Abstract
While restoration of ACE2 activity in the pancreas leads to improvement of glycemia in experimental models of Type 2 diabetes, global deficiency in ACE2 disrupts β-cell function and impairs glucose tolerance in mice, demonstrating the physiological role of ACE2 in glucose homeostasis. Although the contribution of pancreatic ACE2 to glucose regulation has been demonstrated in genetic models of diabetes and in models with overexpression of the renin-angiotensin system (RAS), it is unclear whether islet ACE2 is involved in glycemic control in common models of human Type 2 diabetes. To determine whether diet-induced diabetes deregulates glucose homeostasis via reduction of ACE2 in the pancreatic islets, wild-type (WT) and ACE2 knockout (KO) male mice were fed a high-fat diet (HFD) for 16 wk. ACE2 KO mice were more susceptible than WT mice to HFD-mediated glycemic dysregulation. Islet ACE2 activity and expression of various genes, including ANG II type 1a receptor (mAT1aR) were then assessed. Surprisingly, we observed no change in islet ACE2 activity and expression despite local RAS overactivity, indicated by an upregulation of mAT1aR expression. Despite a predominant expression in islet α-cells, further investigation highlighted a minor role for ACE2 on glucagon expression. Further, pancreatic ACE2 gene therapy improved glycemia in HFD-fed WT mice, leading to enhanced glucose-stimulated insulin secretion, reduced pancreatic ANG II levels, fibrosis, and ADAM17 activity. Altogether, our study demonstrates that HFD feeding increases RAS activity and mediates glycemic dysregulation likely through loss of ACE2 present outside the islets but independently of changes in islet ACE2.
Keywords: high-fat diet, pancreatic islets, ACE2, Type 2 diabetes, renin-angiotensin system
high-fat diet (HFD) feeding increases renin-angiotensin system (RAS) activity (17), which, in turn, disrupts glucose homeostasis and mediates hyperglycemia (9, 10). Interestingly, mice overexpressing angiotensinogen (AGT) in the adipose tissue develop obesity (33), while lack of AGT protects against HFD-induced obesity (34), underscoring the importance of reducing RAS overactivity in metabolic disorders. An accumulating body of evidence demonstrates the presence of RAS components in the pancreas (26–28). Several studies in rodents demonstrated the differential distribution pattern of RAS components, such as angiotensinogen, renin, ACE2, AT1aR, and AT1bR in both exocrine and endocrine portion of the pancreas, including, but not limited to, pancreatic ductal epithelial cells, endothelial cells lining pancreatic blood vessels, and islets (14, 26–28). In addition to rodents, the presence of AT1aR in the β-cells of islets, and in the endothelial cells of pancreatic blood vessels was also demonstrated in humans (47). Moreover, an overactive ACE/ANG II/AT1R axis has been shown to promote β-cell dysfunction (12, 25), and RAS blockers have been suggested to preserve β-cell function through mechanisms affecting pancreatic islet blood flow and oxidative stress (7, 12, 31). Altogether, these observations support the existence of a local angiotensin-generating system in the pancreas and the plausible role exerted by these components on pancreatic islet physiology and function. While the deleterious effects of the ACE/ANG II/AT1R axis in the regulation of glucose homeostasis have been underscored, the efficacy of RAS blockers, such as ACEIs and ARBs, has been under scrutiny due to discrepancies in reports showing the inefficiency or superiority of RAS blockers in controlling hyperglycemic symptoms or complications in humans (1, 5). The beneficial role of the alternate ACE2/ANG-(1–7)/MasR axis has also gained momentum in the context of Type 2 diabetes mellitus (T2DM), both at pancreatic and systemic level, mainly because of its opposing effects on the ACE/ANG II/AT1R axis. Studies using ANG II-infused (48) and global ACE2 knockout (KO) mice (45, 48) have shown the significance of endogenous ACE2 in regulating glycemia because of its potential role in adaptive hyperinsulinemic response in insulin-resistant state and in the regulation of glucose transporters, such as GLUT-1 and GLUT-4 peripherally (2, 45, 48). Our group has reported a downregulation in pancreatic ACE2 in db/db (3) and in ANG II-infusion mouse models (10) of impaired glycemic control, which can be attributed to a sheddase, a disintegrin, and metalloprotease 17 (ADAM17), as observed in other tissues (11, 24, 50). We further demonstrated the beneficial effects of overexpression of pancreatic ACE2 on islet function, insulin content, hyperglycemia, and glucose tolerance (3, 10). Similarly, other groups reported that 1) administration of ANG-(1–7) to diabetic rats improves first-phase insulin secretion and islet insulin content through improved pancreatic microcirculation (51); 2) a positive correlation exists between the ACE2/ANG-(1–7)/MasR axis and glucose-stimulated insulin release under high-glucose conditions in vitro (20); and 3) ACE2 loss of function made the islets more susceptible to HFD-induced apoptosis in vivo, while treatment with ANG-(1–7) in vitro protected MIN6 and MS-1 cocultures from palmitate-induced endothelial dysfunction (30). Altogether, these observations point to the beneficial role played by the compensatory ACE2/ANG-(1–7)/MasR axis of the RAS in maintaining islet function and integrity in a diabetic setting.
Despite the accumulation of evidence demonstrating the therapeutic effects of ACE2 in improving glycemia in transgenic (3) and pharmacological models (10), the modulation of islet-specific ACE2 expression or activity and its association with β-cell dysfunction remains unknown. Hence, we investigated the impact of long-term HFD on islet ACE2 activity and the expression along with modulation of other RAS components, and we have attempted to clarify the targets of ACE2 gene therapy.
METHODS
Animals and diet.
Mice were housed in a temperature- and humidity-controlled facility under a 12:12-h dark-light cycle. Wild-type (WT) male C57BL/6J mice (8–10 wk old) and ACE2 KO (12–15 wk old) mice (19) were fed either a regular diet (RD; Harlan Laboratories, 2019S) or 45% kcal high-fat diet (HFD; OpenSource Diets; D12451) for 16 wk. All procedures were performed according to the National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee at the Louisiana State University Health Sciences Center.
Pancreatic islet isolation.
Islets were isolated from a subset of RD- and HFD-fed mice, as previously described (53), with the following modifications to the original protocol. Briefly, liberase TL (Roche, Indianapolis, IN), dissolved in RPMI 1640 medium (cat. no. 11875-093; Gibco, Carlsbad, CA), was injected into the bile duct. The distended pancreas was then dissected out, digested for 13 min in a 37°C water bath, and passed through a sieve to remove tissue debris. Islets were then separated from exocrine tissue by density gradient separation using Histopaque (Sigma, St. Louis, MO) at 2,400 rpm, 22°C for 20 min. Islets were then carefully hand-picked from the interface using a plastic pipette, washed several times using RPMI 1640 medium supplemented with 10% FBS (Gibco), and manually counted under a dissection microscope.
Pancreatic lenti-mACE2 injection.
At the 13th week of HFD feeding, a subset of mice was injected in the pancreas with a lentivirus-encoding murine ACE2 [Lenti-mACE2; 106 transduction units (TU)/ml; n = 10] (23) diluted in 100 µl of 0.9% saline, while the others were injected with a control lentivirus expressing green fluorescent protein (Lenti-GFP; 106 TU/ml; n = 8), as described previously (3).
Plasma insulin, C-peptide, and ANG II levels.
Blood was collected from mice that were fasted for 4 h, and plasma insulin, as well as C-peptide concentrations were measured using ELISA kits (Crystal Chem, cat. no. 90080; ALPCO Diagnostics, 80-CPTMS-E01) after diluting the samples twice with sample diluent. ANG II levels were also measured by ELISA kit, according to the manufacturer’s protocol (FEK-002-12; Phoenix Pharmaceuticals).
Glucose and insulin tolerance tests.
To evaluate the effect of HFD on glucose tolerance, WT and ACE2 KO mice were fasted overnight, followed by measurement of baseline blood glucose levels (0 min). On the basis of body weight, a total of 2 g/kg of 45% glucose solution in 0.9% saline was injected intraperitoneally into each mouse. Blood glucose readings were taken at 15, 30, 60, and 120 min after intraperitoneal injection.
For the insulin tolerance test, mice were fasted for 4 h (9:00 AM to 1:00 PM), insulin (0.75 U/kg; Humulin R, Eli Lilly, Indianapolis, IN) was administered intraperitoneally, and blood was sampled at 15, 30, 60, and 120 min for glucose measurements.
The total area under the curve was calculated using the trapezoidal rule in Prism5 GraphPad Software (San Diego, CA).
Glucose-stimulated insulin secretion.
