Abstract
No studies have been performed on mitochondria of malaria vector mosquitoes. This information would be valuable in understanding mosquito aging and detoxification of insecticides, two parameters that significantly impact malaria parasite transmission in endemic regions. Here, we report the analyses of respiration and oxidative phosphorylation in mitochondria of cultured cells (ASE line) from Anopheles stephensi, a major vector of malaria in India, Southeast Asia and parts of the Middle East. ASE cell mitochondria shared many features in common with mammalian muscle mitochondria, despite the fact that these cells have a larval origin. However, two major differences with mammalian mitochondria were apparent. One, the glycerol-phosphate shuttle plays a major role in NADH oxidation in ASE cell mitochondria as it does in insect muscle mitochondria. In contrast, mammalian white muscle mitochondria depend primarily on lactate dehydrogenase, whereas red muscle mitochondria depend on the malate-oxaloacetate shuttle. Two, ASE mitochondria were able to oxidize Pro at a rate comparable with that of α-glycerophosphate. However, the Pro pathway appeared to differ from the currently accepted pathway, in that ketoglutarate could be catabolyzed completely by the Krebs cycle or via transamination depending on the ATP need.
Keywords: mitochondria, bioenergetics, mosquito, malaria, proline, Anopheles stephensi
Introduction
Watanabe & Williams [1, 2] were the first to perform biochemical studies on insect sarcosomes. Later studies demonstrated that the citric acid cycle was operational in sarcosomes of the housefly Musca domestica [3–7] and of the blowfly Phormia regina [8]. In general, oxidation was accompanied by the esterification of inorganic phosphate [3–7]. Specific patterns of oxidation were explored in the mitochondrial and supernatant fractions of the honey bee Apis mellifera [9]. Recently, studies from Sohal’s laboratory have provided extensive insights into mitochondrial physiology of Drosophila melanogaster [10–12]. To our knowledge, however, no analogous studies have been performed on mitochondria of malaria vector mosquitoes. Such information would be valuable in understanding the physiology of mosquito aging and detoxification of insecticides, two parameters that significantly impact malaria transmission in endemic regions.
We present here for the first time, results from analyses of respiration and oxidative phosphorylation in mitochondria of cultured cells (ASE line1) from Anopheles stephensi, a major vector of malaria in India, Southeast Asia and parts of the Middle East. We chose to use cultured cells as opposed to mitochondria from whole insects initially to characterize mitochondrial metabolic pathways with samples that could be isolated quickly and cleanly in significant quantities. For our studies, we employed polarographic analyses of oxygen consumption of the respiratory chain of isolated ASE cell mitochondria. The polarographic approach is particularly useful for characterizing respiratory function in mitochondria isolated from tissues, cultured cells, or in whole organisms (e.g., yeast). Polarographic studies, in conjunction with the use of specific inhibitors of the electron transport chain, have been widely used in the field, providing most of our current knowledge of mitochondrial bioenergetics (S1). The goal of our studies was to characterize the metabolic pathways utilized by ASE mitochondria in terms of energy production and substrate use, and compare them to mitochondria from various insects and mammals.
Materials and Methods
Cell maintenance
The immortalized A. stephensi ASE cell line was grown in modified Eagle’s minimal essential medium (“E5”) supplemented with glucose, L-glutamine, vitamin solution, nonessential amino acids, penicillin and streptomycin, and 5% heat-inactivated fetal bovine serum at 28°C with 5% carbon dioxide [13]. The population doubling time of these cells is approximately 18–20 h. The cells were split 1:10 into E5 medium and grown in 50 ml culture flasks until confluent. These flasks were used to seed 500-ml culture flasks to prepare replicates of ~2 x 109 cells for mitochondria preparation. For counting, a single-cell suspension was loaded onto a hemocytometer and counted under a microscope; number of cells per ml was calculated by multiplying by the dilution factor and by the conversion factor for 10 counted fields. Under our culture conditions, ASE cell viability measured by trypan blue exclusion is 85–90%. To concentrate the cells for mitochondria preparation, the cells were gently pipetted to resuspend them then the medium was transferred to a 50 ml tube. Cells were pelleted by centrifugation at 800 g for 5 min. The supernatant was removed to just above the cell pellet, the cells were resuspended in a small amount of medium by gentle pipetting and transferred to a sterile holding tube on ice. This cycle was repeated, with collection of the concentrated cells into one tube, until all flasks were processed.
Isolation of mitochondria
Cells were centrifuged for 1 min at 500 g at 4°C and mitochondria were isolated from pelleted cells using a modified procedure from the Giulivi lab [14]. The pellet was weighed and MSHE buffer was added at a ratio of 3 ml per g of cell wet weight (MSHE: 220 mM mannitol, 70 mM sucrose, 0.5 mM EGTA, 0.1% fatty acid-free bovine albumin, and 2 mM HEPES, pH 7.4). The cells were gently homogenized, centrifuged at 600 g for 5 min at 4°C, the pellet was discarded, and the supernatant was centrifuged at 10,300 g for 10 min at 4°C. The pellet, rich in mitochondria, was resuspended in a small volume of MSHE. Using this procedure the yield was 7.5 ± 0.5 μg mitochondrial protein/106 cells. Protein concentration was determined by using the BCA Protein Assay (Pierce).
Polarographic method for evaluating oxygen uptake
The oxygen consumption of 0.5–1 mg/ml mitochondria was assessed in an oxygraph system [14] by Hansatech Instruments (Norfolk, UK). The chamber contained 0.5 to 1 ml of oxygen-saturated reaction buffer (220 mM sucrose, 50 mM KCl, 5 mM MgCl2, 1 mM EGTA, 10 mM potassium phosphate, 10 mM HEPES, pH 7.4). State 4 respiration was initiated by adding a substrate to the isolated mitochondria, whereas State 3 respiration included the addition of 0.45 or 1mM ADP, as indicated in the text. All reactions were performed with continuous stirring at 20–22°C.
Mass spectrometry analysis and protein identification
LC-MS/MS analyses were performed at the Proteomics Facility of the University of California Genome Center. Tandem mass spectra were extracted by BioWorks version 3.3. Charge state deconvolution and deisotoping were not performed. All MS/MS samples were analyzed using X! Tandem (www.thegpm.org; version 2007.01.01.2). X! Tandem was set up to search the Ensemble Anopheles gambiae protein database (13,740 entries) assuming the digestion enzyme trypsin. X! Tandem was searched with a fragment ion mass tolerance of 0.40 Da and a parent ion tolerance of 1.8 Da. Iodoacetamide derivative of cysteine was specified in X! Tandem as a fixed modification. Deamidation of asparagine and glutamine, oxidation of methionine and tryptophan, sulphone of methionine, tryptophan oxidation to formylkynurenin of tryptophan and acetylation of the n-terminus were specified in X! Tandem as variable modifications.
Scaffold (version Scaffold-01_06_03, Proteome Software Inc., Portland, OR) was used to validate MS/MS based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 90.0% probability as specified by the Peptide Prophet algorithm [15]. Protein identifications were accepted if they could be established at greater than 99.0% probability and contained at least 2 identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm [16]. Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony.
Statistical Analyses
The results represent the mean ± SD of three replicates from one experiment. Each experiment was carried out a minimum of 3 times, and the results shown reflecting all results obtained. The effect of treatment was compared with control values by one-way analysis of variance (ANOVA). Tests were carried out using StatSimple version 2.0.5 from Nidus Technologies (Toronto, Canada).
