Abstract
The ionotropic purine type 2X7 receptor (P2X7R) is a nonspecific cation channel implicated in sleep regulation and brain cytokine release. Many endogenous rhythms covary with sleep, including locomotor activity and core body temperature. Furthermore, brain-hypothalamic cytokines and purines play a role in the regulation of these physiological parameters as well as sleep. We hypothesized that these parameters are also affected by the absence of the P2X7 receptor. Herein, we determine spontaneous expression of body temperature and locomotor activity in wild-type (WT) and P2X7R knockout (KO) mice and how they are affected by sleep deprivation (SD). We also compare hypothalamic, hippocampal, and cortical cytokine- and purine-related receptor and enzyme mRNA expressions before and after SD in WT and P2X7RKO mice. Next, in a hypothesis-generating survey of hypothalamic long noncoding (lnc) RNAs, we compare lncRNA expression levels between strains and after SD. During baseline conditions, P2X7RKO mice had attenuated temperature rhythms compared with WT mice, although locomotor activity patterns were similar in both strains. After 6 h of SD, body temperature and locomotion were enhanced to a greater extent in P2X7RKO mice than in WT mice during the initial 2-3 h after SD. Baseline mRNA levels of cortical TNF-α and P2X4R were higher in the KO mice than WT mice. In response to SD, the KO mice failed to increase hypothalamic adenosine deaminase and P2X4R mRNAs. Further, hypothalamic lncRNA expressions varied by strain, and with SD. Current data are consistent with a role for the P2X7R in thermoregulation and lncRNA involvement in purinergic signaling.
Keywords: purine signaling, P2X7R knockout mice, long noncoding RNA, thermoregulation, adenosine deaminase, cytokines
in the brain, nucleoside receptors (purine type 1 receptors; P1Rs) and nucleotide receptors (purine type 2 receptors; P2Rs) regulate multiple biological systems, including sleep, blood flow, and inflammation (4, 53). Attributing physiological function to specific nucleosides or nucleotides is difficult because they are transient and labile in vivo. In addition, P2R signaling is complex, e.g., in the central nervous system, P2X4Rs are coexpressed with P2X7R in astrocytes and microglia, and P2X4Rs provide ancillary support to P2X7Rs in the release of cytokines (18, 19, 35). A variety of transport systems, anabolic and catabolic enzymes, and kinases and aminases rapidly affect purine levels. Furthermore, intracellular ATP concentrations are ~10,000 times higher than adenosine concentrations; thus substantial changes in ATP-derived adenosine can occur without greatly affecting intracellular ATP levels (39). Accurate measurements of nucleotides, nucleosides, and their metabolites are technically challenging and controversial (8, 17, 22, 25, 27, 40, 67, 69). Regardless, extracellular ATP in the brain extracellular fluid is derived from the release of ATP from neurons and glia as a consequence of cellular activity.
Extracellular ATP signaling is closely linked to extracellular adenosine signaling. Extracellular ATP is catabolized via the ectonucleotidases CD39 and CD73, providing a major source of extracellular adenosine. Substantial evidence supports the contribution of adenosine and P1Rs to sleep regulation (1, 44, 50, 58). Furthermore, a role for ATP and P2Rs in sleep regulation is described (37, 38). For instance, brain levels of P2X7R mRNA change with sleep loss and time of day. Moreover, mice lacking functional P2X7Rs [called P2X7RKO (knockout) mice hereafter] have less non-rapid eye movement sleep (NREMS) and attenuated NREMS electroencephalogram slow-wave activity after sleep deprivation (SD) compared with wild-type (WT) mice (38).
The P2X receptors are ATP-gated ionotropic channels while P2Y receptors are metabotropic receptors (65). There are many distinct purinergic receptors with different functions (6). P2X7Rs promote cytokine processing and release (15). For instance, the P2X7R is necessary for activating IL-1β by caspase-1, cleaving the pro-IL-1β into its mature form (5, 55). P2X7Rs also mediate TNF-α release; microglial TNF-α release is induced by P2X7R agonists (28, 59). Brain expression of IL-1β and TNF-α are cell activity dependent (7, 26), and these cytokines are involved in sleep and body temperature regulation in health and disease (12). Because ATP is released with neuro- and gliotransmission (20, 32, 47, 56) and neuronal activity is greater during wakefulness (49, 64), extracellular ATP is posited to be the brain’s mechanism to track prior cellular activity and hence the duration of wakefulness (37).
Long noncoding RNAs (lncRNAs) are >200-base pair RNAs that do not code for specific proteins. However, they are capable of altering gene expression directly or via microRNA (miRNA) or epigenetic interactions (46). lncRNAs are posited to regulate adaptation to cellular stress (54). Roughly 80% of transcription is dedicated to noncoding RNAs (34), and the majority of tissue-specific lncRNAs are found in the brain (13). Furthermore, although miRNAs are implicated in sleep regulation (9–11), and there are bidirectional lncRNA-miRNA interactions in the brain (30), lncRNAs have not heretofore been systematically linked to sleep or body temperature.
Herein, we extend our prior studies of P2X7R signaling by now showing that P2X7RKO mice have lower core body temperature than WT mice and that hypothalamic mRNAs expressions differ between strains. We demonstrate that after SD, temperature and locomotor activity responses in P2X7RKO mice increase disproportionately vs. the responses observed in WT mice. We also report that several hypothalamic lncRNAs change with mouse strain and SD.
METHODS
Animals
Male homozygous P2X7RKO mice (strain B6.129P2-P2rx7/J; backcrossed on a C57BL/6 background; https://www.jax.org/strain/005576) and WT mice (strain C57BL/6J) were purchased from Jackson Laboratory (Bar Harbor, ME) and bred at Washington State University in an American Association for Laboratory Animal Care-approved vivarium. All mice were housed at room temperature (24 ± 1°C) under a 12:12-h light-dark cycle (light onset = ZT0, dark onset = ZT12) and had free access to food and water. P2X7RKO mice have normal body weight at birth, but are ~16% smaller than WT mice at 9 mo of age. The mice used in the experiments reported here were euthanized at 10 wk of age, a time before the reported growth differences between the P2X7RKO strain and WT mice occur (36). At the conclusion of the studies, mice were genotyped from tail snips by Transnetyx (Cordova, TN); all mice had the expected genotypes. All experiments and procedures were approved by Washington State University’s Institutional Animal Care and Use Committee and were designed to minimize animal pain and discomfort in accordance with National Institutes of Health guidelines.
Experimental Design and Data Analyses
Sixty mice between 8 and 10 wk old (30 P2X7RKO and 30 WT) were randomly assigned to one of two experiments: experiment 1 for body temperature and locomotor activity determinations before and after SD or experiment 2 for mRNA or lncRNA determinations with or without SD. In experiment 1, 20 mice (10 P2X7RKO and 10 WT mice) were anesthetized with intraperitoneal injections of ketamine and xylazine (87 and 13 mg/kg, respectively). Minimitter biotelemetry transmitters (Minimitter, Bend, OR) were calibrated at 37°C, chemically sterilized with Cidex-OPA (Johnson & Johnson, New Brunswick, NJ), and implanted intraperitoneally as described elsewhere (33). Mice were allowed to recover for at least 1 wk following surgeries and were housed individually in cages placed atop Minimitter receiver plates. Data were recorded at 6-min intervals, and data points derived from those values were averaged over 1-h bins for body temperature and locomotor activity. Locomotor activity counts for each hour and each mouse were normalized by dividing hourly values by their total 24-h activity counts and are expressed as a percentage of baseline. Baseline values were recorded for 48 h beginning at ZT12 and averaged to ZT-matched hourly values to represent a 24-h period.