Isolated islets were transferred to a Petri dish containing RPMI 1640 medium with l-glutamine (20 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), and FBS (10%). Following 48 h of incubation in 5% CO2 incubator at 37°C, the islets were processed for glucose-stimulated insulin secretion (GSIS). The islets (n = 20/group in triplicate) were preincubated in 2.8 mM glucose-Krebs-Ringer bicarbonate HEPES buffer (KRBH) solution (in mM: 115 NaCl, 5 KCl, 24 NaHCO3, 2.5 CaCl2, 1 MgCl2, 10 HEPES, and 2% wt/vol BSA, pH 7.4) for 30 min at 37°C. The KRBH solution was then aspirated, and the islets incubated in KRBH solution containing different concentrations of glucose (2.8, 11.1, and 25.0 mM) for 1 h at 37°C. After incubation, the solution was collected and stored at −80°C for insulin assay.
Quantitative real-time PCR.
RNA was isolated from islets using Qiagen RNeasy mini kit (Qiagen, Valencia, CA). One-step quantitative RT-PCR (qRT-PCR) was performed using Power SYBR Green RNA-to-CT one-step kit (Life Technologies, Carlsbad, CA). A total of 5-ng target mGlucagon, mACE2, and mAT1a receptor (mAT1aR) mRNA was added per well and assayed on a LightCycler 480 II (Roche) using 18S rRNA as an internal control. Fold changes in target genes were determined by the 2−ΔΔCt method. The primer sequences were as follows: mACE2: gag gat aag cct aaa atc agc tct tg, tcg gaa cag gaa cat ttc gtt; mAT1aR: tca cca gat caa gtg cat ttt ga, aga gtt aag ggc cat ttt gct tt; mGlucagon: cag agg aga acc cca gat cat t, cct gtg agt ggc gtt tgt ctt; and m18S rRNA: cgg aca gga ttg aca gat tg, caa atc gct cca cca act aa.
Immunohistochemistry.
Immunohistochemical detection in pancreatic sections was performed as reported earlier (10). Briefly, paraffin-embedded 5-µm pancreatic sections were deparaffinized, rehydrated, and subjected to antigen retrieval using sodium citrate antigen retrieval buffer at 100°C for 10 min. Antigen-retrieved pancreatic sections were blocked using 10% normal horse serum for 1 h followed by incubation using primary antibodies targeted against ACE2 (1:200 dilution, Santa Cruz sc-20998), insulin (1:100, Abcam ab7842), or glucagon (1:100, Sigma G2654) overnight at 4°C or 2 h at room temperature. Alexa Fluor 488, Alexa Fluor 546, and Alexa Fluor 594 (1:500 dilution, A11008, A11005, and A11074; Life Technologies) were used as secondary antibodies. After 2 h of incubation, slides were washed and Vectashield mounting medium containing DAPI (Vector Laboratories, Burlingame, CA) was added and covered using coverslips. Images were then taken at ×20 magnification using an Olympus BX51 microscope equipped with cellSens software.
Masson’s trichrome and islet area.
Masson’s trichrome staining was performed using a kit (Sigma-HT15-1KT). Briefly, paraffin-embedded pancreatic sections were deparaffinized in xylene and rehydrated in 100%, 95%, and 70% ethanol before placing the slides in Bouin’s fixative at 56°C for 15 min. Slides were then washed, stained with Weigert’s hematoxylin for 5 min, washed again for 5 min, and stained with Bierbich scarlet acid fuchsin for 5 min. This was followed by staining with phosphotungstic acid plus phosphomolybdic acid solution, mixed in equal proportions, for 5 min. Slides were subsequently stained with aniline blue solution for another 5 min before being placed in 1% acetic acid solution for 2 min. Finally, slides were washed, dehydrated, and covered with a coverslip after mounting with Permount. Images were taken at ×10 magnification using Olympus BX51 microscope, cellSens software.
Average islet area for each mouse was obtained by calculating individual islet area of all the islets from 5-µm pancreatic sections of each mouse. A total of 4 WT and 2 KO mice were analyzed and quantified for average islet area (µm2) using Olympus BX51 microscope, cellSens software.
ACE2 activity.
Pancreatic and islet ACE2 activity were measured using a fluorogenic substrate Mca-APK(Dnp), as described previously (41). Measurements were performed in duplicate for each sample, in the presence and absence of DX600 (ACE2 inhibitor). Specific activity from each sample was then normalized to protein content and presented as fluorescence units (FU) per minute per microgram protein.
ADAM17 activity.
Pancreatic ADAM17 activity was measured using a kit (Sensolyte 520, Anaspec). Measurements were performed in duplicate for each sample. Specific activity from each sample was then normalized to protein content and presented as FU per minute per microgram protein.
Ex vivo islets treatment.
To study the effect of ANG II and ANG-(1–7) on glucagon gene expression, islets isolated from six WT mice were divided into two pools and incubated in RPMI 1640 medium (cat. no. 11875-093, Gibco), supplemented with 10% FBS (Gibco) and penicillin/streptomycin (Gibco) for 24 h. After 24 h, islets were treated with either normal glucose (5 mM) or high glucose (25 mM) dissolved in RPMI 1640 medium (cat. no. 11875-020, Gibco) supplemented with 10% FBS and penicillin/streptomycin in the presence and absence of 100 nM of ANG II (Sigma) and ANG-(1–7) (Bachem, Torrance, CA) for 3 h. Islets were then harvested and mRNA was extracted using Qiagen RNeasy mini kit (Qiagen, Valencia, CA). One-step qRT-PCR was performed using Power SYBR Green RNA-to-CT one-step kit (Life Technologies). A total of 5-ng target mGlucagon was added per well and assayed on a LightCycler 480 II (Roche) using 18S rRNA as an internal control. Each treatment group was assayed in duplicate. Fold changes in target genes were determined by the 2−ΔΔCt method. The primer sequences were as follows: mGlucagon: cag agg aga acc cca gat cat t, cct gtg agt ggc gtt tgt ctt and m18S rRNA: cgg aca gga ttg aca gat tg, caa atc gct cca cca act aa.
Statistics.
Data are presented as means ± SE. Unless otherwise stated, data were analyzed by Student's t-test, one-way ANOVA followed by the Tukey post hoc test for multiple comparisons between means, two-way ANOVA followed by Bonferroni’s post hoc test for multiple comparisons between means, or repeated-measures ANOVA followed by Bonferroni’s post hoc test for multiple comparisons, as appropriate, using Prism5 (GraphPad Software). Differences were considered statistically significant at P < 0.05.
RESULTS
HFD-induced glycemic dysregulation is exacerbated in ACE2 KO mice.
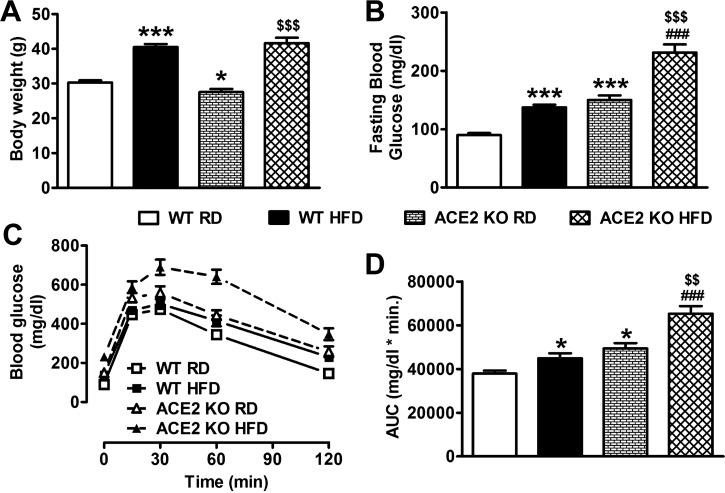
The first goal of our study was to determine the impact of HFD feeding in both WT and ACE2 KO mice. WT mice, fed HFD for 16 wk, showed a significant increase in body weight (40.5 ± 0.9 vs. 30.3 ± 0.6 g, P < 0.001; Fig. 1A), fasting blood glucose (FBG: 137.2 ± 4.8 vs. 89.9 ± 3.2 mg/dl, P < 0.001; Fig. 1B), and glucose intolerance (P < 0.05; Fig. 1, C and D) compared with RD-fed controls. ACE2 KO mice on RD were smaller than WT (27.6 ± 0.9 g, P < 0.05; Fig. 1A) and already displayed increased FBG (149.9 ± 7.9 mg/dl, P < 0.001; Fig. 1B) and glucose intolerance (P < 0.05; Fig. 1, C and D). After 16 wk of HFD, ACE2 KO body weight was no longer different (41.6 ± 1.6 g, P > 0.05; Fig. 1A) from WT, while FBG levels (231.4 ± 13.8 mg/dl, P < 0.001; Fig. 1B) and glucose intolerance (P < 0.001; Fig. 1, C and D) were further increased. Together, these data confirm that lack of ACE2 is deleterious to glycemic control and that hyperglycemia and glucose intolerance are exacerbated by HFD in ACE2 KO mice.