Results and Discussion
Oxidation of Krebs cycle intermediates and related compounds by isolated mitochondria
Several citric acid cycle members and related compounds were tested as substrates for isolated mitochondria from ASE cells (Table I). Significant rates were obtained with α-glycerophosphate, Pro, pyruvate, glutamate, malate, ketoglutarate, fumarate, and succinate. Respiratory control rates (RCR) were high (4 or higher) with these substrates indicating a significant coupling of electron transfer with oxidative phosphorylation.
Table I.
Rates of oxygen consumption by mitochondria from ASE cells with different substrates*
| Substrate | Oxygen Uptake |
|---|---|
| (nmol oxygen/min mg protein) | |
| Pyruvate (5 mM) | 12 |
| Citrate (5 mM) | 22 |
| Pyruvate-Malate (5 mM / 2.5 mM) | 8 |
| Isocitrate (5 mM) | 16 |
| α-Ketoglutarate (10 mM) | 8 |
| Succinate (10 mM) | 6 |
| Fumarate (10 mM) | 7 |
| Glutamate (10 mM) | 10 |
| Glutamate-Malate (10 mM / 1 mM) | 10 |
| Malate (10 mM) | 9 |
| Oxaloacetate (5 mM) | ND |
| α-glycerophosphate (7.5 mM) | 14 |
| TMPD/Ascorbate (0.2 mM / 10 mM) | 33 |
| Acetoacetate (5 mM) | 11 |
| β-Hydroxybutyrate (5 mM) | ND |
| Octanoate (5 mM) | ND |
| Octanoyl-carnitine (20 μM) | 7 |
| Proline (10 mM) | 14 |
| Ornithine (10 mM) | ND |
| Glutamine (10 mM) | ND |
| Threonine (1 mM) | 5 |
| Methionine (1 mM) | ND |
| Lysine (1 mM) | ND |
| Glycine (1 mM) | ND |
| Leucine (1 mM) | ND |
| α-ketobutyrate (1 mM) | ND |
| α-ketoisovalerate (1 mM) | ND |
| α-keto-β-methylglutarate (1 mM) | ND |
| α-ketoisocaproate (1 mM) | ND |
Rates were measured during State 3 in the presence of 0.45 mM ADP. RCR were 4 or higher. Rates of State 4 were 2.3 ± 0.2 (mean ± SD). The numbers represent the mean with SD ≤ 10% mean. ND, not detectable.
Glutamate and malate-glutamate were adequate substrates for these mitochondria. These results indicated that ASE cells share a similar pathway with mammalian mitochondria: malate is transported into mitochondria in exchange for ketoglutarate, followed by the oxidation of malate by malate dehydrogenase, removal of oxaloacetate by the glutamate-aspartate transaminase, and export of aspartate in exchange for glutamate (S1). Glycerophosphate – the other end product of glycolysis in insect flight muscle and, therefore, a physiological substrate for mitochondria – was oxidized at significant rates comparable to pyruvate. The relatively high rate values for the oxidation of glycerophosphate indicated the presence of an active glycerophosphate shuttle.
Addition of malonate, an inhibitor of Complex II, caused a 90% inhibition of pyruvate oxidation, demonstrating that the Krebs cycle is required for pyruvate oxidation. Although it has been amply demonstrated that the mitochondrial fraction of cells contains all the enzymes necessary for the oxidation of pyruvate, most isolated mitochondria oxidize pyruvate poorly unless a primer – succinate, fumarate or malate – is added. Some isolated mitochondria oxidize pyruvate without added primers by carboxylating pyruvate to malate or oxaloacetate. Two enzymes have been described that can catalyze this carboxylation: malic enzyme [17] and pyruvate carboxylase [18]. In addition, other enzymes can catalyze the synthesis of oxaloacetate from phosphoenolpyruvate [19–21], but it appears that these enzymes are not operating at an appreciable rate in most isolated mitochondria. Apart from housefly sarcosomes, no mitochondrial preparations from animal sources have been reported to oxidize pyruvate in the absence of added primers or in the absence of a carboxylating system. However, ASE mitochondria utilized pyruvate at a significant rate indicating that the need to replenish oxaloacetate could be fulfilled by pyruvate carboxylase (PC), which catalyzes the conversion of pyruvate to oxaloacetate and is very abundant in flight muscles of many insect species [22]. In these reports, PC did not co-occur with phosphoenolpyruvate carboxykinase and fructose 1,6-bisphosphatase, so it was concluded that flight muscle PC is not part of the gluconeogenic pathway. Rather, it was proposed that PC may function as an anaplerotic enzyme [22]. Indeed, the amount of oxaloacetate has been shown to increase during flight [23] indicating that this metabolite is synthesized by an additional route. Thus, PC as an anaplerotic enzyme may provide a mechanism for increasing Krebs cycle intermediates via oxaloacetate.
To confirm our inference that pyruvate oxidation could proceed in the absence of priming, pyruvate oxidation was re-analyzed in the presence of malate. Supplementation of mitochondria with malate yielded no significant increase in the rate of oxygen uptake in State 3 (Table I) and, surprisingly, inhibited the response rate by 40%. These results suggested that, as observed in mammalian mitochondria, the transport of pyruvate and malate through the monocarboxylate/proton transporter and malate/citrate transporter, respectively, was also occurring in ASE mitochondria. However, the oxidation of malate to oxaloacetate proceeds through the enzymatic action of malate dehydrogenase in mammalian mitochondria, and because the Keq of this enzyme favors the formation of malate, oxaloacetate must be immediately removed by citrate synthase, a reaction that proceeds in the presence of acetylCoA formed from pyruvate via pyruvate dehydrogenase (S1). The inhibition of malate oxidation by pyruvate addition in ASE mitochondria suggested a feedback inhibition of pyruvate on malic enzyme, indicating that alternative carboxylation reactions to pyruvate oxidation were functional (e.g., via PC) and were similar to housefly sarcosomes. To confirm the involvement of malic enzyme, the effect of tartronic acid, an inhibitor of this enzyme, was tested on malate-only or malate/pyruvate-supplemented mitochondria. Addition of tartronic acid resulted in 92% and 90% inhibition of State 3 oxygen uptake, respectively. This result indicated that malate added exogenously efficiently provides oxaloacetate (through malate dehydrogenase) while pyruvate is provided via the anaplerotic reaction catalyzed by malic enzyme.
Mammalian liver mitochondria are the most important site for generation of ketone bodies (β-hydroxybutyrate and acetoacetate). The liver supplies these compounds as a fuel source to heart and skeletal muscle during diabetes, starvation, and other situations. Although the liver generates ketone bodies, this tissue cannot utilize them for energy and, as such, the liver lacks acetoacetate:succinylCoA CoA transferase, the enzyme required for catabolism of ketone bodies. Mitochondria from ASE cells utilized acetoacetate (Table I), indicating that they are endowed with a pathway to utilize ketone bodies. Conversely, they did not utilize β-hydroxybutyrate, suggesting that the equilibrium of the reaction acetoacetate + NADH ⬄ β-hydroxybutyrate + NAD+ was displaced towards the right. This indicates that the NADH/NAD ratio in these mitochondria is relatively high, probably a result of the high content of endogenous substrates. In agreement with this hypothesis, addition of 0.45 mM ADP to mitochondria without exogenous substrate supplementation (State 2) resulted in values comparable to those obtained with succinate (vide infra Table III).
Table III.
