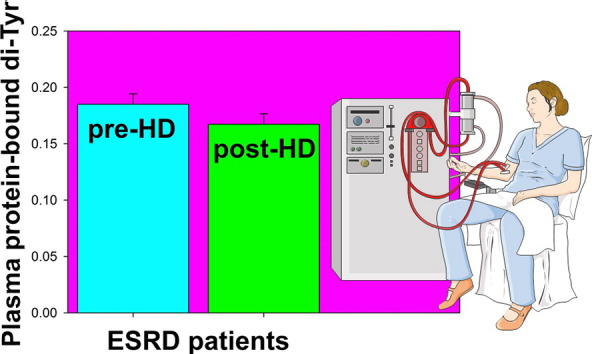
Abstract
Background
Patients with end-stage renal disease (ESRD) undergoing haemodialysis (HD) experience enhanced oxidative stress and systemic inflammation, which are risk factors for cardiovascular disease, the most common cause of excess morbidity and mortality for these patients. Different pathways producing different types of oxidative stress occur in ESRD. The purpose of our study was to determine the effect of HD on plasma levels of protein-bound dityrosine (di-Tyr), a biomarker of protein oxidation.
Methods
Protein-bound di-Tyr formation was measured by size exclusion HPLC coupled to fluorescence detector. Clinical laboratory parameters were measured by standardized methods.
Results
In most ESRD patients, a single HD session decreased significantly the plasma protein-bound di-Tyr level, although the mean post-HD level remained significantly greater than the one in healthy people. Furthermore, pre-HD plasma protein-bound di-Tyr level was positively correlated with pre-HD serum creatinine and albumin concentrations. No significant correlation was found between plasma protein-bound di-Tyr level and serum concentration of C-reactive protein, a biomarker of systemic inflammation.
Conclusions
This study demonstrates that a single HD session does not increase, rather partially decreases, oxidative pathways producing di-Tyr in the haemodialyzed patient.
General significance
The choice of the most pertinent biomarkers of oxidative stress is critical for the development of novel treatments for ESRD. However, the relative importance of oxidative stress and inflammation in ESRD remains largely undetermined, and several questions concerning oxidative stress and inflammation remain poorly defined. These results could stimulate further studies on the use of plasma protein-bound di-Tyr as a long-lasting oxidative stress biomarker in ESRD.
Keywords: Chronic kidney disease, Hemodialysis, Oxidative stress, Biomarker, Protein-bound di-tyrosine, Creatinine
Graphical abstract
Highlights
-
•
Haemodialyzed patients experience oxidative stress and systemic inflammation.
-
•
We assessed haemodialysis (HD) effect on plasma protein-bound dityrosine (di-Tyr).
-
•
In most patients, a single HD session decreased significantly the di-Tyr level.
-
•
Pre-HD di-Tyr level was positively correlated with those of creatinine and albumin.
-
•
No correlation was found between di-Tyr level and C-reactive protein concentration.
1. Introduction
Chronic kidney disease (CKD), or chronic renal failure, is an important public health problem since its prevalence has reached epidemic proportions, with 10–13% of the population affected in different countries around the world [1]. Patients affected by CKD are categorized into five stages according to the glomerular filtration rate and presence of signs of kidney damage [2]. Compared with the general population, CKD patients have a higher risk for premature death, primarily as a result of cardiovascular disease (CVD), and their cardiovascular risk increases continuously with the decrease in kidney function [3]. Thus, most patients with mild to moderate (stages 3–4) CKD die of CVD rather than progress to end stage renal disease (ESRD, or CKD stage 5) [4]. ESRD represents the total inability of kidneys to maintain homeostasis and hence is incompatible with life. Therefore, to ensure survival of patients with ESRD, it is necessary to use methods that substitute for kidney function, including haemodialysis (HD), peritoneal dialysis and kidney transplantation. ESRD patients on maintenance HD too experience a higher risk for CVD and its associated mortality compared to the general population [5].
Patients with CKD are at higher risk for CVD because of higher prevalence of traditional (such as age, diabetes mellitus, left ventricular hypertrophy, dyslipidemia, hypertension) and non-traditional cardiovascular risk factors [6], [7]. The latter include anaemia, uraemia, altered calcium-phosphate metabolism, malnutrition, inflammation and oxidative stress [8], [9], [10], [11]. In patients with ESRD, HD may also impose an additional oxidative stress, mainly attributed to loss of circulating low-molecular-mass dialyzable antioxidants and to the activation of neutrophil NADPH oxidase, provoking inflammation with release of reactive oxygen species [11], [12], [13], [14]. In fact, the extracorporeal treatment itself represents a bioincompatible event in the patient's life: during the HD session, blood is exposed 3 to 4 h to synthetic material, i.e., blood lines and filter. Historically, the first filters used in HD were composed of cellulose: this treatment was so bioincompatible that patients used to experience fever and chills during HD due to complement activation [15]. Currently, with the use of synthetic filters, patients do not experience fever yet, but sub-clinical activation and degranulation of polymorphonuclear neutrophils still occurs [16]. Moreover, intravenous iron therapy in HD patients, even in recommended doses, could further aggravate oxidative stress and atherosclerotic disease. Furthermore, increased total body iron level exacerbates deficiency of lycopene and other lipophilic antioxidants [17].
Four pathways producing different types of “oxidative” stress can be hypothesized in CKD patients, i.e., classical oxidative stress, carbonyl stress, nitrosative stress, and chlorine stress [18]. Increased oxidative stress in patients with ESRD and CKD stage 3 or higher is demonstrated by increase in plasma thiol-specific oxidative stress [19], [20], [21], [22], [23] and protein carbonyls (PCO) [19], [20], [24], [25], [26] and by the presence of plasma advanced oxidation protein products (AOPPs) [26], [27], [28], [29].
AOPPs are considered as potential uremic toxins and inflammatory mediators [30], involved in the activation of polymorphonuclear granulocytes, monocytes and vascular endothelial cells [31], [32]. Chronic accumulation of AOPPs accelerates atherosclerosis by promoting oxidative stress and inflammation [33]. Furthermore, AOPPs directly impair metabolism of high-density lipoproteins, being potent antagonists of their receptor and, therefore, might be directly involved in the development of CVD [34].
AOPPs are a heterogeneous group of dityrosine (di-Tyr)1, pentosidine and carbonyl-containing protein products generated in plasma proteins by both myeloperoxidase (MPO)-dependent (e.g., in ESRD patients) and MPO-independent (e.g., in the predialysis phase of CKD) mechanisms during oxidative/chlorine stress [27], [35]. AOPPs are considered a generic biomarker of protein oxidation because their molecular composition has not yet been precisely defined and their easy spectrophotometric determination is often invalidated by poor reproducibility and accuracy of most colorimetric methods for their detection. Furthermore, measuring AOPPs in diluted plasma as absorbance at 340 nm is a rather nonselective way to determine the level of oxidized proteins; therefore, it is necessary to take precautions to minimize the contribution of species other than AOPPs. Consequently, reliable, validated AOPP reference values in healthy humans are still lacking [27], [34], [35], [36], [37]. In addition, measurement of AOPPs during HD session gave contrasting results [20], [38], [39].
Our preliminary results showed a significant (p < 0.001) increase in di-Tyr fluorescence (normalized to protein concentration) in plasma samples of patients with ESRD undergoing regular maintenance HD as compared to healthy controls [37]. As mentioned above, there is concern that the HD session itself can be, at least in part, responsible of this tremendous oxidative burden [38]. The purpose of the present study was to determine the effect of a single HD session on plasma levels of protein-bound di-Tyr, a biomarker of irreversible protein oxidation, in ESRD patients on maintenance HD. We also examined the potential correlation between plasma protein-bound di-Tyr concentration, taken as a biomarker of oxidative stress and creatinine, albumin, and C-reactive protein (CRP) concentration, taken as biomarkers of systemic inflammation.
2. Materials and methods
2.1. Study participants
Table 1.