At ZT6, mice were manually sleep deprived by experimenters blind to the mouse strain for 6 h until the onset of darkness, ZT12. The gentle handling SD method was implemented by the constant observation of a trained researcher (replaced every hour). Briefly, when a mouse became motionless a soft artist’s paintbrush was used to tap the cage or disturb the bedding around the animal to keep it awake and, when necessary, the back of the mouse was gently stroked with the brush. Sleep-rebound durations after gentle handling of SD are similar to those after voluntary SD induced by protracted intracranial self-stimulation in animals (45). Body temperature and locomotor activity recordings continued for 48 h of recovery. A mixed 2 × 24 ANOVA was used to analyze temperature or locomotor activity during baseline with STRAIN (P2X7RKO vs. WT) as a between-subjects factor and TIME (hours 1–24) as a within-subjects factor. Next, mixed 2 × 2 × 24 ANOVAs for temperature or for locomotor activity were performed as above with an added within-subjects factor of TREATMENT (control sleep vs. SD). A Huynh-Feldt adjustment was used to correct for sphericity bias (the original degrees of freedom are reported). Where appropriate, Student’s t-tests were used for a priori comparisons (pairwise comparisons were restricted to time-matched values).
For experiment 2, 40 mice (20 P2X7RKO and 20 WT mice) were used, and 10 from each strain were randomly selected to be sleep deprived as described above. Immediately following SD at ZT12, mice were decapitated and their brains were removed. The hypothalamus was dissected from the ventral surface of the brain posterior to the optic chiasm and anterior to the mammillary bodies (bregma −0.34 to −2.70 mm). The somatosensory cortex was dissected bilaterally (bregma 1.98 to −2.46 mm) ± 2.0 mm in the dorsal-ventral direction with respect to the parietotemporal ridge. The whole hippocampi from the septal to temporal poles were obtained bilaterally using a custom wire loop dissection instrument. The remaining 10 P2X7RKO mice and 10 WT mice were also euthanized at ZT12, but were allowed spontaneous sleep before death and were used as time-matched controls. Tissues were preserved in RNAlater at 4°C for 24 h and then transferred to −20°C until RNA was isolated for qPCR or lncRNA arrays.
Tissues were thawed and total RNA was extracted in TRIzol reagent (Invitrogen, Grand Island, NY) by mechanical homogenization. Extracts were then treated with DNase digestion, and RNA purity was measured by spectrometry. The RNA was reverse transcribed to cDNA, and qPCR was performed for IL-1β, TNF-α, BDNF, adenosine deaminase (ADA), and P2X4 mRNAs (cDNAs; primers in Table 1) as described elsewhere (61). Values obtained were normalized to the reference gene cyclophilin A (CycA). CycA mRNA is routinely used in sleep studies because it is constitutively expressed and does not vary across the day or with sleep loss (60). The mean of the WT control Ct values were computed, and the ΔCt values were determined by subtracting the average CycA Ct value from control WT, control P2X7RKO, SD WT, and SD P2X7RKO Ct values for each gene. Then, mRNA expression was evaluated using a comparative Ct method (User Bulletin 2; PE Applied Biosystems, Foster City, CA) using the formula: 2 (ΔCt for treatment/strain mean from the control mean) – (ΔCt for control from the control mean). A one-way ANOVA for each selected gene was performed on these values using the statistical software SPSS, version 17. When appropriate, Student's t-tests were used for pairwise comparisons.
Table 1.
Primers used in PCR for BDNF, IL-1β, TNF-α, ADA, P2X4R, and CycA
| Gene | Primer Sequence (Sense) 5′–3′ | Primer Sequence (Antisense) 5′–3′ |
|---|---|---|
| BDNF | GATGAGGACCAGAAGGTTCG | TCCAGCAGAAAGAGCAGAGG |
| IL-1β | ACCCAAGCACCTTCTTTTCC | AGACAGCACGAGGCATTTTT |
| TNF-α | GACAAGGCTGCCCCGACTATGTGCTC | TGATGGCGGAGAGGAGGCTGACTTTC |
| ADA | AAGCATTTGGCATCAAGGTC | CATAGCCACCACGGTCTTCT |
| P2X4R | TCCTGATAAGACCAGCATTT | CAAGAGGGTGAAGTTTTCTG |
| CycA | AGGATTCATGTGCCAGG | TGGACAAGATGCCAGGA |
BDNF, brain-derived neurotrophic factor; ADA, adsenosine deaminase; CycA, cyclophilin A.
Seventy-eight hypothalamic lncRNA concentrations were quantified using mouse RT2 lncRNA PCR arrays (LAMM-004z; Qiagen, Valencia, CA) with probes bioinformatically predicted by the manufacturer to detect inflammatory and autoimmune response-related lncRNAs. Three total RNA extracts from each group (3 WT, 3 P2X7RKO, 3 sleep-deprived WT, and 3 sleep-deprived P2X7RKO mice) were randomly selected from the above samples and were purified using the RNeasy Mini Kit (Qiagen). Purity was assessed with the NanoDrop ND-1000 spectrophotometer (ThermoFisher, Wilmington, DE). All samples had OD260/OD280-nm ratios of 2.0 and OD260/OD230-nm ratios of 1.8–2.1. The on-plate control wells for efficacy of reverse transcriptase, PCR product, and lack of DNA contamination were positive for all 12 plates.
The RT2 First Strand Kit (Qiagen) for cDNA synthesis (1 μg RNA/20 μl RT reaction) and the RT2 lncRNA PCR arrays were used according to the manufacturers' instructions. lncRNA detection was accomplished using RT2 SYBR Green qPCR Mastermix with a CFX96 cycler (Bio-Rad, Nazareth, Belgium). Analysis of the lncRNA data was performed using the open access R statistical version 3.1.3 https://www.r-project.org/. The bioconductor package HTqPCR (16) was then used to process the data as well to identify differentially expressed lncRNAs. Six lncRNAs with raw Ct values >35 or undetected values were excluded from the data set. Normalization of raw Ct values was carried out using the ΔCt method using the average Ct of the housekeeping genes (Actb, B2m, Rplp0, Rn7sk, Snora73b) subtracted from each probe Ct value in the set. Identification of differentially expressed genes was performed using linear models for microarray data with the included wrapper function for the limma R-Bioconductor open source statistical package. Pairwise comparisons between all four of the experimental groups were performed as well as between pooled strain and treatment data. To limit the false discovery rate (FDR), P values ≤0.01 or less were considered significant.
RESULTS
Experiment 1
Temperature and locomotor activity strain comparisons for baseline.