State 3 oxygen uptake of isolated mitochondria from ASE cells with endogenous substrates or proline*
| Additions | STATE 3 OXYGEN UPTAKE (%) | ||
|---|---|---|---|
| EXPECTED | EXPERIMENTAL | EXPERIMENTAL | |
| Endogenous | Pro | ||
| None | 100 | 32 (100) | 100 |
| Malonate | 0 | 4 (3) | 50 |
| Malate | 100 | 24 (75) | 96 |
| Tartronic acid | 0 | <1 (<3) | 10 |
| Pyruvate | 100 | 5 (15) | 15 |
| HydroxyProline | 0 | n.d. | 2 |
Isolated mitochondria were incubated without adding substrates (endogenous) or with 10 mM Pro (Pro). Expected assumes that KG formed from Pro is only catabolyzed via Krebs cycle, with no significant input from malic enzyme unless malate is present. n.d., not determined.
Winged insects can be roughly divided into two groups based on the metabolic fuel that is utilized for flight activity. Insects capable of long-lasting flights, like locusts and butterflies, use fat as the major substrate for flight-muscle activity, whereas insects capable only of short flights, like flies and bees, use carbohydrate as their main source of flight energy [24]. Accordingly, flight-muscle mitochondria isolated from each of these groups of insects exhibit different properties. For example, house fly muscle mitochondria and locust muscle mitochondria differ in (i) their abilities to oxidize pyruvate for short periods in the absence of added Krebs cycle intermediates, (ii) their abilities to oxidize carnitine esters of free fatty acids (but not free fatty acids), (iii) their concentrations of endogenous substrates, and (iv) the ratios of the substrates utilized (Table II). Based on our data, ASE cell mitochondria were more similar to locust muscle mitochondria than house fly muscle mitochondria in all categories (Table II). In addition, other biochemical features of ASE mitochondria make them not only similar to locust muscle mitochondria, but also generally related to mammalian skeletal muscle mitochondria (Table II). These features included the following: the lack of oxidation of octanoate, which in mammalian heart and liver mitochondria occurs via a carnitine-independent pathway with a matrix-associated medium-chain fatty acylCoA synthetase; the rapid oxygen uptake exhibited when ADP was added in the absence of exogenous substrates; the pattern of substrate preferences (Table II); and the use of intermediates associated with ketone body synthesis (Table I).
Table II.
Substrate preference of insect mitochondria*
| Insect Mitochondria | Mammalian Muscle Mitochondria** | |||
|---|---|---|---|---|
| Substrate | House fly flight muscle | Locust flight muscle | ASE cell (this study) | |
| Glycerophosphate | 25 | ~1 | 1.4 | 0.1 – 1.0 |
| Pyruvate + Malate | 25 | ~ 1.4 | 0.8 | 1 – 3 |
| Ketoglutarate | 0.7 | 0.4 | 0.8 | 0.7–2 |
| Octanoylcarnitine† | NA | 1.1 | 0.7 | 1–2 |
| Succinate | 1 | 0.3 | 0.6 | 0.2 – 2 |
Substrate preference was calculated as the ratio of oxygen uptake with the indicated substrate and with glutamate. Values for house fly and locust were calculated from [24, 66].
Data for pigeon breast muscle, human skeletal muscle, and rat skeletal muscle were obtained from our lab (rat and human skeletal muscle) and compiled from [67–70] .
Data for locust were obtained with palmitoylcarnitine only. Mammalian data represent an average using palmitoylcarnitine and butyrylcarnitine. NA, not available.
Mosquitoes normally utilize carbohydrates during flight [25, 26], but when attached to a flight mill, A. gambiae females can fly up to 22 h using both lipids and carbohydrates over this period [25]. Another study reported that female A. gambiae used only a third of the lipid and an equal amount of carbohydrate for short flights (4 h) compared to longer flights [27]. These results suggest that when A. gambiae females are forced to take a long flight, carbohydrates are primarily used during the first few hours [26]. In A. gambiae, it was shown that when carbohydrates are exhausted, lipids are mobilized and used [25]. A simple calculation based on the average amount of fat and glycogen present in female Anopheles mosquitoes (70 μg/female and 25 μg/female; [27]) would indicate that glycogen can sustain a 1h flight, whereas fat reserves can sustain flight for 6–7h2.
Carbohydrates are mobilized mainly from glycogen reserves, resulting in an increased level of soluble carbohydrates in hemolymph. The mechanism for mobilizing lipids is unknown, but some physiological clues suggest possible mechanisms. More than 30 different members of the adipokinetic hormone/red pigment-concentrating hormone (AKH/RPCH) family have been identified from the major insect orders. AKHs induce the mobilization of flight substrates such as lipids in crickets, grasshoppers, locusts and butterflies, sugars in cockroaches, flies and bees, and Pro in tsetse flies and some coleopterans [29]. For several insect species, AKH octapeptides play an important role during flight [30, 31] and other energy consuming activities, such as walking, ball rolling, or swimming [29, 30]. Locusts utilize mainly carbohydrates for short flights, whereas for longer flights, the transition to mobilization of lipids for energy is regulated by AKHs. [31]. By analogy, AKH may mobilize lipids in A. stephensi for utilization by flight muscle during extended periods of energy utilization.
The conversion of glyceraldehyde-3-phosphate into 1,3-diphosphoglycerate during glycolysis requires the conversion of NAD+ into NADH and, to maintain the glycolytic flux, the NADH must be continuously reoxidized. In white vertebrate muscle, NADH reoxidation is achieved by the conversion of pyruvate into lactate, catalyzed by lactate dehydrogenase. Although, the activity of the glycerol-phosphate shuttle is higher in white than in red muscle [32], the activity of this shuttle plays a supplementary role to lactate dehydrogenase.
In muscles that oxidize a considerable amount of pyruvate via the tricarboxylic acid cycle (e.g. insect flight muscle and vertebrate red muscles) NADH is oxidized by means of the mitochondrial electron transport chain. However, the mitochondrial membrane is impermeable to NAD+ and NADH, so indirect means of transporting the cytoplasmic reducing equivalents into the mitochondria exist [33–35]. In red muscles a large proportion of the pyruvate produced from glucose is oxidized to carbon dioxide and water (e.g. more than 80% in the perfused rat heart; [36]). In this tissue, the malate-oxaloacetate shuttle transports reducing equivalents to the mitochondria.
Insect flight muscles are believed to possess an active glycerol-phosphate shuttle that catalyses a unidirectional net oxidation of cytoplasmic NADH by the mitochondrial electron transport chain [37–39]. Further, in most insect flight muscles the maximum glycolytic capacity (estimated from the activities of phosphofructokinase) is approximately the same as the maximum capacity of the glycerol-phosphate shuttle [28]. This suggests that the operation of this cycle in insect flight muscle could account for most, if not all, of the oxidation of NADH produced during glycolysis. However, the higher rates of oxygen uptake found with α-glycerophosphate (glycerol-phosphate shuttle) than those with malate or glutamate-malate (using malate-oxaloacetate shuttle) obtained with ASE mitochondria indicated that the glycerol-phosphate shuttle plays a major role in NADH oxidation in ASE cell mitochondria as it does in insect muscle mitochondria. In contrast, mammalian white muscle mitochondria depend primarily on the lactate dehydrogenase shuttle, whereas red muscle mitochondria depend on the malate-oxaloacetate shuttle for NADH oxidation.