Characteristics of study group. Data are expressed as mean ± SE.
| Haemodialyzed Patients (n = 73) | |
|---|---|
| Age (years) | 69.62 ± 1.48 |
| Sex | 48 male, 25 female |
| Diabetes | 50 nondiabetic, 23 diabetic |
| Length of time on dialysis (years) | 5.71 ± 0.44 |
| CRP (mg/dL) | 0.51 ± 0.06 |
| Albumin (g/dL) | 3.50 ± 0.04 |
| Fibrinogen(mg/dL) | 355.47 ± 9.01 |
| White blood cells (cells/mm3) | 7293.15 ± 257.90 |
| Haemoglobin (g/dL) | 11.02 ± 0.11 |
| Urea (mg/dL) | 150.14 ± 4.76 |
| Creatinine (mg/dL) | 9.16 ± 0.35 |
| Sodium (mmol/L) | 137.80 ± 0.36 |
| Potassium (mmol/L) | 5.22 ± 0.09 |
| Calcium (mmol/L) | 2.23 ± 0.02 |
| Phosphorus (mmol/L) | 1.65 ± 0.05 |
| Ferritin (ng/mL) | 199.90 ± 16.03 |
| Total iron-binding capacity (g/L) | 184.62 ± 7.18 |
Sample collection
From ESRD patients, venous blood samples of 10 ml were collected before HD and 5 ml were obtained after the same HD session. All samples were collected on the long inter-dialytic interval, i.e., two days apart from the previous HD session. Blood was taken from the arteriovenous fistula or central venous catheter. From healthy donors, 10 ml of venous blood was collected from the antecubital vein. K3EDTA was used as anticoagulant in all the blood samples. All the samples were processed within the first hour from blood sampling through centrifugation for 10 min at 1000g, obtaining pre-HD and post-HD plasma aliquots from haemodialyzed patients and plasma aliquots from healthy controls. Such aliquots were stored at − 80 °C until the execution of the assays.
2.3. Plasma protein-bound di-Tyr determination
Protein-bound di-Tyr formation was evaluated by Size Exclusion/Gel Filtration High Performance Liquid Chromatography (GF-HPLC, same as SE-HPLC) on a BioSep-SEC-S4000 column (300 mm × 7.8 mm) with a guard column (SecurityGuard™ GFC-4000, 4 mm length × 3 mm ID) and UV–VIS detector. Plasma samples were diluted 1:15 in 50 mM Tris-HCl, pH 7.4 and 20 μl was loaded into the column for each sample. The mobile phase consisted of Milli-Q water, containing 0.5% (w/v) SDS and was eluted at 1 ml/min. Eluates were monitored both at 215 nm for measuring absorbance of peptide bonds and at 415-nm emission with 325-nm excitation for measuring di-Tyr fluorescence. In the time range between 6 and 9 min, both the area under the 215-nm absorbance chromatogram (A215) and the area under the 415-nm emission fluorescence chromatogram (IF415nm em) were considered (Supplementary Fig. S1). The ratio between total fluorescence and total absorbance (IF415nm em/A215nm) was calculated for each sample.
2.4. Determination of clinical laboratory parameters
CRP, white blood cells count, albumin, creatinine, fibrinogen, haemoglobin, ferritin, total iron-binding capacity, urea, sodium, potassium, calcium, and phosphorus were measured by standardized methods at the clinical laboratory of the Humanitas Clinical and Research Center.
2.5. Statistical analysis
Table 2.
Plasma protein-bound di-Tyr content in male and female ESRD patients. Mean (SE) of plasma protein-bound di-Tyr level in male and female ESRD patients before (pre-HD) and after (post-HD) a single HD session. Sex-specific mean values were compared by independent-sample t-tests.
| N | Mean (SE) | t | df | P | |
|---|---|---|---|---|---|
| di-Tyr pre-HD | |||||
| Males | 48 | 0.193 (0.005) | 2.23 | 71 | 0.029 |
| Females | 25 | 0.170 (0.010) | |||
| di-Tyr post-HD | |||||
| Males | 47 | 0.174 (0.174) | 2.11 | 70 | 0.039 |
| Females | 25 | 0.154 (0.010) | |||
3. Results
3.1. Plasma protein-bound di-Tyr levels in healthy people and ESRD patients on maintenance HD
There are significant differences in total plasma protein and albumin concentrations between healthy subjects and patients with ESRD [23], [40], as well as for each ESRD patient before and after a single HD session [23]. The latter is due to ultrafiltration performed during the dialysis session in order to restore the dry body weight of the patient. This deserves close consideration in the analysis of plasma protein-bound di-Tyr concentrations, since any increase or decrease in total plasma protein and albumin concentration can dramatically affect the di-Tyr measured values. Therefore, in this study we express plasma protein-bound di-Tyr content as IF415nm em/A215nm ratio.
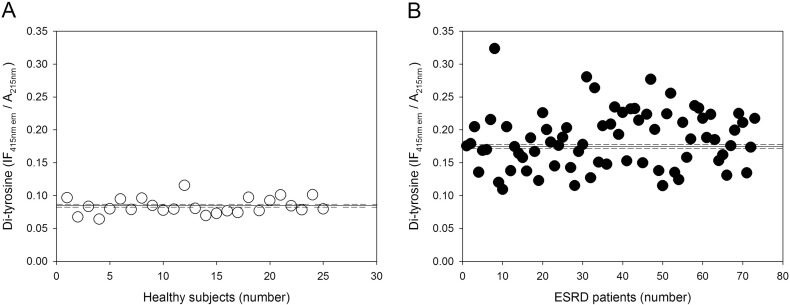
We evaluated the discriminative power of plasma protein-bound di-Tyr content in distinguishing ESRD patients from age-matched healthy subjects by means of receiver operating characteristic (ROC) curve analysis (Supplementary Fig. S2). Plasma protein-bound di-Tyr levels from healthy subjects and ESRD patients yielded an area under the curve (AUC) of 0.99 (95% confidence interval 0.9943 to 1.002; p < 0.0001) (Supplementary Fig. S2A). The cut-off level for plasma protein-bound di-Tyr content as predictor of ESRD was determined by maximizing sensitivity and specificity, at 0.112 IF415nm em/A215nm (Supplementary Fig. S2B). Conversely, plasma protein-bound di-Tyr levels from diabetic (n = 23) and non-diabetic (n = 50) ESRD patients yielded an AUC of 0.54 (not shown), implying that di-Tyr cannot discriminate between diabetic and non-diabetic ESRD patients.
3.2. Effect of a single HD session on the level of plasma protein-bound di-Tyr
3.3. Correlation between pre-HD serum creatinine and albumin concentrations and plasma protein-bound di-Tyr level measured pre-HD
3.4. Correlation between pre-HD serum CRP concentration and plasma protein-bound di-Tyr content measured pre-HD
4. Discussion
The accessibility of plasma proteins for sampling, the relatively long half-lives of many plasma proteins, and the well-characterized biochemical pathways of protein oxidation make plasma proteins an attractive biomarker of oxidative stress in ESRD patients on HD. Biomarkers of protein oxidation can be classified in two types: (i) generic biomarkers, which include oxidation of multiple residues within protein to form several products, e.g., PCO and AOPPs [37], [45]; and (ii) specific biomarkers, which are very specific in both the residue oxidized and the product generated: e.g., oxidation of protein free sulphydryl groups (P-SH) and oxidation of protein Tyr residues to give di-tyrosines [46].