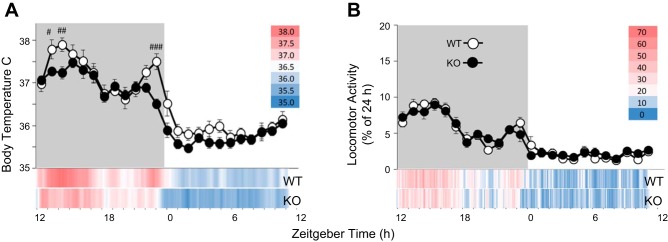
Compared with WT mice, P2X7RKO mice had lower body temperatures by as much as 1°C, particularly during the rodent’s active period (ZT12-24; TIME × STRAIN: F(23, 414) = 2.776, P < 0.01). Specifically, the bimodal core temperature peaks at ZT13-16 and ZT22-23 typically observed in WT mice were attenuated in P2X7RKO mice (Fig. 1A).
Fig. 1.
Purine type 2X7 receptor knockout (P2X7RKO) mice have lower body temperatures. Basal body temperature (A) and locomotor activity (B) in wild -type (WT; white symbols) and P2X7RKO (KO; black symbols) mice are shown. Shadowed background indicates dark period (#P < 0.05, ## P < 0.01, ###P < 0.001; n = 10/strain). Temperature data are presented as means and locomotor activity as a fractional percentage of a 24-h baseline (±SE). At the bottom of each panel, baseline temperature and activity data are also displayed in 6-min increments as a heat map (see legend) for rapid visualization of differences between strains.
Throughout baseline recordings, locomotor activity was not significantly different between strains and was higher in both strains during the dark period (total counts WT = 3,332, P2X7RKO = 2,756) compared with the light period (total counts WT = 1,035, P2X7RKO = 956; Fig. 1B; TIME: F(23, 414) = 33.187, P < 0.001. Although the counts are not significantly different between strains, they are in the same direction as the lower temperatures in the WT. Thus the locomotor activity and body temperature relationships appear to be retained in the P2X7RKO mice. The TIME factor, indicating the expected diurnal variations for either locomotor activity or body temperature, was significant in all subsequent analyses and will not be mentioned hereafter except where significant interactions with STRAIN occurred.
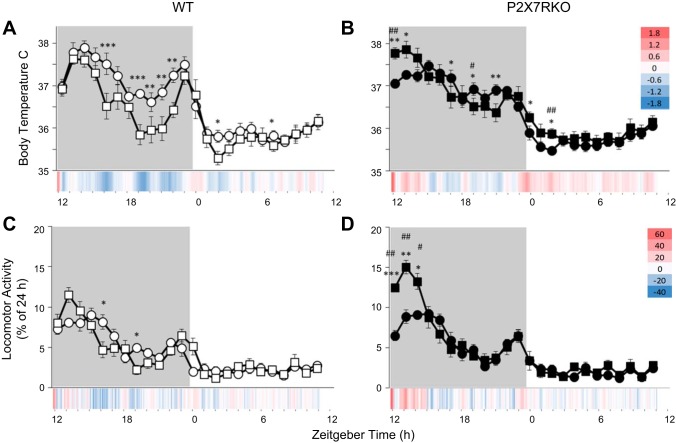
Sleep loss-induced temperature and locomotor activity responses differ by strain.
During the 6-h SD period, locomotor activity and temperature increased with respect to baseline, but no significant strain differences in temperature or locomotor activity were present (data not shown). During the recovery period after SD, temperature was differentially altered in P2X7RKO (Fig. 2B compared with WT mice [Fig. 2; TREATMENT × STRAIN: F(1,18) = 46.013, P < 0.001]. In WT mice, after a brief initial increase, temperature began to gradually decrease from baseline values after SD particularly in the latter half of the dark period [ZT19-23; TIME × STRAIN: F(23,414) = 3.105, P < 0.001; Figs. 1, D and E, and 2A]. Small but statistically significant temperature decreases in WT mice persisted during the subsequent light phase (ZT1.5–3.5; Figs. 1, D and E, and 2A). In contrast, with respect to their own baseline values, P2X7RKO mice exhibited a multiphasic temperature response following sleep loss. An initial increase in temperature immediately after SD (ZT12-14) occurred in P2X7RKO mice and reversed direction to a hypothermic phase at the time when WT mice manifest their largest temperature decreases (ZT16-20). Later in P2X7RKO mice, body temperatures reverted back to higher temperatures observed just after light onset (ZT0-2; Fig. 2B).
Fig. 2.
Sleep loss causes differential body temperature and locomotor activity responses in WT and P2X7RKO mice. Body temperature (A and B) and locomotor activity (C and D) in WT (white symbols) and P2X7RKO (black symbols) mice following 6 h of sleep deprivation (square symbols) or spontaneous sleep (round symbols). Temperature data are presented as means and locomotor activity as fractional percentages of a 24-h baseline (±SE) and at the bottom of each panel in a heat map of differences from baseline values in 6-min increments for ease of visualization. Shadowed background indicates dark period (treatment comparisons: *P < 0.05, **P < 0.01, ***P < 0.001; strain comparisons: #P < 0.05, ##P < 0.01; n = 10/strain).
In WT mice, the initial increase in locomotor activity after sleep loss occurred was not statistically significant. Shortly thereafter, locomotor activity decreased within the corresponding period of maximal temperature decreases (viz. ZT16 and 19; Fig. 2C). Conversely, during the first 3 h post-SD, locomotor activity dramatically increased by 623 counts in P2X7RKO mice above their own baseline values and above the 162 counts [t(18) = 3.35; P = 0.004] induced by sleep loss in WT mice [Fig. 2D; TREATMENT × STRAIN: F(1,18) = 5.820, P < 0.027]. Locomotor activity counts and body temperatures in both strains were comparable to baseline values on the second recovery day (data not shown).
Experiment 2
Effects of strain and sleep loss on hypothalamic, somatosensory, and hippocampal mRNAs.
