na+/H+ exchangers, NHE-1 and NHE-3, of rat intestine. Expression and localization. J Clin Invest 93: 106–113, 1994. doi: 10.1172/JCI116933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford EM, Sartor MA, Gawenis LR, Clarke LL, Shull GE. Reduced NHE3-mediated Na+ absorption increases survival and decreases the incidence of intestinal obstructions in cystic fibrosis mice. Am J Physiol Gastrointest Liver Physiol 296: G886–G898, 2009. doi: 10.1152/ajpgi.90520.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chavez S, Poulsen SB, Soleimani M, Fenton RA, Rieg T. Preserved survival of renal tubulus-specific sodium hydrogen exchanger isoform 3 knockout mice in response to sodium restriction (Abstract). FASEB J, Suppl, 962.4, 2016. [Google Scholar]
- 10.Crajoinas RO, Oricchio FT, Pessoa TD, Pacheco BP, Lessa LM, Malnic G, Girardi AC. Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am J Physiol Renal Physiol 301: F355–F363, 2011. doi: 10.1152/ajprenal.00729.2010. [DOI] [PubMed] [Google Scholar]
- 11.Di Sole F, Escano C, Babich V. Regulation of blood pressure by the calcineurin homologous protein-1 (Abstract). FASEB J, Suppl, 960.26, 2015. [Google Scholar]
- 12.Di Sole F, Vadnagara K, Moe OW, Babich V. Calcineurin homologous protein: a multifunctional Ca2+-binding protein family. Am J Physiol Renal Physiol 303: F165–F179, 2012. doi: 10.1152/ajprenal.00628.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenton RA, Poulsen SB, de la Mora Chavez S, Soleimani M, Busslinger M, Dominguez Rieg JA, Rieg T. Caffeine-induced diuresis and natriuresis is independent of renal tubular NHE3. Am J Physiol Renal Physiol 308: F1409–F1420, 2015. doi: 10.1152/ajprenal.00129.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Y, Song P, Onishi A, Batz BB, Soleimani M, Busslinger M, Vallon V. The diuretic and natriuretic effect of the GLP-1 receptor agonist, exendin-4, is independent of tubular NHE3 (Abstract). J Am Soc Nephrol 26: 188A, 2015. [Google Scholar]
- 15.Girardi AC, Di Sole F. Deciphering the mechanisms of the Na+/H+ exchanger-3 regulation in organ dysfunction. Am J Physiol Cell Physiol 302: C1569–C1587, 2012. doi: 10.1152/ajpcell.00017.2012. [DOI] [PubMed] [Google Scholar]
- 16.He P, Klein J, Yun CC. Activation of Na+/H+ exchanger NHE3 by angiotensin II is mediated by inositol 1,4,5-triphosphate (IP3) receptor-binding protein released with IP3 (IRBIT) and Ca2+/calmodulin-dependent protein kinase II. J Biol Chem 285: 27869–27878, 2010. doi: 10.1074/jbc.M110.133066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He P, Xie Z, Yun C. IRBIT mediates trafficking and activation of Na+,K+-ATPase by angiotensin II (Abstract). FASEB J, Suppl, 969.8, 2015. [Google Scholar]
- 18.He P, Zhang H, Yun CC. IRBIT, inositol 1,4,5-triphosphate (IP3) receptor-binding protein released with IP3, binds Na+/H+ exchanger NHE3 and activates NHE3 activity in response to calcium. J Biol Chem 283: 33544–33553, 2008. doi: 10.1074/jbc.M805534200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He P, Zhao L, No YR, Karvar S, Yun CC. The NHERF1 PDZ1 domain and IRBIT interact and mediate the activation of Na+/H+ exchanger 3 by ANG II. Am J Physiol Renal Physiol 311: F343–F351, 2016. doi: 10.1152/ajprenal.00247.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Healy V, Thompson C, Johns EJ. The adrenergic regulation of proximal tubular Na+/H+ exchanger 3 in the rat. Acta Physiol (Oxf) 210: 678–689, 2014. doi: 10.1111/apha.12181. [DOI] [PubMed] [Google Scholar]