Single HD sessions have different effects on the different types of biomarkers of protein oxidation. For example, in haemodialyzed patients plasma protein oxidation is revealed by decreased P-SH [23], which might result from S-thiolation, the formation of mixed disulphides between P-SH and low-molecular-mass aminothiols, which is considered to be the mechanism protecting P-SH from losing their biological activity by irreversible oxidation [47], [48]. S-thiolated plasma proteins are indeed increased in haemodialyzed patients [21], [22], [23], [40]. However, a single HD session, by removing solutes responsible for increasing ROS production and the low-molecular-mass aminothiols involved in S-thiolation [21], caused transient return of plasma P-SH to the level equal or close to that occurring in healthy subjects [20], [21], [23]. De-thiolation of S-thiolated proteins during a single HD session can be explained by considering the reversible reactions involved in S-thiolation [47]. This is very important in the case of albumin, because its Cys34 thiol represents the largest fraction of all free thiols in plasma, thus attributing to albumin a major role in total plasma antioxidant capacity [41]. Serum albumin de-thiolation during a single HD session may restore transiently its antioxidant activity in haemodialyzed patients [49]. Therefore, S-thiolated proteins represent a useful indicator of thiol-specific reversible oxidative stress in ESRD patients on HD.
Otherwise, by measuring plasma PCO after reaction with 2,4-dinitrophenylhydrazine (DNPH), a number of studies have demonstrated that plasma PCO concentrations increase in ESRD patients [19], [20], [24], [25]. Detection and quantification of PCO by means of DNPH-based methods does not allow for any distinction between primary, or direct, and secondary, or indirect, protein carbonylation [46], [50] and also measure sulphenic acids [51]. Therefore, PCO provide a general and widely used biomarker of severe protein oxidation in ESRD patients.
Increased oxidative stress in ESRD patients is also revealed by the formation of plasma AOPPs [27], [28], [29], which are considered a generic biomarker of protein oxidation and oxidative stress. In this respect, determination of protein-bound di-Tyr by GF-HPLC with fluorometric detection could be taken as a highly specific biomarker of protein oxidation [23], [52], [53], [54]. Protein-bound di-Tyr are final, chemically stable and easily detectable products of tyrosine oxidation in response to oxidative stress induced by both non-enzymatic and peroxidase-catalyzed mechanisms [52], [55]. Myeloperoxidase (MPO), a haemoprotein present in phagocytes, uses hydrogen peroxide to generate di-Tyr from Tyr residues via its peroxidase cycle, in a manner that functions most efficiently at neutral to slightly alkaline pH (7.5–8), near the physiological concentrations of chloride ions and amino acids [56]. In haemodialyzed patients, plasma levels of MPO are significantly higher than the reference value for healthy subjects and further increase during HD [38], [57]. Indeed, the measurement of MPO may serve as a reliable marker of the degree of oxidative stress induced using dialysis membranes of different biocompatibilities [38]. Increased MPO activity could also serve as one mechanistic link between inflammation, oxidative stress and endothelial dysfunction in ESRD [58] and was found to be associated with mortality in ESRD patients undergoing HD [18], [59].
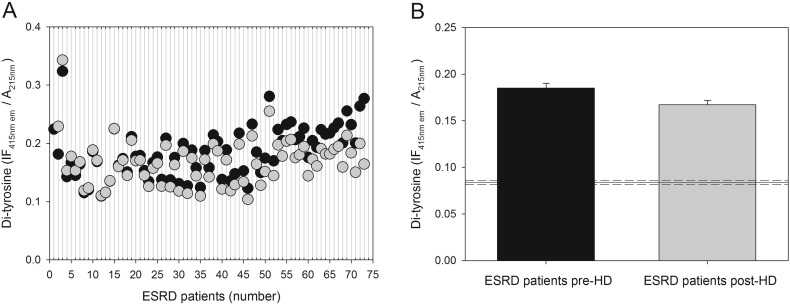
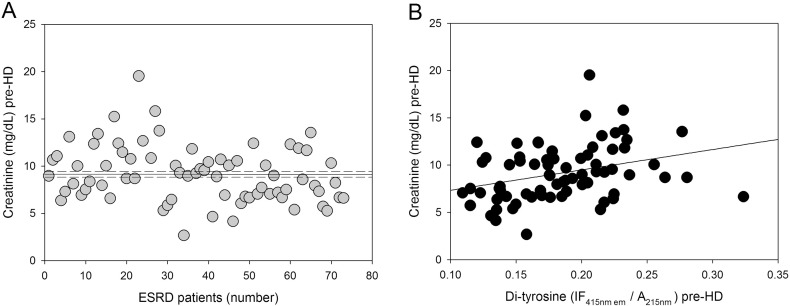
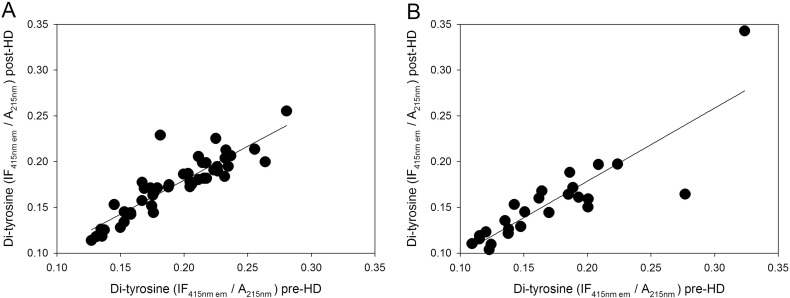
We found significantly higher pre-HD levels of plasma protein-bound di-Tyr in haemodialyzed patients compared with di-Tyr level in age-matched healthy subjects (Fig. 1). The AUC (0.99) indicates that the ROC curve has excellent accuracy and that plasma protein-bound di-Tyr level is good indicator of ESRD due to its high sensitivity and specificity in discriminating between ESRD patients and age-matched healthy subjects (Supplementary Fig. S2). However, the usefulness of plasma protein-bound di-Tyr level in clinical practice is questionable, as there is no need for a new biomarker to dignose ESRD. Rather, the interesting finding is that, in most ESRD patients, a single HD session decreased significantly the plasma protein-bound di-Tyr level, even if the mean level of plasma protein-bound di-Tyr post-HD remained significantly greater in ESRD patients compared to the mean di-Tyr level in age-matched healthy subjects (Fig. 2). Conversely, in a previous study, AOPPs increased during dialysis session, both in patients using a cellulose filter and in patients using polysulphone filters, the latters being the filters also used in our group of patients [38]. In two other studies, a single HD session had no effect on AOPP concentration, which remained significantly greater than normal after the HD session [20], [39]. Given that di-Tyr is a more specific biomarker of protein oxidation and oxidative stress than AOPPs [52], [55], our data challenge the previous findings, suggesting that, in general, the HD session itself does not make the patient's oxidative status worse and can even improve it. It is worthy to note that, in our patients, creatinine levels were correlated with pre-HD plasma protein-bound di-Tyr levels (Fig. 4). Although creatinine is influenced by lean body mass in ESRD patients on HD [60], it is one of the historically used biomarkers of uraemia in HD and its levels efficiently predict mortality in this population [61], [62]. Thus, creatinine is a faithful biomarker of uraemia that, on turn, is known to affect deeply the oxidative status of the patient: this explains the good correlation that we found between di-Tyr levels and creatinine concentration before dialysis. Therefore, we can speculate that the oxidative damage due to uraemic toxins is efficiently improved by the HD session, as demonstrated by reduction of protein-bound di-Tyr. On the other hand, AOPPs may represent a grosser marker of oxidative stress that, in HD patients, is also due the presence of comorbidities, such as diabetes mellitus, and the occurrence of acute clinical events, such as infections. However, future studies with a greater number of patients, inclusive of patients with CKD stages 1–5, are needed to extend these findings, because the usefulness of the ideal biomarker of oxidative damage lies in its ability to provide early indication of disease and/or its progression. As we expected, plasma protein-bound di-Tyr levels measured pre-HD were significantly positively correlated with post-HD plasma protein-bound di-Tyr levels (Fig. 3).
Fig. 1.
Plasma protein-bound di-Tyr level in healthy subjects and ESRD patients. (A) Plasma protein-bound di-Tyr in individual healthy subjects (n = 25). (B) Plasma protein-bound di-Tyr content in individual ESRD patients (n = 73) before HD session (pre-HD). In both (A) and (B), the horizontal solid and dashed lines represent, respectively, the mean and the SE of the plasma protein-bound di-Tyr level.
Fig. 2.