Fig. 1.
Effect of high-fat diet (HFD) feeding on metabolic parameters in wild-type (WT) and ACE2 knockout (KO) mice. Despite a significantly lower body weight in ACE2 KO compared with WT on baseline (A), the significant increase induced by HFD was not different between strains after 16 wk of feeding. In WT mice, HFD induced a significant increase in fasting blood glucose levels (B) and impaired glucose tolerance (C and D) that was exacerbated in ACE2 KO mice. Statistical significance: *P < 0.05, ***P < 0.001 vs. WT RD; $$P < 0.01, $$$P < 0.001 vs. ACE2 KO RD; ###P < 0.001 vs. WT HFD using two-way ANOVA followed by Bonferroni’s multiple-comparison test. Data are from n = 39–49 mice for WT RD and HFD groups; n = 14–19 mice for ACE2 KO RD and HFD groups.
HFD increases RAS activity in the islets of Langerhans, independently of ACE2 levels.
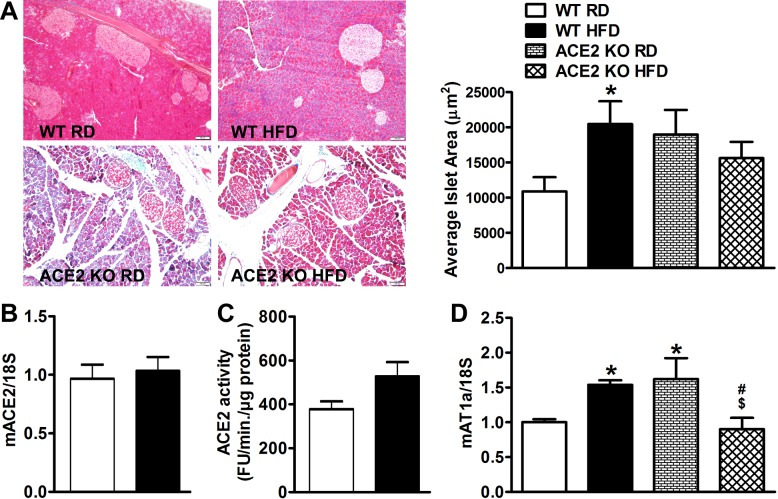
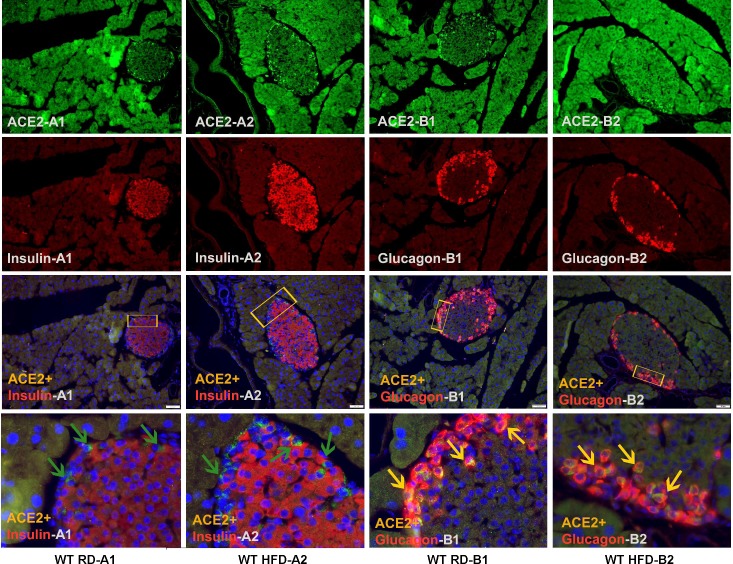
To further assess the impact of HFD on the endocrine pancreas, islet area was measured within each treatment group. WT mice fed HFD displayed higher islet area (20,460 ± 3,240 vs. 10,889 ± 2,014 µm2, P < 0.05; Fig. 2A) compared with their counterparts on RD. On the other hand, both RD-fed and HFD-fed ACE2 KO mice displayed equally higher islet area (18,960 ± 3,503 and 15,615 ± 2,304 µm2, respectively; Fig. 2A). In addition, ACE2 KO mice showed dramatic signs of acinar atrophy throughout the exogenous pancreas. Interestingly, neither ACE2 mRNA expression (Fig. 2B) nor activity (Fig. 2C) showed significant changes in isolated islets of 16-wk HFD-fed WT mice. AT1aR mRNA expression was then assessed as a marker of RAS activity. WT mice on HFD, as well as ACE2 KO on RD, showed a significant increase in AT1aR mRNA in islets (Fig. 2, C and D), suggesting that HFD and lack of ACE2 both contribute to islet RAS activation. Surprisingly, ACE2 KO on HFD appeared to show a paradoxical normalization of islet AT1aR mRNA expression. Since ANG II is known to impair insulin secretion (43), we further measured GSIS in ACE2 KO. We confirmed that GSIS remained impaired in ACE2 KO on HFD (ACE2 KO RD, 2.8 mM: 6.6 ± 2.2 vs. 25 mM: 4.3 ± 1.3 and ACE2 KO HFD, 2.8 mM: 3.1 ± 0.8 vs. 25 mM: 3.3 ± 0.6 ng insulin·h−1·µg protein−1), suggesting that the apparent reduction of AT1aR mRNA is not a reflection of improved glycemic control in these animals. To better understand the relationship between ACE2 and insulin secretion, we performed immunohistochemistry on the islets of WT mice under RD and HFD conditions. Focusing primarily on α- and β-cells as the main cell types involved in glycemic control, we surprisingly observed abundant ACE2 immunofluorescence in glucagon-producing α-cells, but not in insulin-secreting β-cells (Fig. 3) in both RD- and HFD-fed mice, suggesting that ACE2 is localized predominantly in α-cells. In addition, there was no difference in islet ACE2 immunofluorescence between RD and HFD groups, supporting our observation that islet ACE2 expression and activity are unchanged in HFD-fed mice. Together, these data suggest that while the islet RAS is activated by HFD feeding, glycemic dysregulation is independent of islet ACE2 levels.
Fig. 2.
Effect of HFD feeding on islet area and islet ACE2/AT1aR levels in WT and ACE2 KO mice. A: islet area of pancreatic sections from WT (n = 4 mice/group) and ACE2 KO (n = 2 mice/group) on RD and HFD mice stained with Masson’s trichrome and a representative average islet area (µm2) bar graph. HFD-fed WT mice showed no difference in islet mACE2 gene expression levels (n = 5/group) (B) or activity (n = 7 or 8/group) (C) compared with RD-fed control mice. HFD-fed WT mice and RD-fed ACE2 KO mice showed significantly increased mAT1aR gene expression levels compared with RD-fed WT mice (D). Statistical significance: *P < 0.05 vs. WT RD; $P < 0.01 vs. ACE2 KO RD; #P < 0.05 vs. WT HFD using two-way ANOVA followed by Bonferroni’s multiple-comparison test.
Fig. 3.
ACE2 expression in the pancreas. A: double immunofluorescence expression for ACE2 (green) and insulin (red) superimposed image and zoomed in superimposed image of ACE2+Insulin+DAPI (indicated by green arrows) in WT RD (A1), WT HFD (A2) pancreatic sections. B: double immunofluorescence staining of ACE2 (green) and glucagon (red) superimposed image and zoomed in superimposed image of ACE2+Glucagon+DAPI (indicated by yellow arrows) in WT RD (B1) and WT HFD (B2) pancreatic sections. Images were taken at ×20 magnification using a fluorescence microscope. Note that rectangular insets in each superimposed panel point to the region used to obtain zoomed-in superimposed image.
ACE2 does not directly modulate glucagon expression.