- 21.Hoogerwerf WA, Tsao SC, Devuyst O, Levine SA, Yun CH, Yip JW, Cohen ME, Wilson PD, Lazenby AJ, Tse CM, Donowitz M. NHE2 and NHE3 are human and rabbit intestinal brush-border proteins. Am J Physiol 270: G29–G41, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Johansson S, Rosenbaum DP, Knutsson M, Leonsson-Zachrisson M. A phase 1 study of the safety, tolerability, pharmacodynamics, and pharmacokinetics of tenapanor in healthy Japanese volunteers. Clin Exp Nephrol 2016. doi: 10.1007/s10157-016-1302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiela PR, Laubitz D, Larmonier CB, Midura-Kiela MT, Lipko MA, Janikashvili N, Bai A, Thurston R, Ghishan FK. Changes in mucosal homeostasis predispose NHE3 knockout mice to increased susceptibility to DSS-induced epithelial injury. Gastroenterology 137: 965–975, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labonté ED, Carreras CW, Leadbetter MR, Kozuka K, Kohler J, Koo-McCoy S, He L, Dy E, Black D, Zhong Z, Langsetmo I, Spencer AG, Bell N, Deshpande D, Navre M, Lewis JG, Jacobs JW, Charmot D. Gastrointestinal inhibition of sodium-hydrogen exchanger 3 reduces phosphorus absorption and protects against vascular calcification in CKD. J Am Soc Nephrol 26: 1138–1149, 2015. doi: 10.1681/ASN.2014030317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ledoussal C, Lorenz JN, Nieman ML, Soleimani M, Schultheis PJ, Shull GE. Renal salt wasting in mice lacking NHE3 Na+/H+ exchanger but not in mice lacking NHE2. Am J Physiol Renal Physiol 281: F718–F727, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Ledoussal C, Woo AL, Miller ML, Shull GE. Loss of the NHE2 Na+/H+ exchanger has no apparent effect on diarrheal state of NHE3-deficient mice. Am J Physiol Gastrointest Liver Physiol 281: G1385–G1396, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Li HC, Du Z, Barone S, Rubera I, McDonough AA, Tauc M, Zahedi K, Wang T, Soleimani M. Proximal tubule specific knockout of the Na+/H+ exchanger NHE3: effects on bicarbonate absorption and ammonium excretion. J Mol Med (Berl) 91: 951–963, 2013. doi: 10.1007/s00109-013-1015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li XC, Shull GE, Miguel-Qin E, Chen F, Zhuo JL. Role of the Na+/H+ exchanger 3 in angiotensin II-induced hypertension in NHE3-deficient mice with transgenic rescue of NHE3 in small intestines. Physiol Rep 3: e12605, 2015. doi: 10.14814/phy2.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li XC, Shull GE, Miguel-Qin E, Zhuo JL. Role of the Na+/H+ exchanger 3 in angiotensin II-induced hypertension. Physiol Genomics 47: 479–487, 2015. doi: 10.1152/physiolgenomics.00056.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linz B, Hohl M, Reil JC, Böhm M, Linz D. Inhibition of NHE3-mediated sodium absorption in the gut reduced cardiac end-organ damage without deteriorating renal function in obese spontaneously hypertensive rats. J Cardiovasc Pharmacol 67: 225–231, 2016. doi: 10.1097/FJC.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 31.Linz D, Wirth K, Linz W, Heuer HO, Frick W, Hofmeister A, Heinelt U, Arndt P, Schwahn U, Böhm M, Ruetten H. Antihypertensive and laxative effects by pharmacological inhibition of sodium-proton-exchanger subtype 3-mediated sodium absorption in the gut. Hypertension 60: 1560–1567, 2012. doi: 10.1161/HYPERTENSIONAHA.112.201590. [DOI] [PubMed] [Google Scholar]
- 32.McDonough AA. Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure. Am J Physiol Regul Integr Comp Physiol 298: R851–R861, 2010. doi: 10.1152/ajpregu.00002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishinaga H, Komatsu R, Doi M, Fustin JM, Yamada H, Okura R, Yamaguchi Y, Matsuo M, Emoto N, Okamura H. Circadian expression of the Na+/H+ exchanger NHE3 in the mouse renal medulla. Biomed Res 30: 87–93, 2009. doi: 10.2220/biomedres.30.87. [DOI] [PubMed] [Google Scholar]