Effect of a single HD session on the level of plasma protein-bound di-Tyr. (A) Scatter diagram showing plasma protein-bound di-Tyr level in patients with ESRD (n = 73) immediately before (black circles) and after (gray circles) a single HD session. (B) Plasma protein-bound di-Tyr content in ESRD patients immediately before (pre-HD) and after (post-HD) a single HD session. Data are expressed as mean ± SE. The horizontal solid and dashed lines represent, respectively, the mean and the SE of the plasma protein-bound di-Tyr level in age-matched healthy subjects (n = 25).
Fig. 4.
Correlation between pre-HD serum creatinine concentration and pre-HD plasma protein-bound di-Tyr level in ESRD patients. (A) Serum creatinine concentrations in individual ESRD patients (n = 73) before HD session (pre-HD). The horizontal solid and dashed lines represent, respectively, the mean and the SE of the serum creatinine concentration. (B) Positive linear correlation between serum creatinine concentration and plasma protein-bound di-Tyr level pre-HD in ESRD patients (n = 73).
Fig. 3.
Correlations between plasma protein-bound di-Tyr levels in ESRD patients (n = 73) measured immediately before (pre-HD) and after (post-HD) a single HD session. (A) Males (n = 48), (B) females (n = 25). Correlations were investigated using simple linear regression analysis.
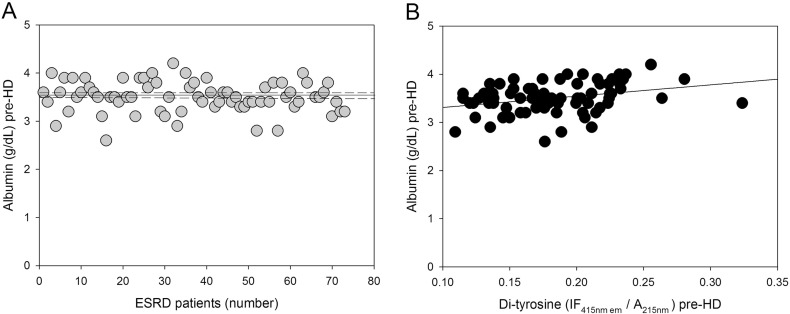
We also found a moderate positive correlation between plasma protein-bound di-Tyr level and pre-dialysis serum albumin concentration (Fig. 5). This result is particularly interesting considering that serum albumin, which is frequently considered a predictor of nutritional status in patients with ESRD [63], is typically low in ESRD patients and hypoalbuminaemia is associated with mortality in haemodialyzed patients [64], [65], [66], [67]. However, other studies suggest that hypoalbuminaemia may be more reflective of inflammation than nutritional status in ESRD patients [58], [68], [69], [70]. In addition, a linkage of hypoalbuminaemia, inflammation, and oxidative stress has been shown in ESRD patients receiving maintenance HD therapy. Indeed, there is a high prevalence of inflammation and oxidative stress in these patients and levels of inflammatory and oxidative stress biomarkers are increased further in hypoalbuminaemic compared with normoalbuminaemic haemodialyzed patients [71]. Therefore, we hypothesized that plasma protein-bound di-Tyr level could correlate with circulating inflammatory biomarkers.
Fig. 5.
Correlation between pre-HD plasma albumin concentration and pre-HD plasma protein-bound di-Tyr level in ESRD patients. (A) Plasma albumin concentrations in individual ESRD patients (n = 73) before HD (pre-HD). The horizontal solid and dashed lines represent, respectively, the mean and the SD of the plasma albumin concentration. (B) Positive linear correlation between plasma albumin concentration and plasma protein-bound di-Tyr content pre-HD in ESRD patients (n = 73).
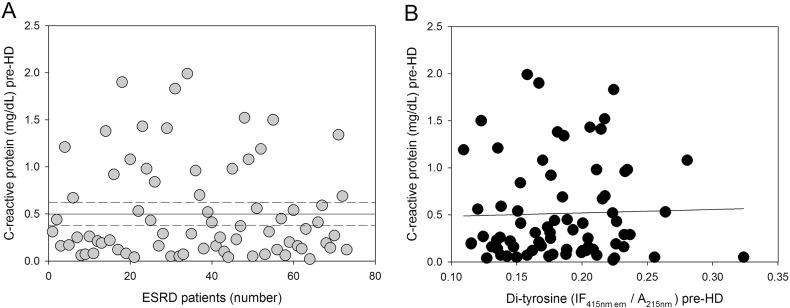
Among the variety of circulating inflammatory biomarkers, CRP, the major acute phase response protein, is elevated in ESRD patients and is the most widely used inflammatory marker predicting future cardiovascular risk and mortality in ESRD patients [42], [72], [73], [74]. In contrast to our hypothesis, the results do not show any statistically significant correlation between plasma protein-bound di-Tyr level and serum concentration of CRP (Fig. 6). These results are in line with others showing no correlations between plasma AOPPs and CRP [28], [75]. Anyway, these results are somewhat surprising considering that inflammation, which is not confined to the kidney, rather it is systemic [76], [77], [78], is a common feature of ESRD patients and both oxidative stress and inflammation are usually inseparably linked and participate in a self-perpetuating vicious circuit. Consequently, the presence and severity of systemic inflammation contribute to ESRD-associated oxidative stress. Indeed, there is evidence to suggest that renal transplantation is associated with almost complete correction of the biomarkers of oxidative stress (measured as PCO) and inflammation (measured as CRP) in patients with ESRD [79], [80]. Thus, our results could suggest that, in haemodialyzed patients, oxidative stress and inflammation may also be in part independent of each other. Otherwise, the absence of correlation between plasma protein-bound di-Tyr level and CRP concentration (measured as “pinpoint marker”) could be explained by the fact that CRP concentration fluctuates substantially over time in patients undergoing HD; therefore, reliable CRP levels can be obtained following regular, repeated measurements [74].
Fig. 6.
Correlation between pre-HD serum CRP concentration and pre-HD plasma protein-bound di-Tyr content in ESRD patients. (A) Serum CRP concentrations in individual ESRD patients (n = 73) before HD (pre-HD). (B) Relationship between serum CRP concentration and plasma protein-bound di-Tyr level pre-HD in ESRD patients (n = 73).
This study has strengths and limitations. Strengths include, firstly, the use of a highly specific biomarker of protein oxidation easy to detect: the intrinsic fluorescence properties of di-Tyr and its chemical stability (fairly unreactive to changes in oxygen and/or pH) allow for its sensitive detection in proteins [23], [52], [53], [54], [56], [57], [58], [59], [39], [55]. Furthermore, plasma protein-bound di-Tyr are obviously not washed out during HD session, unlike some small molecules, such as 4-hydroxynonenal, malonyldialdehyde, and free F2-isoprostanes, which were considered promising biomarkers of oxidative stress in ESRD patients [81], [82], [83]), which are washed out during HD session. In addition, if we consider that protein-bound di-Tyr are carried mainly by albumin in the blood and that albumin half-life in plasma is ~ 19 days [84], protein-bound di-Tyr might serve as long-lasting biomarkers of oxidative stress in ESRD patients. The finding that di-Tyr levels decreased at the end of the dialysis session has to be further discussed, since di-Tyr represents an irreversible oxidative product and, of course, dialysis cannot modify it. However, modern dialyzers have an increased molecular cut-off compared to the older ones, and are capable to dialyze low molecular weigth proteins, such as beta-2 microglobulin, which has a molecular weigth of 11.8 kD and six Tyr residues. Given that we assessed di-Tyr levels as the ratio to total serum proteins, we can speculate that the dialysis of low molecular weigth proteins richer in di-Tyr could have improved the overall content of di-Tyr at the end of the dialysis session. This, of course, does not imply that the di-Tyr content of larger proteins that are not dialyzed, such as albumin, has been modified by the dialysis session. Limitations include the relatively small number of ESRD patients, the fact that the study has been performed in only one single HD centre, and measurements have been made at single time points (“pinpoint marker”). Another limitation is that we assessed the effect of a single HD session using only a single filter type: it would be interesting, in the future, to analyze the influence of different type of dialyzers on plasma protein-bound di-Tyr levels. We thus consider our findings as hypothesis-generating and hope that these results stimulate further studies on the use of plasma protein-bound di-Tyr as a biomarker for oxidative stress in ESRD, with a larger number of haemodialyzed patients enrolled from different dialysis centres.