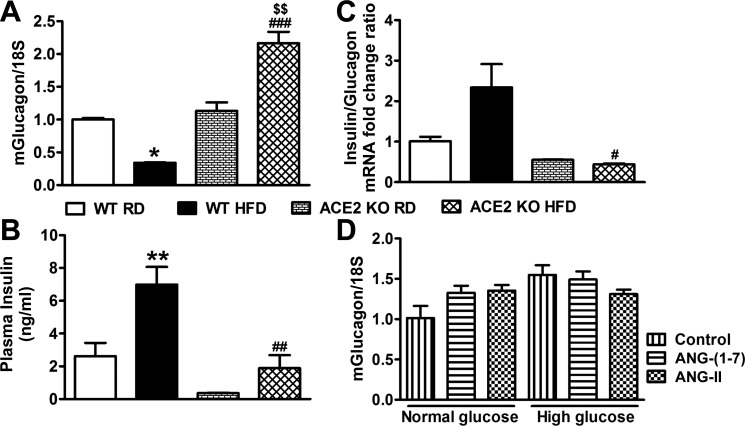
In light of ACE2's prominent expression in α-cells, we first measured glucagon gene expression in ACE2 KO and WT mice to determine whether increased glucagon mRNA levels could explain the exacerbated hyperglycemia in the KO mice. Glucagon gene expression was not different between WT and ACE2 KO mice on RD (Fig. 4A), suggesting that ACE2 does not modulate the expression of this peptide. However, in WT mice on a HFD, glucagon gene expression was reduced by ~70% compared with WT mice on RD, while it was increased by twofold in ACE2 KO (Fig. 4A). As expected, plasma insulin levels were significantly upregulated in WT mice fed a HFD, but since ACE2 KO mice are already known to be hypoinsulinemic (45) and hyperglycemic (4), the compensatory upregulation in plasma insulin levels did not take place in these animals while on HFD (Fig. 4B). Since glycemia is tightly regulated by insulin and glucagon, we then compared the gene expression ratio of these peptides in WT and ACE2 KO under both diet regimens (Fig. 4C). In WT mice on a HFD, we observed a marked elevation in insulin/glucagon gene expression ratio due to an increase in insulin secretion and the repressed glucagon expression. However, in ACE2 KO on HFD, the ratio was dramatically reduced as a result of hypoinsulinemia in these animals likely driving the failed repression of glucagon expression. To further investigate the potential ACE2 role on glucagon expression, isolated islets were treated with ANG II and ANG-(1–7), the product of ANG II hydrolysis by ACE2, in the presence of normal (5 mM) and high (25 mM) glucose. Exposure to a standard concentration (100 nM) of ANG II and ANG-(1–7) had no effect on glucagon gene expression in isolated islets under either normal or high glucose conditions (Fig. 4D). Accordingly, our data suggest that ACE2 does not appear to directly affect glucagon expression.
Fig. 4.
Effect of HFD on glucagon and insulin in WT and ACE2 KO mice. A: HFD-fed WT mice had significantly lower-glucagon mRNA levels, while ACE2 KO mice on HFD had significantly higher levels in the islets. HFD feeding significantly increased plasma insulin levels (B) and elevated insulin/glucagon gene expression ratio (C) in WT mice but significantly decreased plasma insulin levels (B) and insulin/glucagon gene expression ratio (C) in ACE2 KO mice. D: ex vivo treatment with normal (5 mM) and high (25 mM) glucose concentrations in the presence and absence of 100 nM ANG II and ANG-(1–7) for 3 h showed no effect on glucagon mRNA levels in isolated islets of WT mice. Statistical significance, *P < 0.05, **P < 0.01 vs. WT RD; $$P < 0.01 vs. ACE2 KO RD; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. WT HFD using two-way ANOVA followed by Bonferroni’s multiple comparison test.
ACE2 gene therapy improves glycemia in HFD-fed mice.
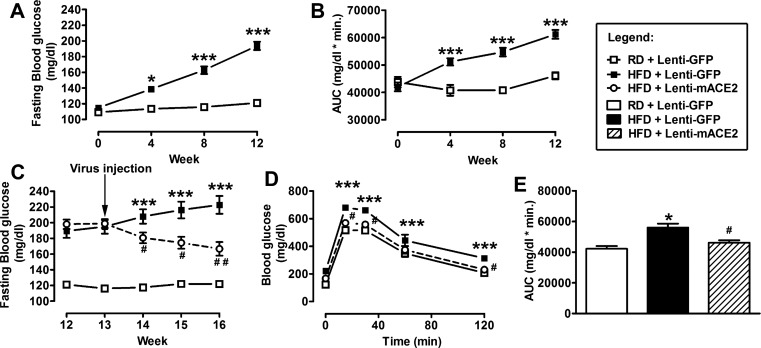
Despite the apparent lack of specific effect for ACE2 in the endocrine pancreas, the exaggerated disruption of glycemic control in ACE2 KO mice supports a rationale for ACE2 therapy in Type 2 diabetes. To investigate the therapeutic role of pancreatic ACE2 in WT HFD-fed mice, Lenti-mACE2 or Lenti-GFP (106 TU/ml, 100 µl) were injected in the pancreas at the 13th week of HFD feeding. By that time, mice on a HFD have already developed a significant increase in body weight (RD: 26.3 ± 0.6 vs. HFD: 35.5 ± 0.5 g, P < 0.001), FBG levels (Fig. 5A), and impaired glucose tolerance (Fig. 5B) compared with RD-fed mice. In addition, WT mice on RD were also injected with the control virus to monitor the lack of effects of that treatment.
Fig. 5.
Effect of Lenti-mACE2 gene therapy on fasting blood glucose and glucose intolerance in HFD-fed mice. Twelve weeks after HFD-feeding, HFD + Lenti-GFP showed significantly higher fasting blood glucose (A) and glucose intolerance (B) compared with RD + Lenti-GFP mice. Three weeks after the pancreatic injection of Lenti-mACE2 (n = 8) or the control lentivirus Lenti-GFP (n = 10), fasting blood glucose levels (C) and glucose intolerance (D and E) were significantly reduced in HFD + Lenti-mACE2 compared with HFD + Lenti-GFP mice. Statistical significance: *P < 0.05, ***P < 0.001 vs. RD + Lenti-GFP; #P < 0.05, ##P < 0.01 vs. HFD + Lenti-GFP using repeated-measures ANOVA followed by Bonferroni’s post hoc test (A–D). One-way ANOVA followed by the Tukey multiple-comparison test (E).
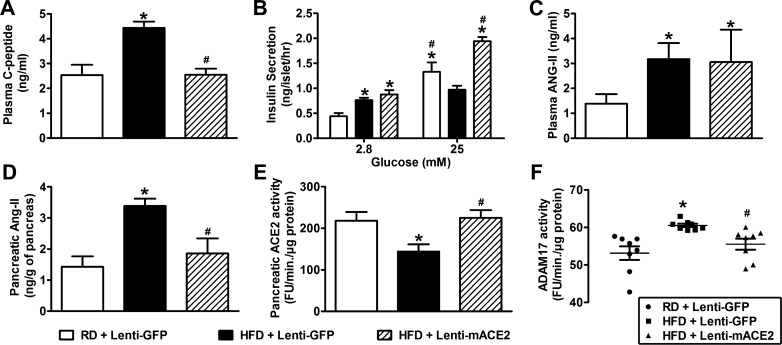
There was a gradual and significant reduction in FBG levels (149.7 ± 7 vs. 222.7 ± 11 mg/dl, Fig. 5C) and an improvement in glucose tolerance (Fig. 5, D and E) in HFD-fed mice treated with Lenti-mACE2 compared with mice injected with the control (Lenti-GFP) virus. Serum C-peptide levels were measured as an index of fasting insulin levels (Fig. 6A). HFD-fed mice treated with Lenti-mACE2 showed a normalization of serum C-peptide levels (2.5 ± 0.2 vs. 4.4 ± 0.2 ng/ml) compared with Lenti-GFP-injected mice, suggesting that pancreatic ACE2 expression could restore the impaired insulin secretion induced by HFD. Moreover, Lenti-mACE2-treated mice exhibited a reduction in fasting plasma insulin levels compared with Lenti-GFP-treated HFD-fed mice (data not shown). This normalization of plasma insulin levels appears to result from improved β-cell function, presumably by correction of pancreatic RAS overactivity as ACE2 therapy did not change insulin sensitivity (HFD+Lenti-GFP: 75 ± 2.3 vs. HFD+Lenti-mACE2: 63.5 ± 1.4% of basal glucose). Additionally, islets isolated from Lenti-mACE2-treated HFD-fed mice exhibited increased GSIS compared with the Lenti-GFP group (Fig. 6B), suggesting that ACE2 expression attenuates the deteriorating effects of a HFD on insulin secretion in the islets. It is important to note that beneficial effects of the Lenti-mACE2 treatment were independent of any change in body weight (HFD+Lenti-GFP: 41.4 ± 0.9 vs. HFD+Lenti-mACE2: 40.3 ± 0.7 g). Overall, these data demonstrate the therapeutic potential of pancreatic ACE2 in reversing HFD-mediated glucose derangements despite unchanged islet ACE2.