- 34.Noonan WT, Woo AL, Nieman ML, Prasad V, Schultheis PJ, Shull GE, Lorenz JN. Blood pressure maintenance in NHE3-deficient mice with transgenic expression of NHE3 in small intestine. Am J Physiol Regul Integr Comp Physiol 288: R685–R691, 2005. doi: 10.1152/ajpregu.00209.2004. [DOI] [PubMed] [Google Scholar]
- 35.Onishi A, Fu Y, Song P, Busslinger M, Soleimani M, Rubera I, Tauc M, Vallon V. A role for NHE3 in renal sodium and acid-base handling in the early proximal tubule and further downstream segments (Abstract). J Am Soc Nephrol 26: 847A, 2015. [Google Scholar]
- 36.Pontes RB, Crajoinas RO, Nishi EE, Oliveira-Sales EB, Girardi AC, Campos RR, Bergamaschi CT. Renal nerve stimulation leads to the activation of the Na+/H+ exchanger isoform 3 via angiotensin II type I receptor. Am J Physiol Renal Physiol 308: F848–F856, 2015. doi: 10.1152/ajprenal.00515.2014. [DOI] [PubMed] [Google Scholar]
- 37.Rieg T, Gerasimova M, Murray F, Masuda T, Tang T, Rose M, Drucker DJ, Vallon V. Natriuretic effect by exendin-4, but not the DPP-4 inhibitor alogliptin, is mediated via the GLP-1 receptor and preserved in obese type 2 diabetic mice. Am J Physiol Renal Physiol 303: F963–F971, 2012. doi: 10.1152/ajprenal.00259.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Routledge FS, McFetridge-Durdle JA, Dean CR; Canadian Hypertension Society . Night-time blood pressure patterns and target organ damage: a review. Can J Cardiol 23: 132–138, 2007. doi: 10.1016/S0828-282X(07)70733-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabbagh Y, Giral H, Caldas Y, Levi M, Schiavi SC. Intestinal phosphate transport. Adv Chronic Kidney Dis 18: 85–90, 2011. doi: 10.1053/j.ackd.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sabbagh Y, O’Brien SP, Song W, Boulanger JH, Stockmann A, Arbeeny C, Schiavi SC. Intestinal npt2b plays a major role in phosphate absorption and homeostasis. J Am Soc Nephrol 20: 2348–2358, 2009. doi: 10.1681/ASN.2009050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saifur Rohman M, Emoto N, Nonaka H, Okura R, Nishimura M, Yagita K, van der Horst GT, Matsuo M, Okamura H, Yokoyama M. Circadian clock genes directly regulate expression of the Na+/H+ exchanger NHE3 in the kidney. Kidney Int 67: 1410–1419, 2005. doi: 10.1111/j.1523-1755.2005.00218.x. [DOI] [PubMed] [Google Scholar]
- 42.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 43.Solocinski K, Richards J, All S, Cheng KY, Khundmiri SJ, Gumz ML. Transcriptional regulation of NHE3 and SGLT1 by the circadian clock protein Per1 in proximal tubule cells. Am J Physiol Renal Physiol 309: F933–F942, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spencer AG, Labonte ED, Rosenbaum DP, Plato CF, Carreras CW, Leadbetter MR, Kozuka K, Kohler J, Koo-McCoy S, He L, Bell N, Tabora J, Joly KM, Navre M, Jacobs JW, Charmot D. Intestinal inhibition of the Na+/H+ exchanger 3 prevents cardiorenal damage in rats and inhibits Na+ uptake in humans. Sci Transl Med 6: 227ra36, 2014. doi: 10.1126/scitranslmed.3007790. [DOI] [PubMed] [Google Scholar]
- 45.Subramanya SB, Rajendran VM, Srinivasan P, Nanda Kumar NS, Ramakrishna BS, Binder HJ. Differential regulation of cholera toxin-inhibited Na-H exchange isoforms by butyrate in rat ileum. Am J Physiol Gastrointest Liver Physiol 293: G857–G863, 2007. doi: 10.1152/ajpgi.00462.2006. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Armando I, Upadhyay K, Pascua A, Jose PA. The regulation of proximal tubular salt transport in hypertension: an update. Curr Opin Nephrol Hypertens 18: 412–420, 2009. doi: 10.1097/MNH.0b013e32832f5775. [DOI] [PMC free article] [PubMed] [Google Scholar]