ABSTRACT
Elevated levels of selenium (Se) cause toxicity in non-accumulator plant species. The primary reasons for toxic Se effect have been considered to be selenoprotein accumulation and oxidative stress. However, based on our recent paper in Plant Cell Reports and previous literature data we suggest that disturbances in the homeostasis of both reactive oxygen and nitrogen species result in selenium-induced nitro-oxidative stress, contributing to toxicity. The most characteristic symptom of Se exposure is the inhibited root elongation which is partly caused by hormonal disturbances. Our recent paper suggests the involvement of cytokinin in selenium stress sensing of the root systemAltogether, the aim of this Addendum is to present reactive nitrogen species and phytohormones as new players in plant selenium toxicity.
KEYWORDS: Cytokinin, nitric oxide, phytohormones, reactive nitrogen species, selenium, toxicity
Selenium toxicity in non-accumulator plants
Selenium (Se) is a naturally occurring element essential to some organisms, including bacteria, some green algae and mammals. Although higher plants do not require selenium, they take it up due to its chemical and physical similarities to sulfur.1 For higher plants, selenium is beneficial at low concentrations, but elevated levels of this element can cause toxicity.2 For non-accumulator plants like Arabidopsis thaliana, tissue Se concentrations higher than 2 mg/kg cause toxicity3 and can result in an approximately 10% reduction of biomass yield without the appearance of visible symptoms.4 However, in general the toxicity of Se inevitably depends on the plant's age, since seedlings are more sensitive to elevated Se levels than mature plants. The threshold value of toxicity depends also on the form of selenium and selenite has been reported to be more toxic than selenate.5 In extreme cases, a large amount of accumulated selenium can give rise to symptoms like chlorosis, necrosis, leaf withering and drying.2 The remarkable inhibition of shoot and root development is a more typical consequence of selenium exposure. As it is the point of contact with the element in the environment, the root system is highly affected by Se. The primary root (PR) elongation is markedly inhibited by excess Se, and this effect has been shown to be concentration/time-dependent6,7,8,9,10 and consequently associated with selenium tolerance. Therefore, the degree of PR growth inhibition reflects the plants' resistance or susceptibility against Se and can be presented as selenium tolerance index (%).9,11,12
Our recent paper13 provided new information about the tissue- and cellular-level mechanisms of the effect of selenium (selenite) on primary root growth. We observed that both the length of the root apical meristem and its cell number are significantly reduced in selenite-exposed roots. Moreover, Se treatment resulted in a notable reduction of cyclinB1 expression, suggesting that the main reason for PR shortening is the inhibition of cell division in the apical meristem.
Known molecular mechanisms of selenium toxicity
The main reason for Se toxicity is the malformation of non-specific selenoproteins.14 Selenoamino acids -selenocysteine or selenomethionine- can be incorporated into proteins in place of cysteine and methionine residues, leading to alterations in protein structure and function.15 Furthermore, the accumulation of these malfunctioning Se-containing proteins results in toxicity; although plants like the Se-hyperaccumulator Stanleya pinnata are able to decrease the levels of these proteins by directing them to the ubiquitin-26S proteasome pathway.16 Recently, the involvement of endoplasmic reticulum-assisted degradation (ERAD) in removal of misfolded selenoproteins and in Se tolerance was proposed.17
It is also known that Se exerts its toxic effect also through inducing oxidative stress.14 Selenium-triggered production of reactive oxygen species (ROS) including hydrogen peroxide and superoxide has been observed in the root and shoot system by several research groups.7,8,11,13,18,19 Recently it was pointed out that superoxide generated by the glutathione-mediated reduction of selenite may partly be responsible for the Se-induced impairment of photosynthesis.9
The primary metabolism and the levels of some essential macro- and microelements are also affected by Se and these changes are associated with selenium-induced growth inhibition.18,20
Novel mechanisms behind selenium toxicity: Reactive nitrogen species and phytohormones
In addition to ROS, Se toxicity also involves changes in the level of reactive nitrogen species (RNS) such as nitric oxide (NO) and peroxynitrite. In our recent publication,13 selenite was shown to decrease NO levels in a concentration-dependent manner in Arabidopsis roots. This selenite-induced NO diminution was also observed in the nitrate reductase-deficient nia1nia2 mutant, suggesting that nitrate reductase activity is not involved in NO level changes (Fig. 1). However, in pea root tips, selenite had the opposite effect on NO levels, and significantly increased peroxynitrite formation and consequently protein tyrosine nitration.19 Similarly, selenate exposure resulted in NO accumulation in Brassica rapa root tips and the effect proved to be concentration- and time-dependent.8 The diverse NO responses can be explained by the variable parameters such as the applied Se forms, concentrations, plant age and growth conditions.