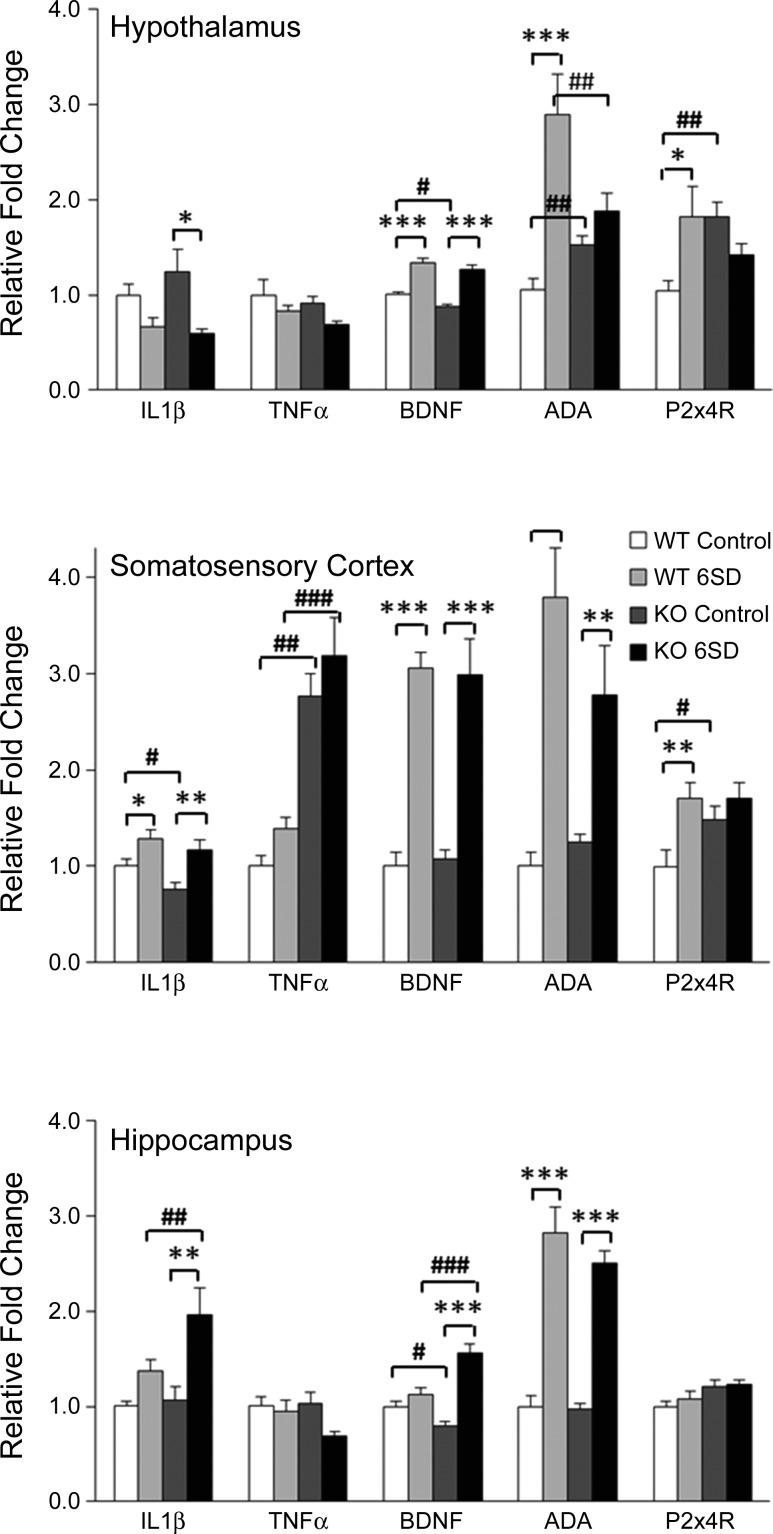
Basal mRNA expressions within some brain regions of P2X7RKO mice differed from those of WT mice (Fig. 3. Hypothalamic ADA [F(3,28) = 10.347, P < 0.001] and P2X4R [F(3,28) = 3.639, P < 0.05] mRNAs and cortical TNF-α [F(3,28) = 22.440, P < 0.001] and P2X4R [F(3,28) = 4.530, P < 0.05] mRNAs were higher in the P2X7RKO mice than in WT mice. Smaller baseline differences between strains with some transcripts were also observed. Thus, compared with WT mice, P2X7RKO mice had lower basal mRNA levels of cortical IL-1β [F(3,29) = 7.317, P < 0.01], hypothalamic, and hippocampal BDNF [F(3,33) = 37.893, P < 0.001; F(3,34) = 20.005, P < 0.001, respectively].
Fig. 3.
P2X7RKO mice have altered brain mRNA expressions before and after sleep deprivation. Relative fold-changes (+SE) in IL-1β, TNF-α, brain-derived neurotrophic factor (BDNF), adenosine deaminase (ADA), and P2X4R mRNA in the hypothalamus, somatosensory cortex, and hippocampus between WT and P2X7RKO (KO) with spontaneous sleep (Control) or after 6 h of sleep deprivation (6SD) are shown. *P < 0.05, **P < 0.01, ***P < 0.001 for treatment-induced differences. #P < 0.05, ##P < 0.01, ###P < 0.001 for strain differences.
Differential strain mRNA responses to SD also occurred (Fig. 3). Of note is that hypothalamic ADA mRNA responses were absent in the P2X7RKO mice but were large in the WT mice. In contrast, hippocampal IL-1β and BDNF mRNAs increased in the P2X7RKO mice compared with WT mice. Although cortical TNF-α mRNA levels in the P2X7RKO mice after SD were much higher than those in corresponding WT mice, they were not significantly above their own baseline levels. Finally, some of the mRNA responses to SD confirmed earlier reports in both strain;, e.g., cortical IL-1β, BDNF, and ADA all increased after SD in both strains (38, 70).
Effects of strain and sleep loss on hypothalamic lncRNAs.
There were differences in hypothalamic lncRNA expressions between strains (Table 2). Six lncRNAs were elevated in P2X7RKO compared with WT mice, including 1700020I14Rik, 9430037G07Rik, A330023F24Rik, Gm15832, Gm17275, and Neat1, whereas Gm15050 and Gm17337 were lower in P2X7RKO compared with WT mice. Notably, the decreased lncRNAs manifest the largest changes in expression magnitude between strains. In WT mice, SD led to increased hypothalamic 4930470G03Rik, A230107N01Rik, C130021I20Rik, Gm17354, and decreased A430010J10Rik expression. Only 6820431F20 levels were elevated by SD in P2X7RKO mice. No lncRNAs that changed with strain were altered by SD in a statistically significant manner; although 6820431F20 and Gm17354 were increased by SD in both strains, our significance criterion of P ≤ 0.01 was not met. Differences in the remaining lncRNAs (see online Supplemental Table 1) between strain or control and SD treatment were not statistically significant or did not meet the inclusion criteria.
Table 2.
Hypothalamic lncRNA concentrations in P2X7RKO mice expressed as fold-change from WT mice and fold-change from control after sleep deprivation in each strain
| KO-WT |
WTSD-WT |
KOSD-KO |
||||
|---|---|---|---|---|---|---|
| IncRNA | Fold-change | P value | Fold-change | P value | Fold-change | P value |
| 1700020I14Rik | 1.417 | 0.001* | 1.045 | 0.595 | 1.102 | 0.253 |
| Neat1 | 1.421 | 0.002* | 1.053 | 0.583 | 0.975 | 0.788 |
| Gm17337 | 0.584 | 0.004* | 0.925 | 0.612 | 1.574 | 0.011 |
| Gm15832 | 1.326 | 0.005* | 1.090 | 0.310 | 1.075 | 0.391 |
| Gm17275 | 1.304 | 0.005* | 1.070 | 0.402 | 0.869 | 0.096 |
| 9430037G07Rik | 1.301 | 0.008* | 1.075 | 0.398 | 0.957 | 0.607 |
| Gm15050 | 0.610 | 0.008* | 1.003 | 0.986 | 1.190 | 0.286 |
| A330023F24Rik | 1.257 | 0.010* | 1.190 | 0.037 | 1.201 | 0.030 |
| A230107N01Rik | 1.084 | 0.256 | 1.281 | 0.003* | 1.087 | 0.241 |
| Gm17354 | 1.181 | 0.017 | 1.226 | 0.005* | 1.168 | 0.023 |
| 4930470G03Rik | 1.197 | 0.018 | 1.232 | 0.008* | 1.055 | 0.432 |
| A430010J10Rik | 0.977 | 0.817 | 0.741 | 0.010* | 1.055 | 0.432 |
| C130021I20Rik | 1.451 | 0.057 | 1.718 | 0.010* | 1.026 | 0.886 |
| 6820431F20Rik | 1.112 | 0.231 | 1.246 | 0.023 | 1.326 | 0.006* |
IncRNA, long noncoding RNA; SD, sleep deprived. *Statistical significance.