Oxidation of amino acids by isolated mitochondria
Following Winteringham's suggestion that free amino acids present in high concentrations in the hemolymph and in the thoracic tissues of insects can function as energy reserves for flight [40], we tested whether several amino acids could serve as respiratory substrates for isolated ASE cell mitochondria. The average oxygen consumption in State 3 observed with glutamate was 10 nmol oxygen/min mg protein. Of the other amino acids tested (Thr, Met, Lys, Gly, Leu, Ile, Val), and the corresponding branched-chain α-ketoacids, only Thr was oxidized with a significant value (Table I). Mammalian liver mitochondria are endowed with the catabolic pathways for Met, Lys, Gly, and Thr, which drive the formation of NADH and/or FADH at several steps of these pathways. Mammalian liver mitochondria also contain Thr dehydrogenase, (and possibly 2-amino-3-oxobutyrate-CoA ligase) and branched chain α-ketoacid dehydrogenase activities, activities that are required for Thr and branched chain ketoacid metabolism. The capacity to oxidize these amino acids (with the exception of Thr) and the corresponding α-ketoacids are absent in ASE mitochondria. As such, if amino acids have a function in energy production for ASE mitochondria (see below exception for Pro) they contribute only to the supply of Krebs cycle intermediates. For example, Glu can be reversibly transaminated to pyruvate.
In many insects the amino acid Pro is present in relatively high concentrations in hemolymph and flight muscles [41]. It was first shown that Pro serves as an energy substrate during flight in the tsetse fly Glossina morsitans and since then, several investigations have led to the conclusion that Pro is either fully or partially oxidized to supply the flight muscles with energy and that Ala is the end product (see [42–44]. High concentrations of Pro can be found in hemolymph, flight muscles and fat body and the use of Pro to power flight in a number of insects has been corroborated. Biochemical pathways of Pro oxidation and resynthesis have been fully described for the tsetse fly [42] and partially for the Colorado potato beetle [45, 46].
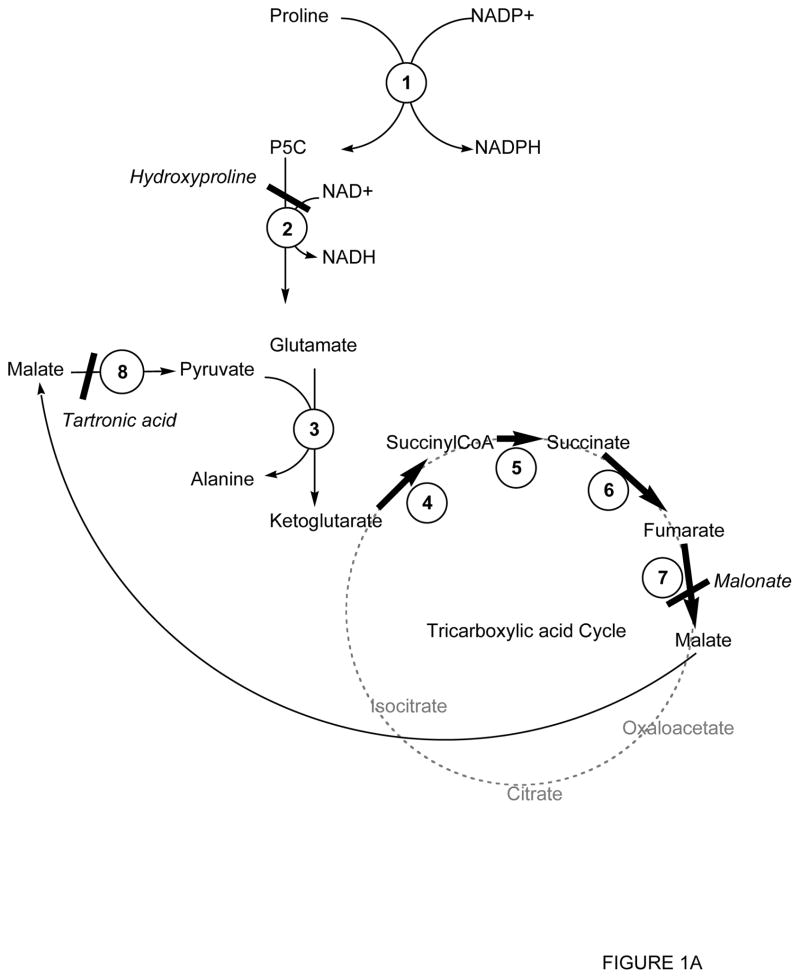
It is believed that during flight, Pro is catabolyzed in the flight muscles in two steps to glutamate (Fig. 1A). Glu subsequently serves as the substrate for alanine aminotransferase (AlaT), providing α-ketoglutarate for oxidation in the Krebs cycle. The 5-carbon moiety is only partially oxidized. Malic enzyme decarboxylates malate from the cycle and produces pyruvate, which is subsequently converted to Ala in the presence of Glu by AlaT [47] . Based on our inference that ASE mitochondria were similar to insect flight muscle mitochondria, we tested whether ASE mitochondria could catabolyze Pro by this pathway. If so, then the following steps would be expected to occur: (i) Pro would be utilized (i.e., addition of ADP to Pro-supplemented mitochondria would result in a significant increase in the oxygen uptake and this would be inhibited by oligomycin and uncoupled by FCCP), (ii) Pro oxidation would be competitively inhibited by hydroxyproline [48, 49], a substrate for Δ1-pyrroline-5-carboxylic dehydrogenase (P5CDH), which generates 4-hydroxyglutamate [50], (iii) the competitive inhibitor of succinate dehydrogenase – malonate – would inhibit Pro oxidation if oxidation of ketoglutarate proceeds exclusively through the Krebs cycle, (iv) malonate inhibition would be reversed by malate because this substrate would provide pyruvate via malic enzyme, (v) subsequent addition of tartronic acid would inhibit malic enzyme and oxygen uptake, and (vi) tartronic acid inhibition would be reversed by the addition of pyruvate (Fig. 1A & Table III).
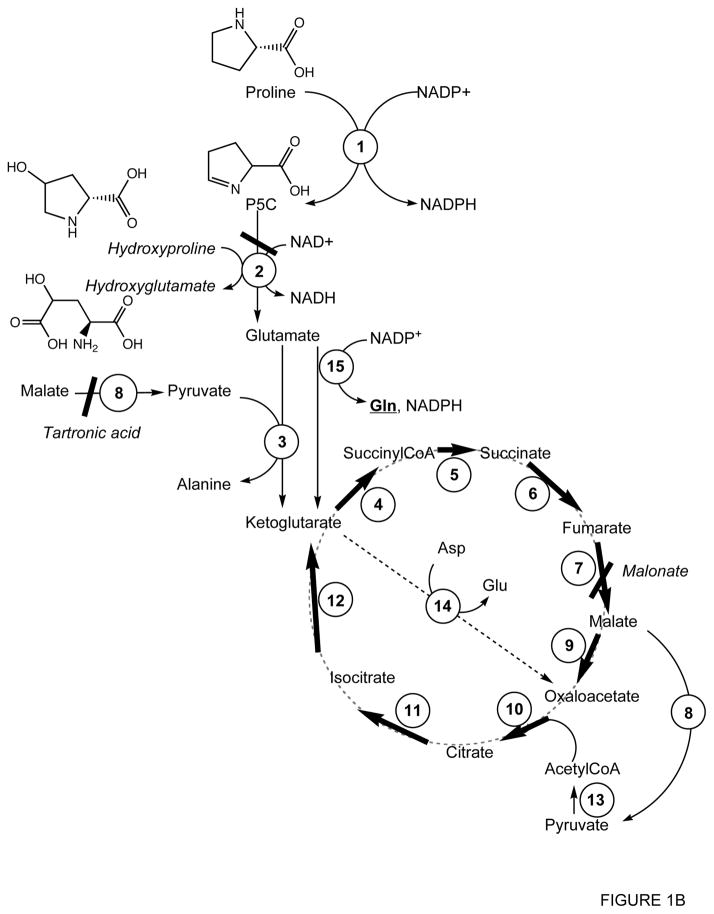
Figure 1.
Schematic representation of proline metabolism in ASE mitochondria
Panel A: Proline metabolism as described by [42–44, 47, 71].