Figure 1.
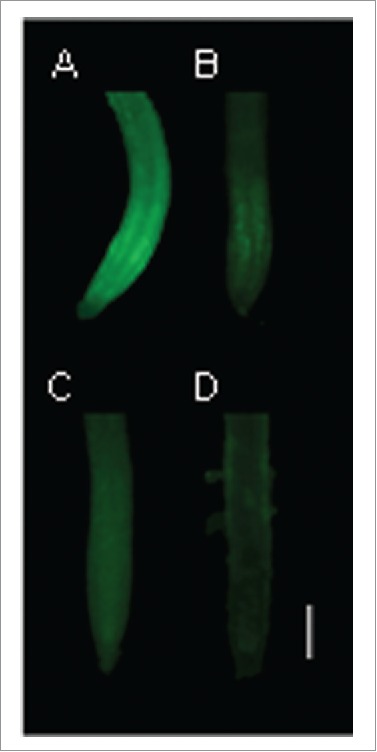
Root tips of wild-type (A,B) and nia1nia2 (C,D) Arabidopsis treated with 0 (control, A,C) or 40 μM sodium-selenite for 4 d. Nitric oxide levels were visualized by staining the root samples with 4-amino-5-methylamino-2′,7′ difluorofluorescein diacetate. Bar = 100 μm.
These results show that the homeostasis of not only ROS but also RNS can be greatly affected by Se. Consequently, besides selenium-induced oxidative stress, the secondary nitrosative stress also contributes to selenium toxicity. Therefore, rather than simply oxidative stress, we urge the consideration of nitro-oxidative stress21 as an underlying mechanism of Se phytotoxicity.
The actions of certain hormones, such as ethylene (ET), jasmonic acid (JA) and salicylic acid, are related to stress resistance. Selenite exposure induced the production of ET and JA and the intensification of their signaling led to enhanced selenite tolerance in Arabidopsis.11
Besides stress hormones, phytohormones like auxin and cytokinin (CK) are major regulators of plant growth and development; ethylene is also able to exert a regulatory effect on growth. Therefore, the involvement of these morphogens in Se-triggered growth arrest cannot be ignored. Previously, selenite was found to decrease auxin-inducible DR5::GUS activity in Arabidopsis root tips, while the in situ expression of ethylene biosynthetic ACS8::GUS was notably enhanced.7
In our recent publication,13 a detailed examination of cytokinin levels and signaling was presented in selenite-exposed Arabidopsis. Our results clearly showed that CK signaling intensified in the root tip, yet was reduced in the aboveground plant parts, suggesting that the root-to-shoot translocation of CK is strongly inhibited by excess selenite. Additionally, the selenite-induced upregulation of CK degrading enzymes (cytokinin oxidase 4 and 5) in the cotyledons and the downregulation of cytokinin oxidase 4 in the root tip may also contribute to the altered distribution of cytokinin. We also suggested that the accumulated cytokinin in the root tips may party be responsible for the root meristem shortening. Our results provided new evidence for the mutually negative relationship between cytokinin and NO in the root system of selenite-stressed Arabidopsis. They also pointed out that overproduction of CK or NO causes selenite insensitivity which suggests the possibility that both molecules are involved in sensing of Se toxicity in plant roots.
Based on the results of our recent paper13 and additional, data in the literature,7,19 we propose an updated concept for the background mechanisms of plant Se toxicity (Fig. 2). Since excess Se disturbs both ROS and RNS homeostasis, it causes secondary nitro-oxidative stress leading to macromolecule modifications and consequently cell death. Furthermore, phytohormones involved in growth regulation and/or stress responses are also affected by selenium. Thus, it is easy to imagine that the imbalance of hormonal homeostasis contributes to the appearance of Se toxicity symptoms.
Figure 2.