DISCUSSION
A new finding reported herein is that P2X7RKO mice have reduced body temperatures compared with WT mice and that these differences were more pronounced during the dark period (Fig. 1A). In contrast, a prior report compared WT and P2X7RKO rectal temperatures at 3-h intervals (sampled within a 6-h period) and found no differences between WT and P2X7RKO mice (3). In the present report, the animals were freely moving when their temperature was recorded. Moreover, temperature was recorded with a 6-min resolution across the day and is represented by the red-to-blue color spectrum at this resolution in heat maps at the bottom of Figs. 1A and 2, A and B (for locomotion in Figs. 1B and 2, C and D). The limited sampling of body temperatures in Ref. 3 could account for differences between those findings and the current study. Irrespective of the inherent methodological issues involved in sampling rectal temperature (e.g., stress and handling), the times of day that temperature was sampled are unclear as the vivarium light cycles were not reported (3). Thus, if the mice were sampled during the latter half of the light phase, their findings are consistent with the lack of temperature differences at those times in our study.
P2XRs are linked to temperature regulation, especially in inflammation (3, 21, 24, 41, 66). After intraventricular injection of nonspecific P2XR antagonists into the rat brain (24), reported elevated body temperatures at ambient temperatures of 25°C, but did not report locomotor activity as an outcome measure. Explanations for the directionality differences in body temperature between that study and the current study may include species specificity, as we used constitutive P2X7KO mice rather than rats, or it could indicate that thermoregulatory P2XR subtypes have differential involvement in maintaining core temperature. Furthermore, pharmacokinetic profiles of the P2R antagonists would likely differ from the constitutive loss of the P2X7R.
In the first few hours during recovery from sleep loss, P2X7RKO mice had higher locomotor activity and body temperatures compared with WT mice (Fig. 2). Temperature and locomotor activity directly correlate with each other in normal mice and both are inversely correlated with sleep in WT mice (31). P2X7RKO mice thermoregulatory mechanisms may be influenced by abnormal ATP signaling in the brain. Disrupted thermoregulatory responses are observed following P2XR antagonists and LPS, suggesting a P2X7R involvement in temperature control (3, 24, 42). Nevertheless, the blunted temperature rhythms during baseline recordings do not stem from a limited response capacity for core hyperthermia in P2X7RKO mice; the SD data clearly demonstrate that P2X7RKO mice can attain higher body temperatures. During the first 2 h after SD, WT mice have ~30 min of extra NREMS, whereas P2X7RKO mice only have 10 min of extra NREMS (38). This finding is consistent with the initial increased locomotor activity and temperature that followed SD in P2X7RKO mice reported herein.
In the current study, higher levels of hypothalamic ADA mRNA following SD in WT mice were found (Fig. 3). This finding is consistent with those previously reported (70). Furthermore, WT mice have higher mean concentrations of ADA mRNA than SD P2X7RKO mice, indicating that P2X7RKO mice have a blunted ADA response to SD. This finding suggests that at least some aspect of ATP catabolism was perturbed by the absence of a functional P2X7R.
Hypothalamic and hippocampal BDNF mRNA were lower in P2X7KO compared with WT mice (Fig. 3). We anticipated that BDNF, IL-1β, and TNF-α mRNAs would decrease after SD in P2X7RKO compared with WT mice because each of these substances is altered by ATP-P2X7R interactions. However, the anticipated results were only observed with IL-1β mRNA in the hypothalamus, and although basal levels of IL-1β were lower in the cortex in P2X7RKO vs. WT mice, they increased in the cortex and hippocampus after SD in both strains. TNF-α mRNA was significantly upregulated in the cortex in P2X7RKO before and after SD compared with WT. The lack of changes in TNF-α mRNA in the hypothalamus observed in this study could be explained by masking that occurs with gross dissections. c-fos mRNA expression, a marker of cellular activity, varies within subdivisions of the hypothalamus with SD or time of day (48). Thus there may be local mRNA expression changes to specific hypothalamic nuclei that are not detected in the whole hypothalamus extracts used here. The role of proinflammatory cytokines in sleep regulation is complex as SD in WT mice attenuates hypothalamic IL-1β and TNF-α mRNAs (70), while in rats, the hypothalamic expression of these two cytokines increases with SD (43, 62).
In WT mice, but not in P2X7RKO mice, hypothalamic and somatosensory cortical P2X4R mRNA was upregulated after SD. Furthermore, in these areas baseline levels of P2X4R mRNA are higher in the P2X7RKO mice. The P2X4R and P2X7R exhibit some homologies; 49% of amino acids in the transmembrane domain and the extracellular loop are identical (65). The P2X7R and P2X4R are coexpressed and colocalize in many cell types (29, 52, 68). Initially, the P2X7R was thought to be the exclusive nonheteromeric P2XR (63). Pharmacoelectrophysiological experiments subsequently indicated the presence of functional P2X7/4R heterotrimers (14, 23), but in situ and cross-linking studies refute the existence of a P2X7/4R heterotrimer complex (2). Regardless, in endothelial cells, downregulation of either P2X4 or P2X7 receptors results in an increase in the other (65). Thus it is plausible that the lack of P2X7Rs in the KO mice led to a compensatory increase in P2X4Rs during development. However, as with all constitutive KO mouse models, developmental differences could account for strain effects.
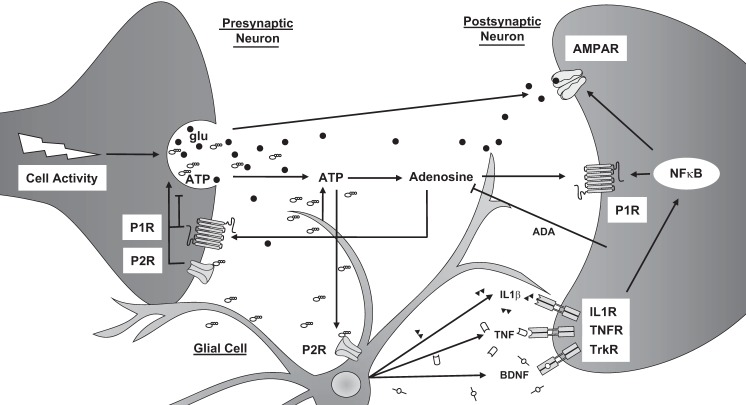
How purines, cytokines, and neurotrophins interact in the brain is complex and involves multiple cell types to affect vigilance states (Fig. 4). The absence of P2X7Rs may confer reduced neuro/glial transmission, decreased glutamate and ATP levels, leading to suppressed locomotor activity and temperatures. With the current data set, it is difficult to know whether the latter responses are indicative of a compromised homeostatic set point that delimits the activity and temperature ranges of the organism, an aberrant regulation of neurotransmitter levels linked to sleep/arousal (e.g., monoamines, catecholamines), or are a combination of other disrupted brain signaling mechanisms (e.g., cortical activity, cytokine secretion) that manifest in the observed responses to sleep loss. Altered cellular physiology is consistent with the reduced EEG amplitude that occurs during recovery sleep in P2X7RKO mice compared with WT mice (38). It is known that purinergic signaling is ubiquitous centrally and systemically and has a diverse array of receptor subtypes and multiple effectors that modulate many physiologies and pathologies (3, 4, 19, 24, 35, 36, 38, 41, 47, 66).