In conclusion, the choice/indication of the most pertinent biomarkers of oxidative stress is a critical step in the development of novel treatment options for ESRD patients. Furthermore, ESRD is associated with other pro-oxidant conditions such as CVD and diabetes mellitus. In this regard, the relative importance of the different types of oxidative stress and inflammation in ESRD remains largely undetermined, and several questions concerning oxidative stress and inflammation remain poorly defined. Additional large-scale studies with the inclusion of clinically relevant endpoints are required to examine the potential correlations between a panel of biomarkers of inflammation and oxidative stress in ESRD patients on HD. This may pave the way for potential therapeutic intervention aimed at reducing the oxidative stress in hemodialysed patients. The widespread use of anti-oxidants cannot be recommended yet, as large studies with hard end-points are currently lacking. However, when taking into account some surrogate end-points, such as albumin for malnutrition, the already available data are encouraging [85]. Moreover, the use of more biocompatible and anti-oxidant filters, such as vitamin E-coated polysulfone membranes, could potentially change the clinical practice in the future [86], [87].
Conflict of interest disclosure statement
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. We further confirm that the manuscript has been read and approved by all named authors and that the order of authors listed in the manuscript has been approved by all of us.
Abbreviations
- AOPPs
advanced oxidation protein products
- AUC
area under the curve
- CDK
chronic kidney disease
- CRP
C-reactive protein
- CVD
cardiovascular disease
- di-Tyr
dityrosine(s)
- DNPH
dinitrophenylhydrazine
- ESRD
end stage renal disease
- HD
haemodialysis
- GF-HPLC
gel filtration high performance liquid chromatography
- MPO
myeloperoxidase
- PCO
protein carbonyls
- P-SH
protein sulphydryl group(s)
- ROC
receiver operating characteristic
Transparency document
Transparency document.
Acknowledgments
The authors acknowledge financial support from the Fondazione Ariel (Grant 5x1000), Rozzano (MI), Italy. The authors are grateful to Dr. Barbara Ponzini and all the personnel at the Analysis Laboratory, Department of Pathophysiology and Transplantation, University of Milan, for their invaluable support in providing blood samples from healthy subjects. Graphical abstract was prepared using and combining medical clip arts available within the Servier Medical Art section, by courtesy of Servier International.
Footnotes
In this manuscript the term dityrosine will refer to 3,3′-dityrosine (3,3′-bityrosine or o,o′-dityrosine)
The Transparency document associated with this article can be found, in online version.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bbacli.2016.12.004.
Appendix A. Supplementary data
Supplementary material
References
- 1.Stenvinkel P. Chronic kidney disease: a public health priority and harbinger of premature cardiovascular disease. J. Intern. Med. 2010;268(5):456–467. doi: 10.1111/j.1365-2796.2010.02269.x. [DOI] [PubMed] [Google Scholar]
- 2.Levey A.S., Atkins R., Coresh J., Cohen E.P., Collins A.J., Eckardt K.U., Nahas M.E., Jaber B.L., Jadoul M., Levin A., Powe N.R., Rossert J., Wheeler D.C., Lameire N., Eknoyan G. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72(3):247–259. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 3.Vanholder R., Massy Z., Argiles A., Spasovski G., Verbeke F., Lameire N., European Uremic Toxin Work Group Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol. Dial. Transplant. 2005;20(6):1048–1056. doi: 10.1093/ndt/gfh813. [DOI] [PubMed] [Google Scholar]
- 4.Sarnak M.J., Levey A.S., Schoolwerth A.C., Coresh J., Culleton B., Hamm L.L., McCullough P.A., Kasiske B.L., Kelepouris E., Klag M.J., Parfrey P., Pfeffer M., Raij L., Spinosa D.J., Wilson P.W., American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108(17):2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 5.United States Renal Data System . 2013. Annual Data Report: Atlas of Chronic Kidney Disease & End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2013. ( http://www.ajkd.org/issue/S0272-6386(13)X0014-9) [Google Scholar]
- 6.Muntner P., He J., Astor B.C., Folsom A.R., Coresh J. Traditional and non-traditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J. Am. Soc. Nephrol. 2005;16(2):529–538. doi: 10.1681/ASN.2004080656. [DOI] [PubMed] [Google Scholar]
- 7.Di Lullo L., House A., Gorini A., Santoboni A., Russo D., Ronco C. Chronic kidney disease and cardiovascular complications. Heart Fail. Rev. 2015;20(3):259–272. doi: 10.1007/s10741-014-9460-9. [DOI] [PubMed] [Google Scholar]
- 8.Himmelfarb J., Stenvinkel P., Ikizler T.A., Hakim R.M. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62(5):1524–1538. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 9.Carrero J.J., Stenvinkel P. Persistent inflammation as a catalyst for other risk factors in chronic kidney disease: a hypothesis proposal. Clin. J. Am. Soc. Nephrol. 2009;4(Suppl. 1):S49–S55. doi: 10.2215/CJN.02720409. [DOI] [PubMed] [Google Scholar]
- 10.Popolo A., Autore G., Pinto A., Marzocco S. Oxidative stress in patients with cardiovascular disease and chronic renal failure. Free Radic. Res. 2013;47(5):346–356. doi: 10.3109/10715762.2013.779373. [DOI] [PubMed] [Google Scholar]
- 11.Modaresi A., Nafar M., Sahraei Z. Oxidative stress in chronic kidney disease. Iran. J. Kidney Dis. 2015;9(3):165–179. [PubMed] [Google Scholar]
- 12.Wratten M.L., Galaris D., Tetta C., Sevanian A. Evolution of oxidative stress and inflammation during hemodialysis and their contribution to cardiovascular disease. Antioxid. Redox Signal. 2002;4:935–944. doi: 10.1089/152308602762197470. [DOI] [PubMed] [Google Scholar]
- 13.Pavone B., Sirolli V., Bucci S., Libardi F., Felaco P., Amoroso L., Sacchetta P., Urbani A., Bonomini M. Adsorption and carbonylation of plasma proteins by dialyser membrane material: in vitro and in vivo proteomics investigations. Blood Transfus. 2010;8(Suppl. 3):s113–s119. doi: 10.2450/2010.018S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glorieux G., Neirynck N., Veys N., Vanholder R. Dialysis water and fluid purity: more than endotoxin. Nephrol. Dial. Transplant. 2012;27(11):4010–4021. doi: 10.1093/ndt/gfs306. [DOI] [PubMed] [Google Scholar]
- 15.Horl W.H., Riegel W., Schollmeyer P., Rautenberg W., Neumann S. Different complement and granulocyte activation in patients dialyzed with PMMA dialyzers. Clin. Nephrol. 1986;25:304–307. [PubMed] [Google Scholar]
- 16.Oldani S., Finazzi S., Bottazzi B., Garlanda C., Baldassarre E., Valaperta S., Cuccovillo I., Albini M., Child M., Montanelli A., Graziani G., Badalamenti S. Plasma pentraxin-3 as a marker of bioincompatibility in hemodialysis patients. J. Nephrol. 2012;25(1):120–126. doi: 10.5301/JN.2011.8432. [DOI] [PubMed] [Google Scholar]