Fig. 6.
Effect of Lenti-mACE2 gene therapy on renin angiotensin system (RAS) overactivity and ADAM17 activity in the pancreas of HFD-fed mice. Three weeks after the pancreatic injection of Lenti-mACE2 (n = 8) or the control lentivirus Lenti-GFP (n = 10), C-peptide levels (A) and glucose-stimulated insulin secretion (GSIS) (B) (20 islets/mouse; n = 3) were determined in RD and HFD-fed mice. HFD-fed mice treated with Lenti-GFP show increased plasma ANG II (C), pancreatic ANG II (D), reduced pancreatic mACE2 activity (E), and increased pancreatic ADAM17 activity (F), while intrapancreatic Lenti-mACE2 injection reduced pancreatic (D) but not plasma (C) ANG II, ADAM17 activity (F), and restored ACE2 activity (E) in the pancreas of the HFD-fed mice. Statistical significance: *P < 0.05 vs. RD + Lenti-GFP; #P < 0.05 vs. HFD + Lenti-GFP using one-way ANOVA followed by the Tukey multiple-comparison test. For GSIS: *P < 0.05 vs. 2.8 mM glucose or RD; #P < 0.05 vs. HFD + Lenti-GFP using two-way ANOVA followed by Bonferroni’s post hoc test.
ACE2 gene therapy normalizes RAS activity in the exogenous pancreas.
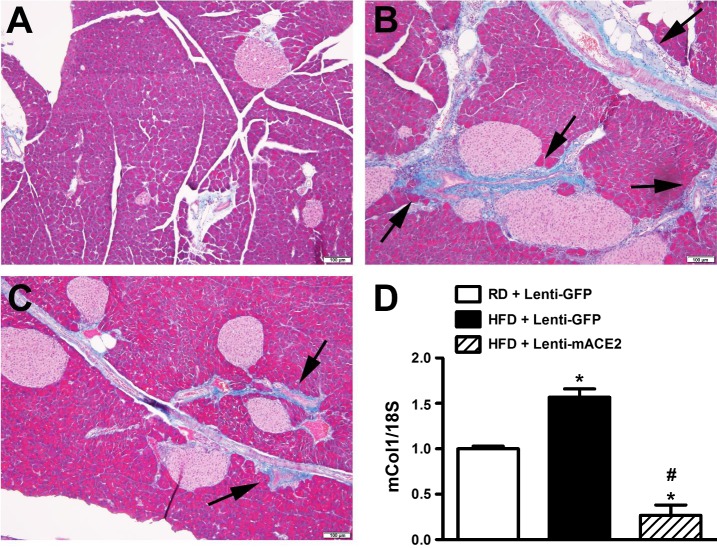
To identify potential mechanisms explaining how ACE2 gene therapy is capable of overcoming glucose dysregulation in diet-induced obesity, we first investigated the impact of this treatment on RAS components in the pancreas. HFD-fed mice exhibited significantly higher plasma (3.2 ± 0.6 vs. 1.4 ± 0.4 ng/ml, P < 0.05; Fig. 6C) and pancreatic (3.4 ± 0.2 vs. 1.43 ± 0.3 ng/g, P < 0.05; Fig. 6D) ANG II levels compared with mice fed with RD, indicating that HFD-feeding increases systemic, as well as local, RAS activity in mice. In addition, consumption of HFD was associated with a 50% reduction in pancreatic ACE2 activity (144.1 ± 17.2 vs. 218 ± 20.9 FU·min−1·µg protein−1, Fig. 6E) compared with RD-fed mice (P < 0.05). While targeted ACE2 gene therapy reversed the effects of HFD feeding in the pancreas (Fig. 6, D and E), it failed to normalize the elevated ANG II levels in the plasma (Fig. 6C). To further support the critical role of an activated pancreatic RAS in HFD-induced Type 2 diabetes and because ANG II is well known to promote fibrosis in various tissues, we examined the effects of HFD on pancreas histology. Besides the enlarged islets area (Fig. 2A), we did not observe any significant morphological change in the endocrine pancreas of HFD-fed animals (Fig. 7). However, fibrosis (Fig. 7, A and B) and collagen expression (Fig. 7D) were significantly increased in the exogenous pancreas of HFD-fed mice. The contribution of elevated ANG II levels to this process was further confirmed by the reversal observed following ACE2 gene therapy (Fig. 7, C and D). Together, our data confirm that the beneficial effects associated with ACE2 expression are mediated by local metabolism of ANG II levels.
Fig. 7.
Effect of ACE2 gene therapy on pancreatic fibrosis in HFD-fed mice. Masson’s trichrome staining of pancreatic sections showing fibrosis (black arrows) in RD + Lenti-GFP (A), HFD + Lenti-GFP (B), and HFD+Lenti-ACE2 (C)-treated groups. Pictures were taken at ×10 magnification. HFD + Lenti-GFP (D) group showed a significant increase in mCol1 gene expression compared with RD + Lenti-GFP group and HFD + Lenti-ACE2-treated group significantly decreased mCol1 mRNA levels in the pancreas. Statistical significance: *P < 0.05 vs. RD + Lenti-GFP; #P < 0.05 vs. HFD + Lenti-GFP using one-way ANOVA followed by Tukey’s multiple-comparison test (n = 3/group).
HFD induces ACE2 shedding in the pancreas.
We previously reported that ADAM17-mediated ACE2 shedding contributes to the development of neurogenic hypertension (50), and other groups have implicated this sheddase in diabetic nephropathy (11). Although we recently showed that ADAM17 does not appear to reduce ACE2 activity in islets (40), we have not considered the contribution of the exogenous pancreas. HFD induced a significant increase in ADAM17 activity in the whole pancreas compared with RD-fed mice and was reversed by ACE2 gene therapy (Fig. 6F). These data suggest that HFD-induced RAS overactivity might have resulted in enhanced ADAM17 activity throughout the whole pancreas.
DISCUSSION
Despite the accumulation of evidence demonstrating the therapeutic effects of ACE2 in improving glycemia in various diabetic models, questions remain regarding the specific contribution of ACE2 expression or activity on β-cells or other cell types in the islets. In addition, the mechanisms by which ACE2 improves glycemia in T2DM remain elusive. In this study, we report that 1) HFD increases islet AT1aR expression but does not alter islet ACE2 levels in mice; 2) ACE2 is predominantly localized in glucagon-producing α-cells; 3) HFD upregulates glucagon mRNA levels in ACE2 KO mice, but ANG II, ANG-(1–7), and ACE2 have no direct effect on glucagon gene expression; 4) HFD-mediated reduction in pancreatic ACE2 levels and increase in local ANG II levels are associated with an increase in pancreatic fibrosis and ADAM17 levels; 5) pancreatic ACE2 gene therapy reduces local ADAM17, fibrosis, and ANG II levels, leading to improved glycemia in HFD-fed mice.
While there are reports underscoring the importance of global ACE2 in a diabetes setting, pancreas-targeted genetic restoration of ACE2 has been shown to improve glycemia in db/db and ANG II infusion mouse models (3, 10). In addition, elevated RAS activity has been associated with glucose dysregulation in HFD-fed mice (15) and downregulation of ACE2 levels in the pancreas (10). As shown previously by Gupte et al. (18), we observed that HFD-fed WT mice exhibit significantly higher plasma ANG II levels compared with RD-fed mice. In addition, the elevation in ANG II levels and concomitant reduction in ACE2 in the pancreas of HFD-fed WT mice highlights the critical role of local RAS overactivity on the inhibition of ACE2 compensatory function. The pivotal role of ACE2 is further evidenced by the rise in mAT1aR gene expression in the islets of ACE2 KO mice on RD. These observations highlight the existence of a fine-tuned balance between the “classic” (i.e., ACE/ANG II/AT1aR) and compensatory (i.e., ACE2/ANG-(1–7)/MasR) RAS within the pancreas, which is thought to contribute to β-cell dysfunction in rodent and human islets (13, 31). However, this vision of a critical pancreatic RAS contrasts with data showing that in ACE2 KO mice, ANG II receptor blockers (45), or ACE inhibitors (2) were unable to reverse β-cell dysfunction. Although we did not look at the early effects of HFD on AT1R in the islets of ACE2 KO mice, it is possible that imbalance between ANG II/ANG-(1–7) peptides in the early phase of disease progression might have induced pathological effects that worsened the development of hyperglycemia and β-cell deficit in these animals. This is supported by our previous findings that glycemic dysfunction in db/db mice could be reversed by ACE2 gene therapy only in the early development of the disease (3). This also suggests that the observed RAS overactivation in islets of ACE2 KO mice at baseline, in the absence of pathological stimulus, could be one possible (but not the only) mechanism that is making them prone to age-dependent impaired glucose homeostasis (37). Therefore, the involvement and role of alternate mechanisms, dependent on the RAS, such as reduction in pancreatic microcirculation, possibly due to reduced formation of ANG-(1–7) (51), or independent of that system cannot be ruled out.