Fig. 4.
Cellular activity-induced links between extracellular ATP and extracellular adenosine, their receptors, and cytokines in the regulation of sleep. Cellular activity leads to the secretion of glutamate (glu) in glutamatergic neurons and other neurotransmitters along with ATP into the extracellular space. ATP is also released by glia. Some ATP is hydrolyzed to adenosine, which activates purine type 1 receptors (P1R). ADA degrades adenosine to inosine, which can suppress proinflammatory cytokine signaling. Extracellular ATP activates P2X7 and P2X4 receptors (P2R). Presynaptic purinergic receptor activation modulates neurotransmission. P2X7R activation enhances processing and release of ATP, IL-1β), TNF-α, and BDNF from glial stores; these somnogenic cytokines bind to their respective receptors (tyrosine kinase receptor B; TrkB) to activate the transcription factors (NF-κB) and increase surface expression of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) and P1Rs.
There were significant strain differences in basal expressions of eight hypothalamic lncRNAs. Unlike microRNAs, the function of lncRNAs is more difficult to determine. Regardless, current results led to the formulation of several hypotheses. lncRNAs can affect the expressions of genes in their vicinity. Although speculative, the 9430037G07Rik lncRNA is located 223 kB from the gene that encodes the ectonucleotidase CD73, which converts ATP to adenosine, thereby regulating purinergic signaling. Gm15832 is within 673 kiss from the mouse IL-1 receptor sequence. Thus two potential lncRNA targets that are related to sleep could be regulated by lncRNAs.
Of the 78 hypothalamic immune functions related lncRNAs probed, six were altered by SD. Other lncRNAs (not measured in the current study) are implicated in sleep regulation. For example, the function of 116HG, a brain-enriched lncRNA, is linked to abnormal sleep in Prader-Willi syndrome and influences circadian gene expression and energy metabolism (51). Furthermore, Drosophila lacking yar, a lncRNA conserved across several insects, have fragmented sleep and attenuated sleep rebound after SD (57). There are limitations to the current lncRNA measurements. First, only three biological replicates per condition were used. Second, the array was restricted to lncRNAs predicted to interact with miRNAs that putatively affect inflammation- or immune-related targets; lncRNAs associated with other functional domains (e.g., plasticity or metabolism) or were not examined. Finally, extracts from only one brain region, the hypothalamus, were assayed. It is likely that additional lncRNAs that function to regulate sleep will be found.
Perspectives and Significance
The activation of P2X7Rs by ATP causes the release of prosomnogenic cytokines. In constitutive P2X7RKO mice, some actions associated with the P2X7R may be preserved by other P2XRs due to shared activity profiles of various P2XRs (Fig. 4). While ATP is rapidly metabolized to other biological active substances, e.g., adenosine-secreted cytokines are more persistent, allowing them to act on slower time scales (minutes to hours) on neighboring cells. Thus P2X7Rs affect both short- and long-term components of sleep/temperature regulation. These pathways are compromised in mice lacking the P2X7R, as previously demonstrated in rats injected with P2X7R antagonists and agonists, and in P2X7RKO mice (38); those findings are supported by the temperature and locomotor activity results reported here.
GRANTS
This work was supported by the National Institutes of Health Grants NS025378 and HD036520 awarded to J. M. Krueger.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.J.D. and J.M.K. provided conception and design of research; C.J.D., P.T., and K.A.H. performed experiments; C.J.D., P.T., J.N.K., and J.M.K. analyzed data; C.J.D., K.A.H., J.N.K., and J.M.K. interpreted results of experiments; C.J.D. prepared figures; C.J.D. drafted manuscript; C.J.D., P.T., K.A.H., J.N.K., and J.M.K. edited and revised manuscript; C.J.D., P.T., K.A.H., J.N.K., and J.M.K. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Stewart Bohnet for assistance with processing tissue extracts and Dr. Bin Shan for helpful discussions in the manuscript preparations.
REFERENCES
- 1.Alam MN, Szymusiak R, Gong H, King J, McGinty D. Adenosinergic modulation of rat basal forebrain neurons during sleep and waking: neuronal recording with microdialysis. J Physiol 521: 679–690, 1999. doi: 10.1111/j.1469-7793.1999.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonio LS, Stewart AP, Xu XJ, Varanda WA, Murrell-Lagnado RD, Edwardson JM. P2X4 receptors interact with both P2X2 and P2X7 receptors in the form of homotrimers. Br J Pharmacol 163: 1069–1077, 2011. doi: 10.1111/j.1476-5381.2011.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barberà-Cremades M, Baroja-Mazo A, Gomez AI, Machado F, Di Virgilio F, Pelegrín P. P2X7 receptor-stimulation causes fever via PGE2 and IL-1β release. FASEB J 26: 2951–2962, 2012. doi: 10.1096/fj.12-205765. [DOI] [PubMed] [Google Scholar]
- 4.Bianco F, Colombo A, Saglietti L, Lecca D, Abbracchio MP, Matteoli M, Verderio C. Different properties of P2X(7) receptor in hippocampal and cortical astrocytes. Purinergic Signal 5: 233–240, 2009. doi: 10.1007/s11302-009-9137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianco F, Pravettoni E, Colombo A, Schenk U, Möller T, Matteoli M, Verderio C. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol 174: 7268–7277, 2005. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- 6.Browne LE, Jiang LH, North RA. New structure enlivens interest in P2X receptors. Trends Pharmacol Sci 31: 229–237, 2010. doi: 10.1016/j.tips.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Churchill L, Rector DM, Yasuda K, Fix C, Rojas MJ, Yasuda T, Krueger JM. Tumor necrosis factor α: activity dependent expression and promotion of cortical column sleep in rats. Neuroscience 156: 71–80, 2008. doi: 10.1016/j.neuroscience.2008.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clapp-Lilly KL, Roberts RC, Duffy LK, Irons KP, Hu Y, Drew KL. An ultrastructural analysis of tissue surrounding a microdialysis probe. J Neurosci Methods 90: 129–142, 1999. doi: 10.1016/S0165-0270(99)00064-3. [DOI] [PubMed] [Google Scholar]