- 17.Drüeke T., Witko-Sarsat V., Massy Z., Descamps-Latscha B., Guerin A.P., Marchais S.J., Gausson V., London G.M. Iron therapy, advanced oxidation protein products, and carotid artery intima-media thickness in end-stage renal disease. Circulation. 2002;106(17):2212–2217. doi: 10.1161/01.cir.0000035250.66458.67. [DOI] [PubMed] [Google Scholar]
- 18.Stenvinkel P., Carrero J.J., Axelsson J., Lindholm B., Heimbürger O., Massy Z. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle? Clin. J. Am. Soc. Nephrol. 2008;3(2):505–521. doi: 10.2215/CJN.03670807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Himmelfarb J., McMonagle E., McMenamin E. Plasma protein thiol oxidation and carbonyl formation in chronic renal failure. Kidney Int. 2000;58(6):2571–2578. doi: 10.1046/j.1523-1755.2000.00443.x. [DOI] [PubMed] [Google Scholar]
- 20.Ward R.A., Ouseph R., McLeish K.R. Effects of high-flux hemodialysis on oxidant stress. Kidney Int. 2003;63(1):353–359. doi: 10.1046/j.1523-1755.2003.00741.x. [DOI] [PubMed] [Google Scholar]
- 21.Włodek P.J., Smolenski O.B., Chwatko G., Iciek M.B., Miłkowski A., Bald E., Włodek L. Disruption of thiol homeostasis in plasma of terminal renal failure patients. Clin. Chim. Acta. 2006;366(1–2):137–145. doi: 10.1016/j.cca.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 22.Khazim K., Giustarini D., Rossi R., Verkaik D., Cornell J.E., Cunningham S.E., Mohammad M., Trochta K., Lorenzo C., Folli F., Bansal S., Fanti P. Glutathione redox potential is low and glutathionylated and cysteinylated hemoglobin levels are elevated in maintenance hemodialysis patients. Transl. Res. 2013;162(1):16–25. doi: 10.1016/j.trsl.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colombo G., Reggiani F., Garavaglia M.E., Portinaro N.M., Badalamenti S., Milzani A., Dalle-Donne I. Plasma protein thiolation index (PTI) as a biomarker of thiol stress in hemodialyzed patients. Free Radic. Biol. Med. 2015;89:443–451. doi: 10.1016/j.freeradbiomed.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 24.Pieniazek A., Brzeszczynska J., Kruszynska I., Gwozdzinski K. Investigation of albumin properties in patients with chronic renal failure. Free Radic. Res. 2009;43(10):1008–1018. doi: 10.1080/10715760903165003. [DOI] [PubMed] [Google Scholar]
- 25.Caimi G., Carollo C., Hopps E., Montana M., Lo P.R. Protein oxidation in chronic kidney disease. Clin. Hemorheol. Microcirc. 2013;54(4):409–413. doi: 10.3233/CH-131739. [DOI] [PubMed] [Google Scholar]
- 26.Tucker P.S., Dalbo V.J., Han T., Kingsley M.I. Clinical and research markers of oxidative stress in chronic kidney disease. Biomarkers. 2013;18(2):103–115. doi: 10.3109/1354750X.2012.749302. [DOI] [PubMed] [Google Scholar]
- 27.Capeillère-Blandin C., Gausson V., Descamps-Latscha B., Witko-Sarsat V. Biochemical and spectrophotometric significance of advanced oxidized protein products. Biochim. Biophys. Acta. 2004;1689(2):91–102. doi: 10.1016/j.bbadis.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Anderstam B., Ann-Christin B.H., Valli A., Stenvinkel P., Lindholm B., Suliman M.E. Modification of the oxidative stress biomarker AOPP assay: application in uremic samples. Clin. Chim. Acta. 2008;393(2):114–118. doi: 10.1016/j.cca.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 29.Hanasand M., Omdal R., Norheim K.B., Gøransson L.G., Brede C., Jonsson G. Improved detection of advanced oxidation protein products in plasma. Clin. Chim. Acta. 2012;413(9–10):901–906. doi: 10.1016/j.cca.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 30.Descamps-Latscha B., Witko-Sarsat V., Nguyen-Khoa T., Nguyen A.T., Gausson V., Mothu N., London G.M., Jungers P. Advanced oxidation protein products as risk factors for atherosclerotic cardiovascular events in nondiabetic predialysis patients. Am. J. Kidney Dis. 2005;45(1):39–47. doi: 10.1053/j.ajkd.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Witko-Sarsat V., Gausson V., Nguyen A.T., Touam M., Drüeke T., Santangelo F., Descamps-Latscha B. AOPP-induced activation of human neutrophil and monocyte oxidative metabolism: a potential target for N-acetylcysteine treatment in dialysis patients. Kidney Int. 2003;64(1):82–91. doi: 10.1046/j.1523-1755.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- 32.Guo Z.J., Niu H.X., Hou F.F., Zhang L., Fu N., Nagai R., Lu X., Chen B.H., Shan Y.X., Tian J.W., Nagaraj R.H., Xie D., Zhang X. Advanced oxidation protein products activate vascular endothelial cells via a RAGE-mediated signaling pathway. Antioxid. Redox Signal. 2008;10(10):1699–1712. doi: 10.1089/ars.2007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu S.X., Hou F.F., Guo Z.J., Nagai R., Zhang W.R., Liu Z.Q., Zhou Z.M., Zhou M., Xie D., Wang G.B., Zhang X. Advanced oxidation protein products accelerate atherosclerosis through promoting oxidative stress and inflammation. Arterioscler. Thromb. Vasc. Biol. 2006;26(5):1156–1162. doi: 10.1161/01.ATV.0000214960.85469.68. [DOI] [PubMed] [Google Scholar]
- 34.Marsche G., Frank S., Hrzenjak A., Holzer M., Dirnberger S., Wadsack C., Scharnagl H., Stojakovic T., Heinemann A., Oettl K. Plasma-advanced oxidation protein products are potent high-density lipoprotein receptor antagonists in vivo. Circ. Res. 2009;104(6):750–757. doi: 10.1161/CIRCRESAHA.108.193169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capeillère-Blandin C., Gausson V., Nguyen A.T., Descamps-Latscha B., Drüeke T., Witko-Sarsat V. Respective role of uraemic toxins and myeloperoxidase in the uraemic state. Nephrol. Dial. Transplant. 2006;21(6):1555–1563. doi: 10.1093/ndt/gfl007. [DOI] [PubMed] [Google Scholar]
- 36.Selmeci L. Advanced oxidation protein products (AOPP): novel uremic toxins, or components of the non-enzymatic antioxidant system of the plasma proteome? Free Radic. Res. 2011;45(10):1115–1123. doi: 10.3109/10715762.2011.602074. [DOI] [PubMed] [Google Scholar]
- 37.Colombo G., Clerici M., Giustarini D., Portinaro N., Badalamenti S., Rossi R., Milzani A., Dalle-Donne I. A central role for intermolecular dityrosine cross-linking of fibrinogen in high molecular weight advanced oxidation protein product (AOPP) formation. Biochim. Biophys. Acta. 2015;1850(1):1–12. doi: 10.1016/j.bbagen.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 38.Wu C.C., Chen J.S., Wu W.M., Liao T.N., Chu P., Lin S.H., Chuang C.H., Lin Y.F. Myeloperoxidase serves as a marker of oxidative stress during single haemodialysis session using two different biocompatible dialysis membranes. Nephrol. Dial. Transplant. 2005;20(6):1134–1139. doi: 10.1093/ndt/gfh764. [DOI] [PubMed] [Google Scholar]
- 40.Fanti P., Giustarini D., Rossi R., Cunningham S.E., Folli F., Khazim K., Cornell J., Matteucci E., Bansal S. Dietary intake of proteins and calories is inversely associated with the oxidation state of plasma thiols in end-stage renal disease patients. J. Ren. Nutr. 2015;25(6):494–503. doi: 10.1053/j.jrn.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colombo G., Clerici M., Giustarini D., Rossi R., Milzani A., Dalle-Donne I. Redox albuminomics: oxidized albumin in human diseases. Antioxid. Redox Signal. 2012;17(11):1515–1527. doi: 10.1089/ars.2012.4702. [DOI] [PubMed] [Google Scholar]