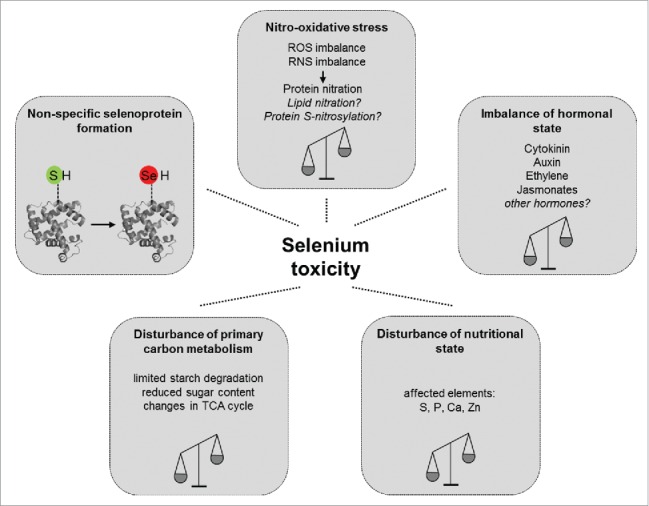
Schematic illustration representing a new concept of processes contributing plant selenium toxicity. See details in the text.
Despite the growing knowledge about plant responses to Se, a number of questions still need clarification. Future research must focus on the underlying mechanisms of selenium-triggered nitrosative stress (e.g. nitration, S-nitrosylation) and the possible involvement of other growth hormones and their interactions with NO in sensing of Se toxicity.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences and by the National Research, Development and Innovation Fund (Grants no. NKFI-6, K120383 and NKFI-1, PD120962). Special thanks to Dr. Tim Crawford for the proofreading.
Funding
This work was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences and by the National Research, Development and Innovation Fund (Grants no. NKFI-6, K120383 and NKFI-1, PD120962).
ORCID
Zsuzsanna Kolbert http://orcid.org/0000-0002-7819-4672
References
- 1.El-Ramady H, Abdalla N, Alshaal T, et al.. Selenium and its role in higher plants. In: Lichtfouse E, et al. (eds.), Pollutants in Buildings, Water and Living Organisms, Environmental Chemistry for a Sustainable World 7; Springer, 2015; pp 238-85 [Google Scholar]
- 2.Terry N, Zayed AM, de Souza MP, Tarun AS. Selenium in higher plants. Annu Rev Plant Physiol Plant Mol Biol 2000; 51:401-32; PMID:15012198; http://dx.doi.org/ 10.1146/annurev.arplant.51.1.401 [DOI] [PubMed] [Google Scholar]
- 3.Mikkelsen RL, Page AL, Bingham FT. Factors affecting selenium accumulation by agricultural crops. In: Selenium in Agriculture and the Environment. Soil Sci Am J 1989; Special publication 23:64-94; http://dx.doi.org/ 10.2136/sssaspecpub23.c4 [DOI] [Google Scholar]
- 4.Kabata-Pendias E. Trace elements in soils and plants, 4th edn. CRC Press/Taylor & Francis Group, LCC, Boca Raton, 2011 [Google Scholar]
- 5.Garousi F. The toxicity of different selenium forms and compounds- Review. Agrártudományi Közlemények 2015; 64:33-8; file:///C:/Users/user/Downloads/acta2015_64_19.pdf [Google Scholar]
- 6.Grant K, Carey NM, Mendoza M, Schulze J, Pilon M, Pilon-Smits EA, van Hoewyk D. Adenosine-5-phosphosulfate reductase (APR2) mutation in Arabidopsis implicates glutathione deficiency in selenite toxicity. Biochem J 2011; 438:325-35; PMID:21585336; http://dx.doi.org/ 10.1042/BJ20110025 [DOI] [PubMed] [Google Scholar]
- 7.Lehotai N, Kolbert ZS, Pető A, Feigl G, Ördög A, Kumar D, Tari I, Erdei L. Selenite-induced hormonal and signalling mechanisms during root growth of Arabidopsis thaliana L. J Exp Bot 2012; 63:5677-87; PMID:22988013; http://dx.doi.org/ 10.1093/jxb/ers222 [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Mo HZ, Hu LB, Li YQ, Chen J, Jang LF. The endogenous nitric oxide mediates selenium-induced phytotoxicity by promoting ROS generation in Brassica rapa. PLoS One 2014; 9(10):e110901; PMID:25333984; http://dx.doi.org/ 10.1371/journal.pone.0110901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher B, Yarmolinsky D, Abdel-Gany S, Pilon M, Pilon-Smits EA, Sagi M, Van Hoewyk D. Superoxide generated from the glutathione-mediated reduction of selenite damages the iron-sulphur cluster of chloroplastic ferredoxin. Plant Phys Biochem 2016; 106:228-35; http://dx.doi.org/ 10.1016/j.plaphy.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 10.Jiang L, Chen Z, Gao Q, Ci L, Cao S, Han Y, Wang W. Loss-of-function mutations in the APX1 gene result in enhanced selenium tolerance in Arabidopsis thaliana. Plant Cell Environ 2016; 39(10):2133-44; PMID:27149098; http://dx.doi.org/18178671 10.1111/pce.12762 [DOI] [PubMed] [Google Scholar]