- 9.Davis CJ, Bohnet SG, Meyerson JM, Krueger JM. Sleep loss changes microRNA levels in the brain: a possible mechanism for state-dependent translational regulation. Neurosci Lett 422: 68–73, 2007. doi: 10.1016/j.neulet.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis CJ, Clinton JM, Krueger JM. MicroRNA 138, let-7b, and 125a inhibitors differentially alter sleep and EEG delta-wave activity in rats. J Appl Physiol (1985) 113: 1756–1762, 2012. doi: 10.1152/japplphysiol.00940.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis CJ, Clinton JM, Taishi P, Bohnet SG, Honn KA, Krueger JM. MicroRNA 132 alters sleep and varies with time in brain. J Appl Physiol (1985) 111: 665–672, 2011. doi: 10.1152/japplphysiol.00517.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis CJ, Krueger JM. Sleep and cytokines. Sleep Med Clin 7: 517–527, 2012. doi: 10.1016/j.jsmc.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG, Lagarde J, Veeravalli L, Ruan X, Ruan Y, Lassmann T, Carninci P, Brown JB, Lipovich L, Gonzalez JM, Thomas M, Davis CA, Shiekhattar R, Gingeras TR, Hubbard TJ, Notredame C, Harrow J, Guigó R. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res 22: 1775–1789, 2012. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubyak GR. Go it alone no more—P2X7 joins the society of heteromeric ATP-gated receptor channels. Mol Pharmacol 72: 1402–1405, 2007. doi: 10.1124/mol.107.042077. [DOI] [PubMed] [Google Scholar]
- 15.Dubyak GR. P2X7 receptor regulation of non-classical secretion from immune effector cells. Cell Microbiol 14: 1697–1706, 2012. doi: 10.1111/cmi.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dvinge H, Bertone P. HTqPCR: high-throughput analysis and visualization of quantitative real-time PCR data in R. Bioinformatics 25: 3325–3326, 2009. doi: 10.1093/bioinformatics/btp578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dworak M, McCarley RW, Kim T, Kalinchuk AV, Basheer R. Replies to commentaries on ATP changes during sleep. Sleep 34: 841–843, 2011. doi: 10.5665/sleep.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer W, Appelt K, Grohmann M, Franke H, Nörenberg W, Illes P. Increase of intracellular Ca2+ by P2X and P2Y receptor-subtypes in cultured cortical astroglia of the rat. Neuroscience 160: 767–783, 2009. doi: 10.1016/j.neuroscience.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 19.Fumagalli M, Brambilla R, D’Ambrosi N, Volonté C, Matteoli M, Verderio C, Abbracchio MP. Nucleotide-mediated calcium signaling in rat cortical astrocytes: role of P2X and P2Y receptors. Glia 43: 218–230, 2003. doi: 10.1002/glia.10248. [DOI] [PubMed] [Google Scholar]
- 20.Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci 8: 1078–1086, 2005. doi: 10.1038/nn1498. [DOI] [PubMed] [Google Scholar]
- 21.Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature 436: 108–111, 2005. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- 22.Grabb MC, Sciotti VM, Gidday JM, Cohen SA, van Wylen DG. Neurochemical and morphological responses to acutely and chronically implanted brain microdialysis probes. J Neurosci Methods 82: 25–34, 1998. doi: 10.1016/S0165-0270(98)00025-9. [DOI] [PubMed] [Google Scholar]
- 23.Guo C, Masin M, Qureshi OS, Murrell-Lagnado RD. Evidence for functional P2X4/P2X7 heteromeric receptors. Mol Pharmacol 72: 1447–1456, 2007. doi: 10.1124/mol.107.035980. [DOI] [PubMed] [Google Scholar]
- 24.Gurin VN, Gurin AV, Melenchuk EV, Spyer KM. The effects of activation and blockade of central P2X receptors on body temperature. Neurosci Behav Physiol 33: 845–851, 2003. doi: 10.1023/A:1025967903081. [DOI] [PubMed] [Google Scholar]
- 25.Haddad GG. Does the brain gain back energy during sleep? But what does it mean? Sleep 34: 835–836, 2011. doi: 10.5665/sleep.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hallett H, Churchill L, Taishi P, De A, Krueger JM. Whisker stimulation increases expression of nerve growth factor- and interleukin-1β-immunoreactivity in the rat somatosensory cortex. Brain Res 1333: 48–56, 2010. doi: 10.1016/j.brainres.2010.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heller HC. Repeatability is not the same as accuracy. Sleep 34: 839, 2011. doi: 10.5665/sleep.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hide I, Tanaka M, Inoue A, Nakajima K, Kohsaka S, Inoue K, Nakata Y. Extracellular ATP triggers tumor necrosis factor-α release from rat microglia. J Neurochem 75: 965–972, 2000. doi: 10.1046/j.1471-4159.2000.0750965.x. [DOI] [PubMed] [Google Scholar]
- 29.Humphreys BD, Dubyak GR. Modulation of P2X7 nucleotide receptor expression by pro- and anti-inflammatory stimuli in THP-1 monocytes. J Leukoc Biol 64: 265–273, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Iyengar BR, Choudhary A, Sarangdhar MA, Venkatesh KV, Gadgil CJ, Pillai B. Non-coding RNA interact to regulate neuronal development and function. Front Cell Neurosci 8: 47, 2014. doi: 10.3389/fncel.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jhaveri KA, Trammell RA, Toth LA. Effect of environmental temperature on sleep, locomotor activity, core body temperature and immune responses of C57BL/6J mice. Brain Behav Immun 21: 975–987, 2007. doi: 10.1016/j.bbi.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jo YH, Role LW. Coordinate release of ATP and GABA at in vitro synapses of lateral hypothalamic neurons. J Neurosci 22: 4794–4804, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapás L, Bohnet SG, Traynor TR, Majde JA, Szentirmai E, Magrath P, Taishi P, Krueger JM. Spontaneous and influenza virus-induced sleep are altered in TNF-double-receptor deficient mice. J Appl Physiol (1985) 105: 1187–1198, 2008. doi: 10.1152/japplphysiol.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R, Willingham AT, Stadler PF, Hertel J, Hackermüller J, Hofacker IL, Bell I, Cheung E, Drenkow J, Dumais E, Patel S, Helt G, Ganesh M, Ghosh S, Piccolboni A, Sementchenko V, Tammana H, Gingeras TR. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science 316: 1484–1488, 2007. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- 35.Kawano A, Tsukimoto M, Noguchi T, Hotta N, Harada H, Takenouchi T, Kitani H, Kojima S. Involvement of P2X4 receptor in P2X7 receptor-dependent cell death of mouse macrophages. Biochem Biophys Res Commun 419: 374–380, 2012. doi: 10.1016/j.bbrc.2012.01.156. [DOI] [PubMed] [Google Scholar]
- 36.Ke HZ, Qi H, Weidema AF, Zhang Q, Panupinthu N, Crawford DT, Grasser WA, Paralkar VM, Li M, Audoly LP, Gabel CA, Jee WS, Dixon SJ, Sims SM, Thompson DD. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol Endocrinol 17: 1356–1367, 2003. doi: 10.1210/me.2003-0021. [DOI] [PubMed] [Google Scholar]
- 37.Krueger JM, Rector DM, Roy S, Van Dongen HP, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci 9: 910–919, 2008. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krueger JM, Taishi P, De A, Davis CJ, Winters BD, Clinton J, Szentirmai E, Zielinski MR. ATP and the purine type 2 X7 receptor affect sleep. J Appl Physiol (1985) 109: 1318–1327, 2010. doi: 10.1152/japplphysiol.00586.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landolt HP. Sleep homeostasis: a role for adenosine in humans? Biochem Pharmacol 75: 2070–2079, 2008. doi: 10.1016/j.bcp.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 40.Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem 79: 463–484, 2001. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- 41.Lister MF, Sharkey J, Sawatzky DA, Hodgkiss JP, Davidson DJ, Rossi AG, Finlayson K. The role of the purinergic P2X7 receptor in inflammation. J Inflamm (Lond) 4: 5, 2007. doi: 10.1186/1476-9255-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu K, Wang J, Hu B, Shi X, Zhou J, Tang Y, Peng Y. Brilliant blue G attenuates lipopolysaccharide-mediated microglial activation and inflammation. Neural Regen Res 8: 599–608, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mackiewicz M, Sollars PJ, Ogilvie MD, Pack AI. Modulation of IL-1 β gene expression in the rat CNS during sleep deprivation. Neuroreport 7: 529–533, 1996. doi: 10.1097/00001756-199601310-00037. [DOI] [PubMed] [Google Scholar]