- 42.Carrero J.J., Stenvinkel P. Inflammation in end-stage renal disease—what have we learned in 10 years? Semin. Dial. 2010;23(5):498–509. doi: 10.1111/j.1525-139X.2010.00784.x. [DOI] [PubMed] [Google Scholar]
- 43.Musunuru K., Kral B.G., Blumenthal R.S., Fuster V., Campbell C.Y., Gluckman T.J., Lange R.A., Topol E.J., Willerson J.T., Desai M.Y., Davidson M.H., Mora S. The use of high-sensitivity assays for C-reactive protein in clinical practice. Nat. Clin. Pract. Cardiovasc. Med. 2008;5(10):621–635. doi: 10.1038/ncpcardio1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lacson E., Jr., Levin N.W. C-reactive protein and end-stage renal disease. Semin. Dial. 2004;17(6):438–448. doi: 10.1111/j.0894-0959.2004.17604.x. [DOI] [PubMed] [Google Scholar]
- 45.Dalle-Donne I., Aldini G., Carini M., Colombo R., Rossi R., Milzani A. Protein carbonylation, cellular dysfunction, and disease progression. J. Cell. Mol. Med. 2006;10(2):389–406. doi: 10.1111/j.1582-4934.2006.tb00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bachi A., Dalle-Donne I., Scaloni A. Redox proteomics: chemical principles, methodological approaches and biological/biomedical promises. Chem. Rev. 2013;113(1):596–698. doi: 10.1021/cr300073p. [DOI] [PubMed] [Google Scholar]
- 47.Dalle-Donne I., Milzani A., Gagliano N., Colombo R., Giustarini D., Rossi R. Molecular mechanisms and potential clinical significance of S-glutathionylation. Antioxid. Redox Signal. 2008;10(3):445–473. doi: 10.1089/ars.2007.1716. [DOI] [PubMed] [Google Scholar]
- 48.Rossi R., Giustarini D., Milzani A., Dalle-Donne I. Cysteinylation and homocysteinylation of plasma protein thiols during ageing of healthy human beings. J. Cell. Mol. Med. 2009;13(9B):3131–3140. doi: 10.1111/j.1582-4934.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soejima A., Kaneda F., Manno S., Matsuzawa N., Kouji H., Nagasawa T., Era S., Takakuwa Y. Useful markers for detecting decreased serum antioxidant activity in hemodialysis patients. Am. J. Kidney Dis. 2002;39(5):1040–1046. doi: 10.1053/ajkd.2002.32787. [DOI] [PubMed] [Google Scholar]
- 50.Colombo G., Clerici M., Garavaglia M.E., Giustarini D., Rossi R., Milzani A., Dalle-Donne I. A step-by-step protocol for assaying protein carbonylation in biological samples. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016;1019:178–190. doi: 10.1016/j.jchromb.2015.11.052. [DOI] [PubMed] [Google Scholar]
- 51.Dalle-Donne I., Carini M., Orioli M., Vistoli G., Regazzoni L., Colombo G., Rossi R., Milzani A., Aldini G. Protein carbonylation: 2,4-dinitrophenylhydrazine reacts with both aldehydes/ketones and sulfenic acids. Free Radic. Biol. Med. 2009;46(10):1411–1419. doi: 10.1016/j.freeradbiomed.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 52.DiMarco T., Giulivi C. Current analytical methods for the detection of dityrosine, a biomarker of oxidative stress, in biological samples. Mass Spectrom. Rev. 2007;26(1):108–120. doi: 10.1002/mas.20109. [DOI] [PubMed] [Google Scholar]
- 53.Lim P.S., Cheng Y.M., Yang S.M. Impairments of the biological properties of serum albumin in patients on haemodialysis. Nephrology (Carlton) 2007;12(1):18–24. doi: 10.1111/j.1440-1797.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 54.Al-Hilaly Y.K., Williams T.L., Stewart-Parker M., Ford L., Skaria E., Cole M., Bucher W.G., Morris K.L., Sada A.A., Thorpe J.R., Serpell L.C. A central role for dityrosine crosslinking of amyloid-β in Alzheimer's disease. Acta Neuropathol. Commun. 2013;1:83. doi: 10.1186/2051-5960-1-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heinecke J.W., Li W., Daehnke H.L., 3rd, Goldstein J.A. Dityrosine, a specific marker of oxidation, is synthesized by the myeloperoxidase-hydrogen peroxide system of human neutrophils and macrophages. J. Biol. Chem. 1993;268(6):4069–4077. [PubMed] [Google Scholar]
- 57.Arsov S., Trajceska L., van Oeveren W., Smit A.J., Dzekova P., Stegmayr B., Sikole A., Rakhorst G., Graaff R. The influence of body mass index on the accumulation of advanced glycation end products in hemodialysis patients. Eur. J. Clin. Nutr. 2015;69(3):309–313. doi: 10.1038/ejcn.2014.261. [DOI] [PubMed] [Google Scholar]
- 58.Kaysen G.A., Eiserich J.P. The role of oxidative stress-altered lipoprotein structure and function and microinflammation on cardiovascular risk in patients with minor renal dysfunction. J. Am. Soc. Nephrol. 2004;15(3):538–548. doi: 10.1097/01.asn.0000111744.00916.e6. [DOI] [PubMed] [Google Scholar]
- 59.Kalantar-Zadeh K., Brennan M.L., Hazen S.L. Serum myeloperoxidase and mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 2006;48(1):59–68. doi: 10.1053/j.ajkd.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 39.Kuchta A., Pacanis A., Kortas-Stempak B., Cwiklińska A., Ziętkiewicz M., Renke M., Rutkowski B. Estimation of oxidative stress markers in chronic kidney disease. Kidney Blood Press. Res. 2011;34(1):12–19. doi: 10.1159/000321508. [DOI] [PubMed] [Google Scholar]
- 55.Giulivi C., Traaseth N.J., Davies K.J. Tyrosine oxidation products: analysis and biological relevance. Amino Acids. 2003;25(3–4):227–232. doi: 10.1007/s00726-003-0013-0. [DOI] [PubMed] [Google Scholar]
- 60.Noori N., Kovesdy C.P., Bross R., Lee M., Oreopoulos A., Benner D., Mehrotra R., Kopple J.D., Kalantar-Zadeh K. Novel equations to estimate lean body mass in maintenance hemodialysis patients. Am. J. Kidney Dis. 2011;57(1):130–139. doi: 10.1053/j.ajkd.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fink J.C., Burdick R.A., Kurth S.J., Blahut S.A., Armistead N.C., Turner M.S., Shickle L.M., Light P.D. Significance of serum creatinine values in new end-stage renal disease patients. Am. J. Kidney Dis. 1999;34(4):694–701. doi: 10.1016/S0272-6386(99)70395-1. [DOI] [PubMed] [Google Scholar]
- 62.Dwyer J.T., Larive B., Leung J., Rocco M.V., Greene T., Burrowes J., Chertow G.M., Cockram D.B., Chumlea W.C., Daugirdas J., Frydrych A., Kusek J.W., HEMO Study Group Are nutritional status indicators associated with mortality in the Hemodialysis (HEMO) Study? Kidney Int. 2005;68(4):1766–1776. doi: 10.1111/j.1523-1755.2005.00593.x. [DOI] [PubMed] [Google Scholar]
- 63.Gama-Axelsson T., Heimbürger O., Stenvinkel P., Bárány P., Lindholm B., Qureshi A.R. Serum albumin as predictor of nutritional status in patients with ESRD. Clin. J. Am. Soc. Nephrol. 2012;7(9):1446–1453. doi: 10.2215/CJN.10251011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herselman M., Esau N., Kruger J.M., Labadarios D., Moosa M.R. Relationship between serum protein and mortality in adults on long-term hemodialysis: exhaustive review and meta-analysis. Nutrition. 2010;26(1):10–32. doi: 10.1016/j.nut.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 65.Mehrotra R., Duong U., Jiwakanon S., Kovesdy C.P., Moran J., Kopple J.D., Kalantar-Zadeh K. Serum albumin as a predictor of mortality in peritoneal dialysis: comparisons with hemodialysis. Am. J. Kidney Dis. 2011;58(3):418–428. doi: 10.1053/j.ajkd.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Markaki A., Gkouskou K., Ganotakis E., Margioris A., Daphnis E. A longitudinal study of nutritional and inflammatory status in patients on dialysis. J. Ren. Care. 2014;40(1):14–22. doi: 10.1111/jorc.12048. [DOI] [PubMed] [Google Scholar]