Panel B: Proline metabolism expanded and modified according to the experimental results found in this study.
Inhibitors are shown in italics. 1, Δ1-pyrroline-5-carboxylate reductase; 2, Δ1-pyrroline-5-carboxylate dehydrogenase; 3, Glutamate-pyruvate transaminase; 4, ketoglutarate dehydrogenase; 5, succinylCoA synthetase; 6, succinate dehydrogenase; 7, fumarate reductase; 8, malic enzyme; 9, malate dehydrogenase; 10, citrate synthase; 11, aconitase; 12, isocitrate dehydrogenase; 13, pyruvate dehydrogenase; 14, aspartate-oxaloacetate transaminase; 15, glutamate dehydrogenase.
Our experimental results obtained with Pro did not agree with the expected results in terms of the partial inhibition achieved by malonate addition and the partial reversal of this inhibition by pyruvate (Table III). Further, our results not only ruled out an exclusive role for the Krebs cycle in Pro oxidation, but also suggested that the Krebs cycle and another oxidative pathway contributed equally to the oxidation of Pro. This alternative pathway would catabolyze the ketoglutarate not utilized by the Krebs cycle. We suggest that ketoglutarate is transaminated to Glu (via aspartate transaminase) and the oxaloacetate formed in this process is recycled to ketoglutarate through the Krebs cycle (Fig. 1B). In support of this argument, malonate inhibition was reversed by the addition of aspartate, the substrate of aspartate transferase. It could also be argued, however, that pyruvate addition after tartronic acid-mediated inhibition of malic enzyme could have had another effect on Pro oxidation. Specifically, pyruvate addition could dose-dependently activate Ala transaminase to generate ketoglutarate. However, the low percentage of oxygen uptake obtained upon addition of pyruvate suggested that this substrate was mainly functioning as an inhibitor of malic enzyme (Table III).
In intact insects, Ala that results from the catabolism of Pro is released from flight muscles into the hemolymph and is transported to the fat body where it serves as precursor for resynthesis of Pro [42]. In this pathway, Pro functions not only as a substrate for muscle contraction but also as a transporter for disposal of ammonia via the fat body. Waste nitrogen can also be disposed of via the amino acid glutamine. Given that glutamine and ornithine can form Glu via glutaminase and transamination/P5CDH, respectively, we tested whether these substrates were utilized by ASE mitochondria when excess Glu was present. ASE mitochondria did not catabolyze Gln or ornithine, suggesting that ammonia formed during protein catabolism is transported out as Ala and Gln. In an analogous situation, significant increases in Gln have been found in the hemolymph of Locusta migratoria during flight [51]. Taken together, the Pro-Ala pathway resembles the mammalian glucose-Ala pathway in which Ala released following transamination of pyruvate in muscle is recovered as glucose by gluconeogenesis in liver. This process sustains glucose levels during exercise and functions to dispose of excess nitrogen. Similarly waste nitrogen released as Ala is recovered by the fat body and converted to Pro, which is utilized as a principle flight muscle energy source. In accord with our other observations, the presence of this catabolic pathway for Pro is further evidence that ASE mitochondria are endowed with pathways that can be attributed to muscle cells.
Effect of inhibitors on respiration and oxidative phosphorylation
The effects of electron transport chain inhibitors and uncouplers on ASE cell mitochondria were similar to effects of these compounds on mammalian mitochondria (Table IV). The inhibition of α-glycerophosphate by oligomycin constituted a reliable criterion for the tightness of the coupling of respiratory-chain phosphorylation. The subsequent addition of FCCP, an uncoupler that completely dissipates the chemiosmotic gradient, released the inhibition of oligomycin as expected and restored oxygen uptake to levels comparable to those obtained with α-glycerophosphate. Rotenone blocked oxygen uptake between Complex I and III, whereas addition of antimycin A blocked the rate between Complex I or Complex II and Complex III. As observed in mammalian mitochondria, azide and cyanide were equally effective at inhibiting Complex IV.
Table IV.
Effect of inhibitors and uncouplers on oxidative phosphorylation in ASE cell mitochondria*
| Substrate and additions | Inhibition of State 3 rate (%) |
|---|---|
| Pyruvate-Malate (5 mM /2.5 mM)/5 μM rotenone | > 90 |
| Pyruvate-Malate/ 10 mM malonate | 50 |
| Succinate (10 mM)/ malonate | 50 |
| α-glycerophosphate (7.5 mM)/ (1 μg/ml) Oligomycin | 80 |
| α-glycerophosphate/oligomycin/ 4 μM FCCP | ~ 0 |
| Glutamate/0.2 μM rotenone | 92 |
| α-glycerophosphate/ 5 μg/ml antimycin A | 90 |
| 0.2 mM TMPD/10 mM ascorbate/ 1 mM NaN3 | 93 |
| TMPD/ascorbate/ 1 mM KCN | 100 |
All State 3 rates were evaluated with 0.45 mM ADP, with the exception of TMPD/ascorbate which was evaluated with 1 mM ADP.
On the tissue of origin for the mitochondria characterized in this study
Our data indicate that the biochemical features of ASE mitochondria are similar to those of muscle mitochondria. This statement should not be interpreted to mean that ASE cells are similar to mammalian muscle cells or that ASE cells were derived from A. stephensi muscle tissue. Following our isolation procedure and the cell growth conditions (described under Materials and Methods), the majority of ASE mitochondria exhibited biochemical patterns that have been described for muscle mitochondria. There are several possible explanations for our findings.
The immortalized mosquito cell line utilized in this study was originally derived from minced first stage larvae of A. stephensi var. mysorensis as culture No. 43 (Mos. 43; [52]). The primary culture contained epithelial-type, fibroblast-type, and occasional giant cells in a distribution that similar to that reported for a primary culture from Aedes novalbopictus [53] – 80% epithelial cells, 5–10% fibroblasts, and a small percentage of giant cells. However, the Mos. 43 cell type distribution shifted at the 15th serial subculture to a majority representation of fibroblast-like cells, probably due to their faster growth rate. Despite this morphological shift, the persistence of different isoenzymes (e.g. lactate dehydrogenase; [53]) in Mos. 43 indicated that multiple cell types remained in culture. The specific origin of these cell types and, hence, the origin of the ASE cell derivative of Mos. 43 is unknown.
Even if the tissue origin of a cell line is known, cell culture conditions can alter the expression of metabolic pathways so that they no longer reflect metabolism in vivo. For example, some of our substrate assays suggested that the ASE cells lack several glycolytic enzymes (hexokinase, glucose-phosphate isomerase, glutamate-oxaloacetate transaminase, and phosphoglucomutase; [54]) whereas other assays indicated the presence and activity of several dehydrogenases that would not function in the absence of glycolysis (pentose phosphate shunt, Krebs cycle, glycolysis; [53]). The presence of the these dehydrogenases – specifically, those for malate, α–glycerophosphate, isocitrate, β-hydroxybutyrate, and glutamate dehydrogenases – are further supported by studies in the Luckhart lab that identified these proteins in Anopheles gambiae Sua 5B cells via LC-MS/MS (Table V). The presence of these enzymes in A. gambiae is highly predictive of their presence in A. stephensi, given.the significant conservation of these enzymes across disparate species.
Table V.