- 44.Murillo-Rodriguez E, Blanco-Centurion C, Gerashchenko D, Salin-Pascual RJ, Shiromani PJ. The diurnal rhythm of adenosine levels in the basal forebrain of young and old rats. Neuroscience 123: 361–370, 2004. doi: 10.1016/j.neuroscience.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 45.Oonk M, Krueger JM, Davis CJ. Voluntary sleep loss in rats. Sleep 39: 1467–1479, 2016. doi: 10.5665/sleep.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ørom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G, Lai F, Zytnicki M, Notredame C, Huang Q, Guigo R, Shiekhattar R. Long noncoding RNAs with enhancer-like function in human cells. Cell 143: 46–58, 2010. doi: 10.1016/j.cell.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science 310: 113–116, 2005. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 48.Peterfi Z, Churchill L, Hajdu I, Obál F Jr, Krueger JM, Parducz A. Fos-immunoreactivity in the hypothalamus: dependency on the diurnal rhythm, sleep, gender, and estrogen. Neuroscience 124: 695–707, 2004. doi: 10.1016/j.neuroscience.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 49.Pigarev IN, Nothdurft HC, Kastner S. Evidence for asynchronous development of sleep in cortical areas. Neuroreport 8: 2557–2560, 1997. doi: 10.1097/00001756-199707280-00027. [DOI] [PubMed] [Google Scholar]
- 50.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science 276: 1265–1268, 1997. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Powell WT, Coulson RL, Crary FK, Wong SS, Ach RA, Tsang P, Alice Yamada N, Yasui DH, Lasalle JM. A Prader-Willi locus lncRNA cloud modulates diurnal genes and energy expenditure. Hum Mol Genet 22: 4318–4328, 2013. doi: 10.1093/hmg/ddt281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raouf R, Chabot-Doré AJ, Ase AR, Blais D, Séguéla P. Differential regulation of microglial P2X4 and P2X7 ATP receptors following LPS-induced activation. Neuropharmacology 53: 496–504, 2007. doi: 10.1016/j.neuropharm.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Skaper SD, Debetto P, Giusti P. The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J 24: 337–345, 2010. doi: 10.1096/fj.09-138883. [DOI] [PubMed] [Google Scholar]
- 54.Solé C, Nadal-Ribelles M, de Nadal E, Posas F. A novel role for lncRNAs in cell cycle control during stress adaptation. Curr Genet 61: 299–308, 2015. doi: 10.1007/s00294-014-0453-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem 276: 125–132, 2001. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 56.Song Z, Sladek CD. Site of ATP and phenylephrine synergistic stimulation of vasopressin release from the hypothalamo-neurohypophyseal system. J Neuroendocrinol 18: 266–272, 2006. doi: 10.1111/j.1365-2826.2006.01411.x. [DOI] [PubMed] [Google Scholar]
- 57.Soshnev AA, Ishimoto H, McAllister BF, Li X, Wehling MD, Kitamoto T, Geyer PK. A conserved long noncoding RNA affects sleep behavior in Drosophila. Genetics 189: 455–468, 2011. doi: 10.1534/genetics.111.131706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strecker RE, Morairty S, Thakkar MM, Porkka-Heiskanen T, Basheer R, Dauphin LJ, Rainnie DG, Portas CM, Greene RW, McCarley RW. Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behav Brain Res 115: 183–204, 2000. doi: 10.1016/S0166-4328(00)00258-8. [DOI] [PubMed] [Google Scholar]
- 59.Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci 24: 1–7, 2004. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taishi P, Bredow S, Guha-Thakurta N, Obál F Jr, Krueger JM. Diurnal variations of interleukin-1 beta mRNA and beta-actin mRNA in rat brain. J Neuroimmunol 75: 69–74, 1997. doi: 10.1016/S0165-5728(97)00002-7. [DOI] [PubMed] [Google Scholar]
- 61.Taishi P, Davis CJ, Bayomy O, Zielinski MR, Liao F, Clinton JM, Smith DE, Krueger JM. Brain-specific interleukin-1 receptor accessory protein in sleep regulation. J Appl Physiol (1985) 112: 1015–1022, 2012. doi: 10.1152/japplphysiol.01307.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taishi P, Gardi J, Chen Z, Fang J, Krueger JM. Sleep deprivation increases the expression of TNF alpha mRNA and TNF 55kD receptor mRNA in rat brain. Physiologist 42: A4, 1999. [Google Scholar]
- 63.Torres GE, Egan TM, Voigt MM. Hetero-oligomeric assembly of P2X receptor subunits. Specificities exist with regard to possible partners. J Biol Chem 274: 6653–6659, 1999. doi: 10.1074/jbc.274.10.6653. [DOI] [PubMed] [Google Scholar]
- 64.Vyazovskiy VV, Cirelli C, Tononi G. Electrophysiological correlates of sleep homeostasis in freely behaving rats. Prog Brain Res 193: 17–38, 2011. doi: 10.1016/B978-0-444-53839-0.00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weinhold K, Krause-Buchholz U, Rödel G, Kasper M, Barth K. Interaction and interrelation of P2X7 and P2X4 receptor complexes in mouse lung epithelial cells. Cell Mol Life Sci 67: 2631–2642, 2010. doi: 10.1007/s00018-010-0355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilhelm K, Ganesan J, Müller T, Dürr C, Grimm M, Beilhack A, Krempl CD, Sorichter S, Gerlach UV, Jüttner E, Zerweck A, Gärtner F, Pellegatti P, Di Virgilio F, Ferrari D, Kambham N, Fisch P, Finke J, Idzko M, Zeiser R. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat Med 16: 1434–1438, 2010. doi: 10.1038/nm.2242. [DOI] [PubMed] [Google Scholar]
- 67.Wilson DF. Measuring in vivo metabolite levels in brain. Sleep 34: 837, 2011. doi: 10.5665/sleep.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilson HL, Varcoe RW, Stokes L, Holland KL, Francis SE, Dower SK, Surprenant A, Crossman DC. P2X receptor characterization and IL-1/IL-1Ra release from human endothelial cells. Br J Pharmacol 151: 96–127, 2007. doi: 10.1038/sj.bjp.0707213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wisor JP, Schmidt MA, Clegern WC. Evidence for neuroinflammatory and microglial changes in the cerebral response to sleep loss. Sleep 34: 261–272, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zielinski MR, Taishi P, Clinton JM, Krueger JM. 5′-Ectonucleotidase-knockout mice lack non-REM sleep responses to sleep deprivation. Eur J Neurosci 35: 1789–1798, 2012. doi: 10.1111/j.1460-9568.2012.08112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.