An interesting finding of our study is that the presence of ACE2 in the endocrine pancreas might not be as critical as originally thought. First, ACE2 does not seem majorly expressed in the islets. Data regarding the distribution pattern of endogenous ACE2 in β- and non-β-islet cells and its subsequent role in the regulation of the two main islet hormones, insulin and glucagon, involved in the blood glucose regulation, are controversial. In rat islets, ACE2 has been reported to be localized in insulin-producing β-cells and in somatostatin-producing δ-cells (14), while in human islets, β-cells showed higher ACE2 mRNA levels compared with α-cells (6). Surprisingly, in mice, we observed that ACE2 was predominantly colocalized with glucagon in the α-cells rather than with insulin in the β-cells. This qualitative assessment of ACE2 levels in different islet cell types using immunohistochemistry is in agreement with our recent findings that ACE2 mRNA and activity are predominantly higher in non-β-cells compared with β-cells (40). Although more work is required, validation of these species-specific differences, would not support the use of mice as a good model to study the role of islet ACE2 in β-cell dysfunction. Although mouse islets consist of 60–80% of insulin-secreting β-cells, the remaining 20–40% of cell types in these islets can contribute significantly toward glucose homeostasis (36, 46). Predominant expression of ACE2 in α-cells suggests a possible association between ACE2 and glucagon. Previous studies demonstrated that ACE2 can be a key component in the maintenance of euglycemia because of its potential effects on glucoregulatory pathways involved in insulin secretion and action (3, 10, 37, 45, 48). In addition to its effects on insulin, our study suggests a negative correlation between ACE2 and glucagon, as evidenced by an increased glucagon gene expression in the islets of HFD-fed ACE2 KO mice. Although the beneficial effects of ACE2 in the pancreas stem from the balance between ANG II and ANG-(1–7) (3, 10), neither ANG-II nor ANG-(1–7) had an effect on glucagon gene expression in isolated islets, both in normal and high glucose conditions. It is important to note that glucagon gene expression remained unaltered in the islets of ACE2 KO mice on a RD as well. Therefore, our findings do not support a potential direct role for ACE2 on glucagon gene expression. The second argument dampening the importance of endocrine ACE2 relates to its expression in diabetes. Indeed, we did not observe any change in endogenous ACE2 levels in the islets of hyperglycemic WT mice. These findings are in line with our previous observations showing the lack of change in endogenous islet ACE2 levels in T2DM db/db mice (40). In addition, despite a lack of functional endogenous ACE2, upregulation in AT1aR levels was not different from hyperglycemic WT mice, thereby ruling out the possibility of islet ACE2 as being a primary counterregulatory enzyme for the upregulation of AT1aR and thus, β-cell function in mouse islets. Accordingly, and in keeping with the idea that ACE2 seems to be an important player in glycemic regulation, the detrimental effects seen in ACE2 global KO mice would most likely stem from a lack of ACE2 present outside the islets. It is interesting to note that ACE2 KO mice displayed significant atrophy of the pancreas with smaller and loose acini compared with WT mice, a major histopathological change associated with diabetes in various species (16, 35). This reiterates that ACE2 may be important not only in glycemic regulation but also in maintaining pancreas morphology and probably pancreas mass. Together, the absence of change in islet ACE2 in vivo coupled with a lack of effect of ANG II and ANG-(1–7) on glucagon expression ex vivo suggests the possibility of exocrine pancreatic ACE2 playing a role in the regulation of glycemia by pancreatic ACE2 gene therapy. While the contribution of exocrine ACE2, on endocrine function has not been established, studies have reported some of the nonendocrine actions of the RAS that might indirectly affect β-cell function. Of particular interest is the association of overactive RAS (32) and reduction in pancreatic ACE2/ACE ratio (29) to chronic pancreatitis and hypoxia (8). These conditions not only affect exocrine function but also endocrine function and blood glucose (32, 49). In view of these findings, it may be interesting to investigate the role of the exocrine RAS, particularly ACE2, on endocrine function more closely in diabetic pathophysiology.
We have shown previously that the beneficial effects of ACE2 on glycemia are mediated through both ANG-(1–7)/MasR signaling (3) and decreased ANG II/AT1R signaling (10) in the pancreas. Identification of ANG II in the exocrine pancreas (26) coupled with reduction in pancreatic ANG II levels upon Lenti-mACE2 injection suggests the likelihood of ACE2 present in the exocrine portion of the pancreas degrades exocrine ANG II and, thereby, prevents the deleterious effects of the ANG-II/AT1R axis on β-cell function. This can be supported by the fact that pancreatic ACE2 gene therapy improved glycemia in HFD-fed mice by enhancing GSIS from the β-cells of the pancreas. Our results are also consistent with the observations made by Shoemaker et al. (45), indicating a role for ACE2 in regulating insulinemia in HFD. Altogether, identification of ACE2 in the exocrine pancreas (3, 29), coupled with beneficial effects of ACE2 gene therapy, suggests the likely role of nonendocrine ACE2 in diabetes.
In this study, we also observed that HFD feeding upregulated pancreatic ADAM17 activity, and we (50) and others (17, 38) previously demonstrated that ANG II mediates the activation of this sheddase, in various tissues. Therefore, upregulation of ADAM17 in the pancreas might have promoted ACE2 shedding (50), leading to a downregulation in total pancreatic ACE2 levels. Treatment with Lenti-mACE2 prevented ADAM17 upregulation in the pancreas, possibly by enhancing pancreatic ANG II degradation. Together, we speculate that pancreatic ACE2 gene therapy might have shown its beneficial effects on β-cell dysfunction and fasting glycemia levels, possibly via downregulation of ANG II and its downstream signaling present in the pancreas. This could be evidenced by a reduction in pancreatic fibrosis after treatment with Lenti-mACE2 in HFD-fed mice. Fibrosis is one of the many ANG II-mediated mechanisms (22, 42, 44), which can contribute to β-cell dysfunction (21) and against which activation of the ACE2/ANG-(1–7)/MasR axis has been proven beneficial in various tissues (39, 52). Hence, the observed reduction in pancreatic fibrosis upon Lenti-mACE2 treatment could result from the reduction in pancreatic ANG II levels, similar to the previously reported reduction of fibrosis by RAS blockers (42, 44).
Perspectives and Significance
We have previously shown that ACE2 present in the pancreas is downregulated in db/db and ANG II-infused mice and that ACE2 gene therapy to the pancreas was able to restore this loss of ACE2 and improve glycemia (3, 10). While our previous work strongly supports the role of pancreatic ACE2 in the regulation of β-cell function, our current study sheds light on the role of ACE2 present in the islets on glycemic regulation. In this study, we demonstrate that ACE2 present in the islets is not altered in the HFD-induced diabetic state. Although ACE2 is predominantly localized in glucagon-producing α-cells, ANG-(1–7), the product of ANG II hydrolysis by ACE2, has no effect on glucagon expression. While ACE2 in general seems to be an important player in glycemia regulation, we conclude that islet ACE2 is not associated with HFD-induced glucose dysregulation. On the other hand, it is possible that ACE2 present in the nonendocrine portion of the pancreas may exert a beneficial role in HFD-induced hyperglycemic state, mostly by combatting ANG II-induced detrimental effects. Given the fact that our assumptions were drawn using in vivo and ex vivo experimental mouse models, further investigation is warranted to extrapolate these findings to human islets. In addition, more work is also needed to better tease out the potential paracrine effects of α-cells or nonendocrine ACE2 on insulin secretion in normal and diabetic conditions in humans.
GRANTS
This work was supported by research grants from the American Heart Association (11PRE6320006 to K. H. Chhabra, 14PRE18830012 to H. Chodavarapu, and 12EIA8030004 to E. Lazartigues), the National Center for Research Resources (RR-018766), and the National Institute of General Medical Sciences (GM-103514 and GM-106392).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.C., K.H.C., and E.L. conceived and designed the research; H.C., K.H.C., H.X., and X.Y. performed experiments; H.C. and K.H.C. analyzed data; H.C., K.H.C., X.Y., and E.L. interpreted results of experiments; H.C., K.H.C., and E.L. prepared figures; H.C. and K.H.C. drafted manuscript; H.C., K.H.C., H.X., V.S., X.Y., and E.L. edited and revised manuscript; H.C., K.H.C., H.X., V.S., X.Y., and E.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Kim Brint Pedersen and Dr. Srinivas Sriramula of the Louisiana State University Health Sciences Center, New Orleans, for their suggestions on experimental design, Dr. Bernardo Ruiz (Dept. of Pathology) for pathological expertise, and Dr. Mohan K. Raizada, University of Florida, for providing the Lenti-mACE2 virus.