Metabolic enzymes of A. gambiae Sua 5B cells identified by LC-MS/MS*
| Pathway | Enzyme |
|---|---|
| Glycolysis and glycogen metabolism | Aldolase |
| Glyceraldehyde-3-phosphate dehydrogenase | |
| Phosphoglycerate kinase | |
| Enolase | |
| Krebs cycle and oxidative phosphorylation | UDP-glucose pyrophosphorylase |
| Pyruvate dehydrogenase and Krebs cycle | Pyruvate dehydrogenase |
| Citrate synthase | |
| Aconitase | |
| Isocitrate dehydrogenase | |
| α-ketoglutarate dehydrogenase | |
| SuccinylCoA synthetase | |
| Fumarase | |
| Malate dehydrogenase | |
| Electron transport chain | Complex I (75K, 51K, 42K) |
| Electron-transfer-flavoprotein, α polypeptide | |
| Cytochrome c oxidase (Va, VIb, IV, Vb) | |
| Cytochrome c1 and c2 | |
| Ubiquinol-cytochrome c reductase hinge protein (14K) | |
| ATP synthase (α, β, δ, ε) | |
| Fatty acid metabolism | Carnitine-acyl transferase I |
| Acyl-CoA dehydrogenase | |
| 3-HydroxyacylCoA dehydrogenase | |
| Thiolase | |
| 3-hydroxybutyrate dehydrogenase | |
| Malic enzyme | |
| Amino acid metabolism | Glutamate dehydrogenase |
| Glutaminase | |
| Aspartate aminotransferase |
Proteins extracted from the cells were identified by LC-MS/MS. From those that pass the filters and statistical analyses, this table contains only those proteins relevant to the present study and do not necessarily represent all of those detected by mass spectrometry. Other experimental details were described in detail under Materials and Methods.
Finally, it has been proposed that cell culture media, pH, osmotic pressure, and oxygen tension in combination with the mitotic potential of diverse cells in a primary culture select for survival of hemocytes, which become the predominant or only cell type during prolonged passaging and maintenance [55]. Indeed, cell lines are difficult to establish from embryonic tissue before hemocytes have appeared, but it has been suggested that the hypoxic conditions of cell culture select for survival of cells from the hemocyte lineage [56]. As such, many of the existing mosquito cell lines have been described as “hemocyte-like” based on their immune-reactive behavior to stimulation with foreign antigens [55]. It will be interesting to continue our studies of these cells from a biochemical perspective – such data could help to resolve the physiological identity of immortalized mosquito cell lines.
Conclusions
Although ASE cell mitochondria share many features in common with mammalian muscle mitochondria, two major differences are apparent. Specifically, ASE cell mitochondria and mammalian muscle cell mitochondria appear to differ in the oxidation of cytosolic NADH and on the use of Pro as a substrate.
In the case of NADH, the glycerol-phosphate shuttle played a major role in NADH oxidation, whereas in mammalian muscle mitochondria, lactate dehydrogenase or the malate-oxaloacetate shuttle functions in that role.
In the case of Pro, ASE mitochondria were able to oxidize this substrate at a rate comparable with that of α-glycerophosphate (Table I). However, this oxidation appears to have occurred via a pathway (Fig. 1B) that is different from that which is currently accepted. Ketoglutarate can be catabolyzed by complete oxidation through the Krebs cycle or via transamination. The first pathway will likely have a higher yield of ATP and is probably favored by the allosteric effect of fumarate on the malic enzyme when higher levels of ATP are needed.
The degree of Pro utilization for flight ranges from the ‘sparker’ function in the blowfly Phormia regina [57] to the combined use of Pro and carbohydrates in the Colorado potato beetle Leptinotarsa decemlineata [58], the African fruit beetle Pachnoda sinuata [59, 60], and the blister beetle Decapotoma lunata ([61], to exclusive catabolism of Pro in the tsetse fly [42] and possibly some scarab beetles [62, 63]. Given that there are no known specialized storage organs for Pro, this amino acid must, therefore, be synthetically produced during flight. The fat body is the site of Pro resynthesis in the tsetse fly [42] and the Colorado potato beetle [46]. Comparing the rates of oxygen consumption in the presence of pyruvate to those of Pro and octanoylcarnitine, we speculate that ASE mitochondria can utilize carbohydrates and Pro as substrates in a fashion analogous to the use of these substrates for flight in some insect species.
Finally, the biochemical characteristics of ASE mitochondria provide not only a critical foundation for larger studies to be performed with mitochondria from different mosquito tissues, but they also represent completely new physiological insights for anopheline mosquitoes. For instance, knowledge of mitochondria bioenergetics can be applied in studies of mosquito aging [64, 65] and perhaps be useful in understanding the detoxification of insecticides. Specifically, insecticide resistance may result from alterations in mosquito mitochondrial physiology that can be adapted to track the development of insecticide resistance in mosquito populations. And by extension, differences in mitochondrial physiology between mammals and mosquitoes may highlight novel targets for the development of new insecticides.
Supplementary Material
Acknowledgments
This study was supported by the University of California, Mosquito Research Program (UC MRP grant #07-019-3-1). We thank Dr R. A. Freedland for his excellent contributions when reviewing this manuscript.
Footnotes
Abbreviations: ASE, Anopheles stephensi Mos. 43 cell line; MSHE, mannnitol-sucrose-Hepes-EDTA buffer; RCR, respiratory control ratio or rates; PC, pyruvate carboxylase; AKH/RPCH, adipokinetic hormone/red pigment-concentrating hormone; FCCP, carbonylcyanide-p-trifluoromethoxyphenylhydrazone; P5CDH, Δ1-pyrroline-5-carboxylic dehydrogenase; TMPD, N,N,N’,N’-tetramethyl-p-phenylenediamine.
Oxygen uptake of flying mosquito (20 nmol/min per mosquito) was calculated from values published for locust and assuming an average body weight of mosquitoes of 1 mg/insect and that the muscle weight represents 20% of the body weight. Fat weight was estimated to be mainly comprised of palmitic acid, and that the stoichiometry of 1 mol of oxidized palmitic acid requires 23 moles of oxygen. Moles of glucose were calculated by dividing the weight of glycogen with 180 g/mol glucose weight was calculated and assuming a stoichiometry of 1 mol of oxidized glucose requires 6 moles of oxygen. Final numbers for fat and glucose were 6 and 0.8 nmol oxygen consumed/mosquito. These data were adapted from published studies [25, 27, 28].