- 11.Tamaoki M, Freeman JL, Pilon-Smits EAH. Cooperative ethylene and jasmonic acid signaling regulates selenite resistance in Arabidopsis. Plant Physiol 2008; 146:1219-30; PMID:18178671; http://dx.doi.org/ 10.1104/pp.107.110742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Hoewyk D, Takahashi H, Inoue E, Hess A, Tamaoki M, Pilon-Smits EAH. Transcriptome analyses give insights into selenium-stress responses and selenium tolerance mechanisms in Arabidopsis. Physiol Plant 2008; 132:236-53; PMID:18251864; http://dx.doi.org/ 10.1111/j.1399-3054.2007.01002.x [DOI] [PubMed] [Google Scholar]
- 13.Lehotai N, Feigl G, Koós Á, Molnár Á, Ördög A, Pető A, Erdei L, Kolbert ZS. Nitric oxide-cytokinin interplay influences selenite sensitivity in Arabidopsis. Plant Cell Rep 2016; 35(10):2181-95; PMID:27449496 [DOI] [PubMed] [Google Scholar]
- 14.Van Hoewyk D. A tale of two toxicities: malformed selenoproteins and oxidative stress both contribute to selenium stress in plants. Ann Bot 2013; 112:965-72; PMID:23904445; http://dx.doi.org/ 10.1093/aob/mct163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown T, Shrift A. Selenium: toxicity and tolerance in higher plants. Biol Rev 1982; 57:59-84; http://dx.doi.org/ 10.1111/j.1469-185X.1982.tb00364.x [DOI] [Google Scholar]
- 16.Sabbagh M, Van Hoewyk D. Malformed selenoproteins are removed by the ubiquitin–proteasome pathway in Stanleya pinnata. Plant Cell Physiol 2012; 53(3):555-64; PMID:22323770; http://dx.doi.org/ 10.1093/pcp/pcs015 [DOI] [PubMed] [Google Scholar]
- 17.Van Hoewyk D. Defects in endoplasmic reticulum-associated degradation (ERAD) increase selenate sensitivity in Arabidopsis. Plant Signal Behav 2016; http://dx.doi.org/ 10.1080/15592324.2016.1171451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dimkovikj A, Van Hoewyk D. Selenite activates the alternative oxidase pathway and alters primary metabolism in Brassica napus roots: evidence of a mitochondrial stress response. BMC Plant Biol 2014; 14:259; PMID:25267309; http://dx.doi.org/ 10.1186/s12870-014-0259-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehotai N, Lyubenova L, Schröder P, Feigl G, Ördög A, Szilágyi K, Erdei L, Kolbert ZS. Nitro-oxidative stress contributes to selenite toxicity in pea (Pisum sativum L.). Plant Soil 2016; 400:107-22; http://dx.doi.org/ 10.1007/s11104-015-2716-x [DOI] [Google Scholar]
- 20.Riberio DM, Júnior DDS, Barcellos Cardoso F, Martins AO, Silva WA, Nascimento VL, Araújo WL. Growth inhibition by selenium is associated with changes in primary metabolism and nutrient levels in Arabidopsis thaliana. Plant Cell Environ 2016; 39(10):2235-46 [DOI] [PubMed] [Google Scholar]
- 21.Corpas FJ, Barroso JB. Nitro-oxidative stress vs. oxidative and nitrosative stress in higher plants. New Phytol 2013; 199(3):633-5; PMID:23763656 [DOI] [PubMed] [Google Scholar]