REFERENCES
- 1.Bangalore S, Fakheri R, Toklu B, Messerli FH. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ 352: i438, 2016. doi: 10.1136/bmj.i438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernardi S, Tikellis C, Candido R, Tsorotes D, Pickering RJ, Bossi F, Carretta R, Fabris B, Cooper ME, Thomas MC. ACE2 deficiency shifts energy metabolism towards glucose utilization. Metabolism 64: 406–415, 2015. doi: 10.1016/j.metabol.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Bindom SM, Hans CP, Xia H, Boulares AH, Lazartigues E. Angiotensin I-converting enzyme type 2 (ACE2) gene therapy improves glycemic control in diabetic mice. Diabetes 59: 2540–2548, 2010. doi: 10.2337/db09-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bindom SM, Lazartigues E. The sweeter side of ACE2: physiological evidence for a role in diabetes. Mol Cell Endocrinol 302: 193–202, 2009. doi: 10.1016/j.mce.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bokhari S, Israelian Z, Schmidt J, Brinton E, Meyer C. Effects of angiotensin II type 1 receptor blockade on beta-cell function in humans. Diabetes Care 30: 181, 2007. doi: 10.2337/dc06-1745. [DOI] [PubMed] [Google Scholar]
- 6.Bramswig NC, Everett LJ, Schug J, Dorrell C, Liu C, Luo Y, Streeter PR, Naji A, Grompe M, Kaestner KH. Epigenomic plasticity enables human pancreatic α- to β-cell reprogramming. J Clin Invest 123: 1275–1284, 2013. doi: 10.1172/JCI66514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlsson PO, Berne C, Jansson L. Angiotensin II and the endocrine pancreas: effects on islet blood flow and insulin secretion in rats. Diabetologia 41: 127–133, 1998. doi: 10.1007/s001250050880. [DOI] [PubMed] [Google Scholar]
- 8.Chan WP, Fung ML, Nobiling R, Leung PS. Activation of local renin-angiotensin system by chronic hypoxia in rat pancreas. Mol Cell Endocrinol 160: 107–114, 2000. doi: 10.1016/S0303-7207(99)00258-0. [DOI] [PubMed] [Google Scholar]
- 9.Chhabra K, Lazartigues E. ACE2 gene therapy improves glucose tolerance and insulin sensitivity in high fat diet-fed mice (Abstract). FASEB J 25: 848.2, 2011. [Google Scholar]
- 10.Chhabra KH, Xia H, Pedersen KB, Speth RC, Lazartigues E. Pancreatic angiotensin-converting enzyme 2 improves glycemia in angiotensin II-infused mice. Am J Physiol Endocrinol Metab 304: E874–E884, 2013. doi: 10.1152/ajpendo.00490.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chodavarapu H, Grobe N, Somineni HK, Salem ES, Madhu M, Elased KM. Rosiglitazone treatment of Type 2 diabetic db/db mice attenuates urinary albumin and angiotensin converting enzyme 2 excretion. PLoS One 8: e62833, 2013. doi: 10.1371/journal.pone.0062833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu KY, Leung PS. Angiotensin II Type 1 receptor antagonism mediates uncoupling protein 2-driven oxidative stress and ameliorates pancreatic islet beta-cell function in young Type 2 diabetic mice. Antioxid Redox Signal 9: 869–878, 2007. doi: 10.1089/ars.2007.1590. [DOI] [PubMed] [Google Scholar]
- 13.Cole BK, Keller SR, Wu R, Carter JD, Nadler JL, Nunemaker CS. Valsartan protects pancreatic islets and adipose tissue from the inflammatory and metabolic consequences of a high-fat diet in mice. Hypertension 55: 715–721, 2010. doi: 10.1161/HYPERTENSIONAHA.109.148049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang HJ, Yang JK. Tissue-specific pattern of angiotensin-converting enzyme 2 expression in rat pancreas. J Int Med Res 38: 558–569, 2010. doi: 10.1177/147323001003800218. [DOI] [PubMed] [Google Scholar]
- 15.Frantz ED, Crespo-Mascarenhas C, Barreto-Vianna AR, Aguila MB, Mandarim-de-Lacerda CA. Renin-angiotensin system blockers protect pancreatic islets against diet-induced obesity and insulin resistance in mice. PLoS One 8: e67192, 2013. doi: 10.1371/journal.pone.0067192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujisawa H, Zhang Z, Sun W, Huang M, Kobayashi J, Yasuda H, Kinoshita Y, Ando R, Tamura K. Histopathological changes in the pancreas from a spontaneous hyperglycemic cynomolgus monkey. J Toxicol Pathol 25: 215–219, 2012. doi: 10.1293/tox.25.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupte M, Boustany-Kari CM, Bharadwaj K, Police S, Thatcher S, Gong MC, English VL, Cassis LA. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol 295: R781–R788, 2008. doi: 10.1152/ajpregu.00183.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupte M, Thatcher SE, Boustany-Kari CM, Shoemaker R, Yiannikouris F, Zhang X, Karounos M, Cassis LA. Angiotensin converting enzyme 2 contributes to sex differences in the development of obesity hypertension in C57BL/6 mice. Arterioscler Thromb Vasc Biol 32: 1392–1399, 2012. doi: 10.1161/ATVBAHA.112.248559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurley SB, Allred A, Le TH, Griffiths R, Mao L, Philip N, Haystead TA, Donoghue M, Breitbart RE, Acton SL, Rockman HA, Coffman TM. Altered blood pressure responses and normal cardiac phenotype in ACE2-null mice. J Clin Invest 116: 2218–2225, 2006. doi: 10.1172/JCI16980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Härdtner C, Mörke C, Walther R, Wolke C, Lendeckel U. High glucose activates the alternative ACE2/Ang-(1-7)/Mas and APN/Ang IV/IRAP RAS axes in pancreatic β-cells. Int J Mol Med 32: 795–804, 2013. doi: 10.3892/ijmm.2013.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayden MR, Sowers JR. Isletopathy in Type 2 diabetes mellitus: implications of islet RAS, islet fibrosis, islet amyloid, remodeling, and oxidative stress. Antioxid Redox Signal 9: 891–910, 2007. doi: 10.1089/ars.2007.1610. [DOI] [PubMed] [Google Scholar]
- 22.Hiromura M, Mori Y, Kohashi K, Terasaki M, Shinmura K, Negoro T, Kawashima H, Kogure M, Wachi T, Watanabe R, Sato K, Kushima H, Tomoyasu M, Nakano Y, Yamada Y, Watanabe T, Hirano T. Suppressive effects of glucose-dependent insulinotropic polypeptide on cardiac hypertrophy and fibrosis in angiotensin II-infused mouse models. Circ J 80: 1988–1997, 2016. doi: 10.1253/circj.CJ-16-0152. [DOI] [PubMed] [Google Scholar]
- 23.Huentelman MJ, Grobe JL, Vazquez J, Stewart JM, Mecca AP, Katovich MJ, Ferrario CM, Raizada MK. Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp Physiol 90: 783–790, 2005. doi: 10.1113/expphysiol.2005.031096. [DOI] [PubMed] [Google Scholar]
- 24.Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AI, Hooper NM, Turner AJ. Tumor necrosis factor-α convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol Chem 280: 30113–30119, 2005. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung KK, Leung PS. Effects of hyperglycemia on angiotensin II receptor type 1 expression and insulin secretion in an INS-1E pancreatic beta-cell line. JOP 9: 290–299, 2008. [PubMed] [Google Scholar]
- 26.Leung PS, Chan HC, Wong PY. Immunohistochemical localization of angiotensin II in the mouse pancreas. Histochem J 30: 21–25, 1998. doi: 10.1023/A:1003210428276. [DOI] [PubMed] [Google Scholar]
- 27.Leung PS, Chan HC, Fu LX, Wong PY. Localization of angiotensin II receptor subtypes AT1 and AT2 in the pancreas of rodents. J Endocrinol 153: 269–274, 1997. doi: 10.1677/joe.0.1530269. [DOI] [PubMed] [Google Scholar]