References
- 1.Watanabe M. Mitochondria in the flight muscles of insects. 1. Chemical composition and enzymatic content. J Gen Physiol. 1951;34(5):675–689. doi: 10.1085/jgp.34.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watanabe MI, Williams CM. Mitochondria in the flight muscles of insects 2. Effects of the medium on the size, form, and organization of isolated sarcosomes. J Gen Physiol. 1953;37:71–90. doi: 10.1085/jgp.37.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chefurka W. The occurrence of a direct oxidative pathway of carbohydrate metabolism in the fly Musca domestica L. Biochim. Biophys Acta. 1955;17:294–296. doi: 10.1016/0006-3002(55)90375-9. [DOI] [PubMed] [Google Scholar]
- 4.Sacktor B. Investigations on the mitochondria of the housefly, Musca domestica L. 2. Oxidative enzymes with special reference to malic oxidase. Arch Biochem Biophys. 1953;45:349–365. doi: 10.1016/s0003-9861(53)80012-3. [DOI] [PubMed] [Google Scholar]
- 5.Sacktor B. Investigations on the mitochondria of the house fly, Musca domestica L. 1. Adenosinetriphosphatases. J Gen Physiol. 1953;36:371–387. doi: 10.1085/jgp.36.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sacktor B. Investigations on the mitochondria of the housefly, Musca domestica L. 3. Requirements for oxidative phosphorylation. J Gen Physiol. 1954;37:343–359. doi: 10.1085/jgp.37.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacktor B. Cell structure and the metabolism of insect flight muscle. J Biophys Biochem Cytol. 1955;1:29–46. doi: 10.1083/jcb.1.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis SE, Slater EC. Oxidative phosphorylation in insect sarcosomes. Biochem J. 1954;58:207–217. doi: 10.1042/bj0580207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoskins DD, Cheldelin VH, Newburgh RW. Oxidative enzyme systems of the honey bee, Apis mellifera L. J. Gen. Physiol. 1956;39:705–713. doi: 10.1085/jgp.39.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwong LK, Sohal RS. Tissue-specific mitochondrial production of H2O2: Its dependence on substrates and sensitivity to inhibitors. Methods Enzymol. 2002;349:341–346. doi: 10.1016/s0076-6879(02)49349-4. [DOI] [PubMed] [Google Scholar]
- 11.Radyuk SN, Michalak K, Rebrin I, Sohal RS, Orr WC. Effects of ectopic expression of drosophila DNA glycosylases dogg1 and rps3 in mitochondria. Free Radic Biol Med. 2006;41:757–764. doi: 10.1016/j.freeradbiomed.2006.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtti TJ, Munderloh UG. Advances in the definition of culture media for mosquito cells. Invertebrate Cell Systems Applications. 1989;1:21–29. [Google Scholar]
- 14.Giulivi C, Poderoso JJ, Boveris A. Production of nitric oxide by mitochondria. J Biol Chem. 1998;273:11038–11043. doi: 10.1074/jbc.273.18.11038. [DOI] [PubMed] [Google Scholar]
- 15.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 16.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 17.Ochoa S, Mehler AH, Kornberg A. Biosynthesis of dicarboxylic acids by carbon dioxide fixation 1. Isolation and properties of an enzyme from pigeon liver catalyzing the reversible oxidative decarboxylation of l-malic acid. J Biol Chem. 1948;174:979–1000. [PubMed] [Google Scholar]
- 18.Utter MF, Keech DB. Pyruvate carboxylase. 1 Nature of reaction. J Biol Chem. 1963;238:2603–2608. [PubMed] [Google Scholar]
- 19.Bandurski RS, Greiner CM. The enzymatic synthesis of oxalacetate from phosphoryl-enolpyruvate and carbon dioxide. J Biol Chem. 1953;204:781–786. [PubMed] [Google Scholar]
- 20.Siu PML, Wood HG. Phosphoenolpyruvic carboxytransphosphorylase, a CO2 fixation enzyme from propionic acid bacteria. J Biol Chem. 1962;237:3044-&. [PubMed] [Google Scholar]
- 21.Utter MF, Kurahashi K. Mechanism of action of oxalacetic carboxylase. J Biol Chem. 1954;207:821–841. [PubMed] [Google Scholar]
- 22.Crabtree B, Newsholme EA. The activities of phosphorylase, hexokinase, phosphofructokinase, lactate dehydrogenase and the glycerol 3-phosphate dehydrogenases in muscles from vertebrates and invertebrates. Biochem J. 1972;126:49–58. doi: 10.1042/bj1260049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beenakkers MT, Van der Horst AD, Van Marrewijk DJ, Wil JA. Insect lipids and lipoproteins, and their role in physiological processes. Prog Lipid Res. 1985;24:19–67. doi: 10.1016/0163-7827(85)90007-4. [DOI] [PubMed] [Google Scholar]
- 24.Van den Bergh SG. Insect mitochondria. Methods Enzymol. 1967;X:117–122. [Google Scholar]
- 25.Kaufmann C, Briegel H. Flight performance of the malaria vectors Anopheles gambiae and Anopheles atroparvus. J Vector Ecol. 2004;29:140–153. [PubMed] [Google Scholar]
- 26.Clements AN. The biology of mosquitoes. Chapman & Hall; London: 1992. [Google Scholar]
- 27.Kaufmann C, Brown MR. Regulation of carbohydrate metabolism and flight performance by a hypertrehalosaemic hormone in the mosquito Anopheles gambiae. J Insect Physiol. 2008;54:367–377. doi: 10.1016/j.jinsphys.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crabtree B, Newsholme EA. The activities of phosphorylase, hexokinase, phosphofructokinase, lactate dehydrogenase and the glycerol 3-phosphate dehydrogenases in muscles from vertebrates and invertebrates. Biochem J. 1972a;126:49–58. doi: 10.1042/bj1260049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gäde G, Hoffmann KH, Spring JH. Hormonal regulation in insects: Facts, gaps, and future directions. Physiol Rev. 1997;77:963–1032. doi: 10.1152/physrev.1997.77.4.963. [DOI] [PubMed] [Google Scholar]
- 30.Gäde G, Simek P, Marco HG. A novel adipokinetic peptide in a water boatman (Heteroptera, Corixidae) and its bioanalogue in a saucer bug (Heteroptera, Naucoridae) Peptides. 2007;28:594–601. doi: 10.1016/j.peptides.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Van der Horst DJ. Insect adipokinetic hormones: Release and integration of flight energy metabolism. Comp Biochem Physiol B Biochem Mol Biol. 2003;136:217–226. doi: 10.1016/s1096-4959(03)00151-9. [DOI] [PubMed] [Google Scholar]
- 32.Pette D. Mitochondrial enzyme activities. Regul. Metab. Processes Mitochondria, Proc. Symp; 1965; 1966. pp. 28–50. [Google Scholar]
- 33.Borst JGG. Hypertension explained by Starlings theory of circulatory homoeostasis. Lancet. 1963;2:1064–1065. doi: 10.1016/s0140-6736(63)91443-0. [DOI] [PubMed] [Google Scholar]
- 34.Chappell JB. Systems used for transport of substrates into mitochondria. Brit Med Bull. 1968;24:150–157. doi: 10.1093/oxfordjournals.bmb.a070618. [DOI] [PubMed] [Google Scholar]
- 35.Krebs HA. Mitochondrial generation of reducing power. Colloquium of the Third Meeting of the Federation of European Biochemical Societies on the biochemistry of mitochondria; 4–7 April, 1966; Warsaw, Pol. 1967. pp. 105–113. [Google Scholar]
- 36.Morgan HE, Neely JR, Brineaux JP, Park CR. Regulation of glucose transport rat. Colloquium on Metabolic Control and Symposium on Control of Energy Metabolism; Philadelphia, Perm. 20–21 May, 1965; 1965. pp. 347–355. [Google Scholar]
- 37.Klingenberg M, Bucher T. Biological oxidations. Ann Rev Biochem. 1960;29:669–708. doi: 10.1146/annurev.bi.29.070160.003321. [DOI] [PubMed] [Google Scholar]