- 67.Sousa-Martins P., Moura A., Madureira J., Alija P., Oliveira J.G., Lopez M., Filgueiras M., Amado L., Sameiro-Faria M., Miranda V., Mesquita E., Teixeira L., Poveda V., Lobato L., Santos-Silva A., Costa E. Risk factors for mortality in end-stage kidney disease patients under online-hemodiafiltration: three-year follow-up study. Biomarkers. 2016;30:1–7. doi: 10.3109/1354750X.2016.1160428. 10.3109/1354750X.2016.1160428 ([Epub ahead of print]) [DOI] [PubMed] [Google Scholar]
- 68.de Mutsert R., Grootendorst D.C., Indemans F., Boeschoten E.W., Krediet R.T., Dekker F.W. Netherlands Cooperative Study on the adequacy of dialysis-II study group. Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J. Ren. Nutr. 2009;19(2):127–135. doi: 10.1053/j.jrn.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 69.Silverstein D.M. Inflammation in chronic kidney disease: role in the progression of renal and cardiovascular disease. Pediatr. Nephrol. 2009;24(8):1445–1452. doi: 10.1007/s00467-008-1046-0. [DOI] [PubMed] [Google Scholar]
- 70.Friedman A.N., Fadem S.Z. Reassessment of albumin as a nutritional marker in kidney disease. J. Am. Soc. Nephrol. 2010;21(2):223–230. doi: 10.1681/ASN.2009020213. [DOI] [PubMed] [Google Scholar]
- 71.Danielski M., Ikizler T.A., McMonagle E., Kane J.C., Pupim L., Morrow J., Himmelfarb J. Linkage of hypoalbuminemia, inflammation, and oxidative stress in patients receiving maintenance hemodialysis therapy. Am. J. Kidney Dis. 2003;42(2):286–294. doi: 10.1016/s0272-6386(03)00653-x. [DOI] [PubMed] [Google Scholar]
- 72.Kato A., Takita T., Furuhashi M., Maruyama Y., Hishida A. Comparison of serum albumin, C-reactive protein and carotid atherosclerosis as predictors of 10-year mortality in hemodialysis patients. Hemodial. Int. 2010;14(2):226–232. doi: 10.1111/j.1542-4758.2009.00432.x. [DOI] [PubMed] [Google Scholar]
- 73.Bazeley J., Bieber B., Li Y., Morgenstern H., de Sequera P., Combe C., Yamamoto H., Gallagher M., Port F.K., Robinson B.M. C-reactive protein and prediction of 1-year mortality in prevalent hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2011;6(10):2452–2461. doi: 10.2215/CJN.00710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meuwese C.L., Stenvinkel P., Dekker F.W., Carrero J.J. Monitoring of inflammation in patients on dialysis: forewarned is forearmed. Nat. Rev. Nephrol. 2011;7(3):166–176. doi: 10.1038/nrneph.2011.2. [DOI] [PubMed] [Google Scholar]
- 75.Marques de Mattos A., Marino L.V., Ovidio P.P., Jordão A.A., Almeida C.C., Chiarello P.G. Protein oxidative stress and dyslipidemia in dialysis patients. Ther. Apher. Dial. 2012;16(1):68–74. doi: 10.1111/j.1744-9987.2011.01009.x. [DOI] [PubMed] [Google Scholar]
- 76.Yao Q., Axelsson J., Stenvinkel P., Lindholm B. Chronic systemic inflammation in dialysis patients: an update on causes and consequences. ASAIO J. 2004;50(6):lii–lvii. doi: 10.1097/01.mat.0000147958.87989.eb. [DOI] [PubMed] [Google Scholar]
- 77.Yilmaz M.I., Carrero J.J., Axelsson J., Lindholm B., Stenvinkel P. Low-grade inflammation in chronic kidney disease patients before the start of renal replacement therapy: sources and consequences. Clin. Nephrol. 2007;68(1):1–9. doi: 10.5414/cnp68001. [DOI] [PubMed] [Google Scholar]
- 78.Akchurin O.M., Kaskel F. Update on inflammation in chronic kidney disease. Blood Purif. 2015;39(1–3):84–92. doi: 10.1159/000368940. [DOI] [PubMed] [Google Scholar]
- 79.Simmons E.M., Langone A., Sezer M.T., Vella J.P., Recupero P., Morrow J.D., Ikizler T.A., Himmelfarb J. Effect of renal transplantation on biomarkers of inflammation and oxidative stress in end-stage renal disease patients. Transplantation. 2005;79(8):914–919. doi: 10.1097/01.tp.0000157773.96534.29. [DOI] [PubMed] [Google Scholar]
- 80.Aveles P.R., Criminácio C.R., Gonçalves S., Bignelli A.T., Claro L.M., Siqueira S.S., Nakao L.S., Pecoits-Filho R. Association between biomarkers of carbonyl stress with increased systemic inflammatory response in different stages of chronic kidney disease and after renal transplantation. Nephron Clin. Pract. 2010;116(4):c294–c299. doi: 10.1159/000318792. [DOI] [PubMed] [Google Scholar]
- 81.Karamouzis I., Sarafidis P.A., Karamouzis M., Iliadis S., Haidich A.B., Sioulis A., Triantos A., Vavatsi-Christaki N., Grekas D.M. Increase in oxidative stress but not in antioxidant capacity with advancing stages of chronic kidney disease. Am. J. Nephrol. 2008;28(3):397–404. doi: 10.1159/000112413. [DOI] [PubMed] [Google Scholar]
- 82.Ferretti G., Bacchetti T., Masciangelo S., Pallotta G. Lipid peroxidation in hemodialysis patients: effect of vitamin C supplementation. Clin. Biochem. 2008;41(6):381–386. doi: 10.1016/j.clinbiochem.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 83.Wiswedel I., Peter D., Gardemann A., Carluccio F., Hampl H., Siems W. Serum concentrations of F2-isoprostanes and 4-hydroxynonenal in hemodialysis patients in relation to inflammation and renal anemia. Biomark. Insights. 2008;3:419–428. doi: 10.4137/bmi.s363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peters T., Jr. Biochemistry, Genetics and Medical Applications. first ed. Academic Press Inc.; San Diego, CA, USA: 1996. All about albumin; pp. 223–234. [Google Scholar]
- 85.Rattanasompattikul M., Molnar M.Z., Lee M.L., Dukkipati R., Bross R., Jing J., Kim Y., Voss A.C., Benner D., Feroze U., Macdougall I.C., Tayek J.A., Norris K.C., Kopple J.D., Unruh M., Kovesdy C.P., Kalantar-Zadeh K. Anti-inflammatory and anti-oxidative nutrition in hypoalbuminemic dialysis patients (AIONID) study: results of the pilot-feasibility, double-blind, randomized, placebo-controlled trial. J. Cachex. Sarcopenia Muscle. 2013;4(4):247–257. doi: 10.1007/s13539-013-0115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Andrulli S., Di Filippo S., Manzoni C., Stefanelli L., Floridi A., Galli F., Locatelli F. Effect of synthetic vitamin E-bonded membrane on responsiveness to erythropoiesis-stimulating agents in hemodialysis patients: a pilot study. Nephron Clin. Pract. 2010;115(1):c82–c89. doi: 10.1159/000294281. [DOI] [PubMed] [Google Scholar]
- 87.Bargnoux A.S., Cristol J.P., Jaussent I., Chalabi L., Bories P., Dion J.J., Henri P., Delage M., Dupuy A.M., Badiou S., Canaud B., Morena M. Vitamin E-coated polysulfone membrane improved red blood cell antioxidant status in hemodialysis patients. J. Nephrol. 2013;26(3):556–563. doi: 10.5301/jn.5000195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.
Supplementary material