- 28.Leung PS, Chan WP, Wong TP, Sernia C. Expression and localization of the renin-angiotensin system in the rat pancreas. J Endocrinol 160: 13–19, 1999. doi: 10.1677/joe.0.1600013. [DOI] [PubMed] [Google Scholar]
- 29.Liu R, Qi H, Wang J, Wang Y, Cui L, Wen Y, Yin C. Angiotensin-converting enzyme (ACE and ACE2) imbalance correlates with the severity of cerulein-induced acute pancreatitis in mice. Exp Physiol 99: 651–663, 2014. doi: 10.1113/expphysiol.2013.074815. [DOI] [PubMed] [Google Scholar]
- 30.Lu CL, Wang Y, Yuan L, Li Y, Li XY. The angiotensin-converting enzyme 2/angiotensin (1-7)/Mas axis protects the function of pancreatic β cells by improving the function of islet microvascular endothelial cells. Int J Mol Med 34: 1293–1300, 2014. doi: 10.3892/ijmm.2014.1917. [DOI] [PubMed] [Google Scholar]
- 31.Lupi R, Del Guerra S, Bugliani M, Boggi U, Mosca F, Torri S, Del Prato S, Marchetti P. The direct effects of the angiotensin-converting enzyme inhibitors, zofenoprilat and enalaprilat, on isolated human pancreatic islets. Eur J Endocrinol 154: 355–361, 2006. doi: 10.1530/eje.1.02086. [DOI] [PubMed] [Google Scholar]
- 32.Madro A, Kurzepa J, Celinski K, Slomka M, Czechowska G, Kurzepa J, Kazmierak W, Buszewicz G, Ciesielka M, Madro R. Effects of renin-angiotensin system inhibitors on fibrosis in patients with alcoholic chronic pancreatitis. J Physiol Pharmacol 67: 103–110, 2016. [PubMed] [Google Scholar]
- 33.Massiéra F, Bloch-Faure M, Ceiler D, Murakami K, Fukamizu A, Gasc JM, Quignard-Boulange A, Negrel R, Ailhaud G, Seydoux J, Meneton P, Teboul M. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J 15: 2727–2729, 2001. doi: 10.1096/fj.01-0457fje. [DOI] [PubMed] [Google Scholar]
- 34.Massiera F, Seydoux J, Geloen A, Quignard-Boulange A, Turban S, Saint-Marc P, Fukamizu A, Negrel R, Ailhaud G, Teboul M. Angiotensinogen-deficient mice exhibit impairment of diet-induced weight gain with alteration in adipose tissue development and increased locomotor activity. Endocrinology 142: 5220–5225, 2001. doi: 10.1210/endo.142.12.8556. [DOI] [PubMed] [Google Scholar]
- 35.McIlwrath SL, Westlund KN. Pharmacological attenuation of chronic alcoholic pancreatitis induced hypersensitivity in rats. World J Gastroenterol 21: 836–853, 2015. doi:10.1210/10.3748/wjg.v21.i3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nadal A, Quesada I, Soria B. Homologous and heterologous asynchronicity between identified α-, β- and δ-cells within intact islets of Langerhans in the mouse. J Physiol 517: 85–93, 1999. doi: 10.1111/j.1469-7793.1999.0085z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niu M-J, Yang J-K, Lin S-S, Ji X-J, Guo L-M. Loss of angiotensin-converting enzyme 2 leads to impaired glucose homeostasis in mice. Endocrine 34: 56–61, 2008. doi: 10.1007/s12020-008-9110-x. [DOI] [PubMed] [Google Scholar]
- 38.Ohtsu H, Dempsey PJ, Frank GD, Brailoiu E, Higuchi S, Suzuki H, Nakashima H, Eguchi K, Eguchi S. ADAM17 mediates epidermal growth factor receptor transactivation and vascular smooth muscle cell hypertrophy induced by angiotensin II. Arterioscler Thromb Vasc Biol 26: e133–e137, 2006. doi: 10.1161/01.ATV.0000236203.90331.d0. [DOI] [PubMed] [Google Scholar]
- 39.Österreicher CH, Taura K, De Minicis S, Seki E, Penz-Osterreicher M, Kodama Y, Kluwe J, Schuster M, Oudit GY, Penninger JM, Brenner DA. Angiotensin-converting-enzyme 2 inhibits liver fibrosis in mice. Hepatology 50: 929–938, 2009. doi: 10.1002/hep.23104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pedersen KB, Chodavarapu H, Porretta C, Robinson LK, Lazartigues E. Dynamics of ADAM17-mediated shedding of ACE2 applied to pancreatic islets of male db/db mice. Endocrinology 156: 4411–4425, 2015. doi: 10.1210/en.2015-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pedersen KB, Sriramula S, Chhabra KH, Xia H, Lazartigues E. Species-specific inhibitor sensitivity of angiotensin-converting enzyme 2 (ACE2) and its implication for ACE2 activity assays. Am J Physiol Regul Integr Comp Physiol 301: R1293–R1299, 2011. doi: 10.1152/ajpregu.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salama ZA, Sadek A, Abdelhady AM, Darweesh SK, Morsy SA, Esmat G. Losartan may inhibit the progression of liver fibrosis in chronic HCV patients. Hepatobiliary Surg Nutr 5: 249–255, 2016. doi: 10.21037/hbsn.2016.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sauter NS, Thienel C, Plutino Y, Kampe K, Dror E, Traub S, Timper K, Bédat B, Pattou F, Kerr-Conte J, Jehle AW, Böni-Schnetzler M, Donath MY. Angiotensin II induces interleukin-1β-mediated islet inflammation and β-cell dysfunction independently of vasoconstrictive effects. Diabetes 64: 1273–1283, 2015. doi: 10.2337/db14-1282. [DOI] [PubMed] [Google Scholar]
- 44.Shen Y, Miao NJ, Xu JL, Gan XX, Xu D, Zhou L, Xue H, Zhang W, Lu LM. N-acetylcysteine alleviates angiotensin II-mediated renal fibrosis in mouse obstructed kidneys. Acta Pharmacol Sin 37: 637–644, 2016. doi: 10.1038/aps.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shoemaker R, Yiannikouris F, Thatcher S, Cassis L. ACE2 deficiency reduces β-cell mass and impairs β-cell proliferation in obese C57BL/6 mice. Am J Physiol Endocrinol Metab 309: E621–E631, 2015. doi: 10.1152/ajpendo.00054.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steiner DJ, Kim A, Miller K, Hara M. Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets 2: 135–145, 2010. doi: 10.4161/isl.2.3.11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tahmasebi M, Puddefoot JR, Inwang ER, Vinson GP. The tissue renin-angiotensin system in human pancreas. J Endocrinol 161: 317–322, 1999. doi: 10.1677/joe.0.1610317. [DOI] [PubMed] [Google Scholar]
- 48.Takeda M, Yamamoto K, Takemura Y, Takeshita H, Hongyo K, Kawai T, Hanasaki-Yamamoto H, Oguro R, Takami Y, Tatara Y, Takeya Y, Sugimoto K, Kamide K, Ohishi M, Rakugi H. Loss of ACE2 exaggerates high-calorie diet-induced insulin resistance by reduction of GLUT4 in mice. Diabetes 62: 223–233, 2013. doi: 10.2337/db12-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang N, Khan SA, Prabhakar NR, Nanduri J. Impairment of pancreatic β-cell function by chronic intermittent hypoxia. Exp Physiol 98: 1376–1385, 2013. doi: 10.1113/expphysiol.2013.072454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia H, Sriramula S, Chhabra KH, Lazartigues E. Brain angiotensin-converting enzyme type 2 shedding contributes to the development of neurogenic hypertension. Circ Res 113: 1087–1096, 2013. doi: 10.1161/CIRCRESAHA.113.301811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan L, Li Y, Li G, Song Y, Gong X. Ang(1-7) treatment attenuates β-cell dysfunction by improving pancreatic microcirculation in a rat model of Type 2 diabetes. J Endocrinol Invest 36: 931–937, 2013. doi: 10.3275/8951. [DOI] [PubMed] [Google Scholar]
- 52.Zhong J, Basu R, Guo D, Chow FL, Byrns S, Schuster M, Loibner H, Wang XH, Penninger JM, Kassiri Z, Oudit GY. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation 122: 717–728, 2010. doi: 10.1161/CIRCULATIONAHA.110.955369. [DOI] [PubMed] [Google Scholar]
- 53.Zmuda EJ, Powell CA, Hai T. A method for murine islet isolation and subcapsular kidney transplantation. J Vis Exp 50: e2096, 2011. doi:10.3791/2096. [DOI] [PMC free article] [PubMed] [Google Scholar]