- 38.Sacktor B. Insecta energetics and respiratory metabolism of muscular contraction. In: Rockstein M, editor. The Physiology of Insecta. Academic Press; New York: 1965. pp. 483–580. [Google Scholar]
- 39.Sacktor B. Regulation of intermediary metabolism with special reference to the control mechanisms in insect flight muscle. In: Beament JWL, Treherne JE, Wigglesworth VB, editors. Advances in Insect Physiology. Vol. 7. Academic Press; London: 1970. pp. 268–347. [Google Scholar]
- 40.Winteringham FPW, Hellyer GC, McKay MA. Effects of methyl bromide on phosphorus metabolism in the adult housefly, Musca domestica L. Biochem J. 1958;69:640–648. doi: 10.1042/bj0690640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wheeler CH. Mobilization and transport of fuels to the flight muscles. In: Goldsworthy GJ, Wheeler CH, editors. Insect Flight. CRC Press; Boca Raton, FL: 1989. pp. 273–303. [Google Scholar]
- 42.Bursell E. The role of proline in energy metabolism. In: Downer RGH, editor. Energy Metabolism of Insects. Plenum Press; New York: 1981. pp. 135–154. [Google Scholar]
- 43.Hansford RG, Johnson RN. The nature and control of the tricarboxylate cycle in beetle flight muscle. Biochem J. 1975;148:389–401. doi: 10.1042/bj1480389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subramanian MA, Varadaraj G. Differences in the haemolymph free amino acids of the dragonfly Mesogomphus lineatus (Selys) (Anisoptera: Gomphidae) Odonatologica. 1985;14:152–154. [Google Scholar]
- 45.Weeda E, de Kort CAD, Beenakkers AMT. Oxidation of proline and pyruvate in flight muscle mitochondria of the colorado beetle Leptinotarsa decemlineata Say. Insect Biochem. 1980a;10:305–311. [Google Scholar]
- 46.Weeda E, Koopmanshap AB, de Kort CAD, Beenakkers AMT. Proline synthesis in fat body of Leptinotarsa decemlineata. Insect Biochem. 1980b;10:631–636. [Google Scholar]
- 47.Auerswald L, Gade G. Effects of metabolic neuropeptides from insect corpora cardiaca on proline metabolism of the African fruit beetle, Pachnoda sinuata. J Insect Physiol. 1999;45:535–543. doi: 10.1016/s0022-1910(98)00160-7. [DOI] [PubMed] [Google Scholar]
- 48.Strecker HJ. The interconversion of glutamic acid and proline. I The formation of delta1-pyrroline-5-carboxylic acid from glutamic acid in Escherichia coli. J Biol Chem. 1957;225:825–834. [PubMed] [Google Scholar]
- 49.Fahmy AS, Mohamed SA, Girgis RB, Abdel-Ghaffar FA. Enzymes of delta 1-pyrroline-5-carboxylate metabolism in the camel tick Hyalomma dromedarii during embryogenesis. Purification and characterization of delta 1-pyrroline-5-carboxylate dehydrogenases. Comp Biochem Physiol B Biochem Mol Biol. 1997;118:229–237. doi: 10.1016/s0305-0491(97)00053-9. [DOI] [PubMed] [Google Scholar]
- 50.Adams E, Goldstone A. Hydroxyproline metabolism. IV Enzymatic synthesis of ggr-hydroxyglutamate from dgr1-pyrroline-3-hydroxy-5-carboxylate. J Biol Chem. 1960;235:3504–3512. [PubMed] [Google Scholar]
- 51.Van der Horst DJ, Houben NMD, Beenakkers AMT. Dynamics of energy substrates in the haemolymph of Locusta migratoria during flight. J Insect Physiol. 1980;26:441–448. [Google Scholar]
- 52.Pudney M, Varma MGR. Anopheles stephensi varMysorensis: Establishment of a larval cell line (Mos 43) Exp Parasitol. 1971;29:7–12. doi: 10.1016/0014-4894(71)90003-8. [DOI] [PubMed] [Google Scholar]
- 53.Herrera RJ, Mukherjee AB. Electrophoretic characterization and comparison of dehydrogenases from eight permanent insect cell lines. Comp Biochem Physiol Part B: Biochem Mol Biol. 1982;72:359–366. [Google Scholar]
- 54.Bhat UKM, Guru PY. Aedes novalbopictus: Establishment and characterization of larval cell lines. Exp Parasitol. 1973;33:105–113. doi: 10.1016/0014-4894(73)90015-5. [DOI] [PubMed] [Google Scholar]
- 55.Fallon AM, Sun D. Exploration of mosquito immunity using cells in culture. Insect Biochem Mol Biol. 2001;31:263–278. doi: 10.1016/s0965-1748(00)00146-6. [DOI] [PubMed] [Google Scholar]
- 56.Landureau JC, Lenoir-Rousseaux JJ. New culture media for insect cells. In: Kuroda Y, Kurstak E, Maramorosch K, editors. Invertebrate and Fish Tissue Culture. Springer Verlag; New York: 1988. pp. 23–37. [Google Scholar]
- 57.Sacktor B. Utilization of fuels by muscle. In: Candy DJ, Kilby BA, editors. Insect Biochemistry and Function. Chapman and Hall; London: 1975. pp. 1–81. [Google Scholar]
- 58.Weeda E, de Kort CAD, Beenakkers AMT. Fuels for energy metabolism in the colorado potato beetle Leptinotarsa decemlineata Say. J Insect Physiol. 1979;25:951–955. [Google Scholar]
- 59.Zebe E, Gäde G. Flight metabolism in the African fruit beetle Pachnoda sinuata. J Comp Physiol B. 1993;163:107–112. [Google Scholar]
- 60.Auerswald L, Schneider P, Gäde G. Utilisation of substrates during tethered flight with and without lift in the African fruit beetle Pachnoda sinuata (Cetoniinae) J Exp Biol. 1998;201:2333–2342. doi: 10.1242/jeb.201.15.2333. [DOI] [PubMed] [Google Scholar]
- 61.Auerswald L, Gäde G. Energy substrates for flight in the blister beetle Decapotoma lunata (Meloidae) J Exp Biol. 1995;198:1423–1431. doi: 10.1242/jeb.198.6.1423. [DOI] [PubMed] [Google Scholar]
- 62.Gäde G. Hyperprolinaemia caused by novel members of the AKH/RPCH-family of peptides isolated from corpora cardiaca of onitine beetles. Biochem J. 1997a;321:201–206. doi: 10.1042/bj3210201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gäde G. Distinct sequences of AKH/RPCH family members in beetle (Scarabaeus species) corpus cardiacum contain three aromatic amino acid residues. Biochem Biophys Res Commun. 1997b;230:16–21. doi: 10.1006/bbrc.1996.5872. [DOI] [PubMed] [Google Scholar]
- 64.Kang MA, Mott TM, Tapley EC, Lewis EE, Luckhart S. Insulin regulates aging and oxidative stress in Anopheles stephensi. J Exp Biol. 2008;211:741–748. doi: 10.1242/jeb.012955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luckhart S, Riehle MA. The insulin signaling cascade from nematodes to mammals: Insights into innate immunity of Anopheles mosquitoes to malaria parasite infection. Dev Comp Immunol. 2007;31:647–656. doi: 10.1016/j.dci.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vandenbergh SG, Slater EC. Respiratory activity and permeability of housefly sarcosomes. Biochem J. 1962;82:362–371. doi: 10.1042/bj0820362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Azzone GF, Carafoli E. Biochemical properties of skeletal muscle mitochondria. I Oxidative phosphorylation. Exp Cell Res. 1960;21:447–455. doi: 10.1016/0014-4827(60)90278-0. [DOI] [PubMed] [Google Scholar]
- 68.Azzone GF, Carafoli E, Muscatello U. Biochemical properties of skeletal muscle mitochondria. II The ATPase activity and the albumin effect. Exp Cell Res. 1960;21:456–467. doi: 10.1016/0014-4827(60)90279-2. [DOI] [PubMed] [Google Scholar]
- 69.Azzone GF, Eeg-Olofsson O, Ernster L, Luft R, Szabolcsi G. Studies on isolated human skeletal muscle mitochondria. Exp Cell Res. 1961;22:415–436. doi: 10.1016/0014-4827(61)90119-7. [DOI] [PubMed] [Google Scholar]
- 70.Bode C, Klingenberg M. The oxidation of fatty acids in isolated mitochondria. Biochem Z. 1965;341:271–299. [PubMed] [Google Scholar]
- 71.Gäde G, Auerswald L. Beetles’ choice—proline for energy output: Control by AKHs. Comp Biochem Physiol B: Biochem Mol Biol. 2002;132:117–129. doi: 10.1016/s1096-4959(01)00541-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.