Abstract
Accidental or medical radiation exposure of the salivary glands can gravely impact oral health. Previous studies have shown the importance of Tousled-like kinase 1 (TLK1) and its alternate start variant TLK1B in cell survival against genotoxic stresses. Through a high-throughput library screening of natural compounds, the phenolic phytochemical, gallic acid (GA), was identified as a modulator of TLK1/1B. This small molecule possesses anti-oxidant and free radical scavenging properties, but in this study, we report that in vitro it promotes survival of human salivary acinar cells, NS-SV-AC, through repair of ionizing radiation damage. Irradiated cells treated with GA show improved clonogenic survival compared to untreated controls. And, analyses of DNA repair kinetics by alkaline single-cell gel electrophoresis and γ-H2AX foci immunofluorescence indicate rapid resolution of DNA breaks in drug-treated cells. Study of DR-GFP transgene repair indicates GA facilitates homologous recombinational repair to establish a functional GFP gene. In contrast, inactivation of TLK1 or its shRNA knockdown suppressed resolution of radiation-induced DNA tails in NS-SV-AC, and homology directed repair in DRGFP cells. Consistent with our results in culture, animals treated with GA after exposure to fractionated radiation showed better preservation of salivary function compared to saline-treated animals. Our results suggest that GA-mediated transient modulation of TLK1 activity promotes DNA repair and suppresses radiation cytoxicity in salivary gland cells.
Keywords: salivary, acinar, Tousled, radiation, DNA repair, homologous recombination, xerostomia, gallic acid
Graphical abstract
INTRODUCTION
Head and neck cancer accounts for nearly 0.5 million new cases each year, worldwide. Radiotherapy alone or in conjunction with surgery and, or, chemotherapy is the standard of care for most patients treated with curative intent. A debilitating side–effect of regional radiation is salivary hypofunction. Within the first weeks from onset of radiotherapy, patients experience oral dryness with a decline in salivary function that continues well beyond the cessation of therapy. Poor salivary function is attributed to dysfunction and death of the fluid-producing acinar cells. Unrepaired DNA damage threatens genomic integrity and cell survival, and timely repair can avert the loss of salivary gland function. Since temporary relief of symptoms with sialogogues or pro-secretory drugs is the standard of care for dry mouth, cancer treatment planning centers around its prevention whilst new treatments are explored.
A human homolog of A. thaliana Tousled is Tousled-like kinase 1 (TLK1)1. The TLK1 protein variant, TLK1B, utilizes an alternate translation start site, and encodes a shorter protein that shares an identical kinase domain. When expressed in normal epithelial cells, TLK1B increases cell survival against genotoxic stresses, and the identification of its substrates, histone H3, anti-silencing factor (Asf1), and Rad9 provided clues to its association with chromatin dynamics2, 3, 4. Subsequent studies confirmed the role of TLK1B in DNA damage response and in chromatin remodeling at sites of DNA breaks5,6. Cells expressing the kinase-defective mutant are sensitive to radiation, and it suggests an important role of the kinase in cell survival after genotoxic stress7. Radiation-induced activation of checkpoint kinase, Chk1, was essential for transient downregulation of TLK1 and the establishment of replication checkpoint. TLK1 activity dropped soon after radiation, and recovered quickly. An inverse correlation was demonstrated between Chk1 and TLK1 activities8. In the current study, we identified a naturally occurring small molecule, GA, as an activator of TLK1B. Since the full-length and the shorter variant of the protein have identical C-terminal kinase domains, they exhibit same substrate specificities. It is, therefore, anticipated that the pharmacological agent will activate TLK1 as well.
Gallic acid (3,4,5-trihydroxybenzoic acid) is a naturally occurring phytochemical that has a pyrogallol moiety similar to epigallocatechin-3-gallate (ECGC), a constituent of green tea. It is abundant in a number of plant foods such as strawberries, blueberries, grape seeds, gallnuts, witch hazel, and processed beverages such as tea and red wine. GA is a part of the ECGC ester, and the pyrogallol moiety of these compounds is attributed to formation of topoisomerase-DNA complexes9. Similar to ECGC, GA possesses anti-oxidant and anti-carcinogenic activities. The anti-proliferative and apoptotic effects of the compound on cancer cells have been demonstrated10–13. And, advantageously utilizing its anti-oxidant property, reports showed that pretreatment protects cellular DNA against radiation-induced oxidative damage. In animal studies, high doses of the drug protected against chromosomal breaks and aberrations in bone marrow cells after total body radiation14,15. The radioprotective effect of GA was confirmed in a clinical trial where its supplementation in diet reduced the number of oxidized bases in lymphocytes and increased their resistance to reactive oxygen species damage16. To circumvent the influence of the compound’s antioxidant effect in our study, GA treatment commenced after radiation to study its effect on TLK1-influenced DNA repair and cell survival.
MATERIALS AND METHODS
Library Screen
Compounds from the NIH Clinical Collection, the Prestwick Chemical Library, the ChemDiv Library, and the Enzo Life Sciences Redox Library were screened for modulators of TLK1B. Bacterially isolated recombinant protein TLK1B was reacted with its phosphorylation substrate, Rad9, in Kinase Assay buffer (ADP-Hunter Plus Assay, Discoverx). The ADP generated during the kinase reaction was measured by the formation of florescent Resorufin, at 590 nM using Synergy 4 Hybrid Microplate Reader (Biotek).
In vitro kinase Assay
Bacterially isolated recombinant proteins, 0.5 µg TLK1B and 3 µg histone H3 (New England Biolabs) were incubated in kinase buffer (15 mM HEPES pH 7.5, 20 mM NaCl, 10 mM MgCl2, 1mM EGTA, 0.02% Tween 20, and 200 µM ATP) with or without GA (Sigma, 50 µM) for indicated times at room temperature. Reactions were stopped with the addition of Lammeli buffer, heated, and loaded on a SDS-PAG. Immunoblots were reacted with anti-serum to phospho-S-10 Histone H3 (Millipore) followed by HRP-conjugated anti-rabbit antibody (Vector Laboratories).
Cell Culture
The human submandibular acinar cells, NS-SV-AC, were a kind gift from Dr. Masayuki Azuma (University of Tokushima School of Dentistry, Tokushima, Japan)17. The immortalized cell line was cultured in complete keratinocyte growth medium 2 (KGM-2; Lonza) supplemented with antibiotic/ antimycotic (Invitrogen) at 37 °C in a humidified CO2 incubator. Knockdown of TLK1/1B in NS-SV-AC was achieved by transfecting cells with human 29-mer TLK1 shRNA (Origene; ATTACTTCATCTGCTTGGTAGAGGTGGCT), and selecting a multiclonal cell population under puromycin (1 g/ ml) challenge. GA was dissolved in water, and 10 mM solutions were made fresh for each experiment.
MTS assay
To quantitatively determine cell viability, a colorimetric assay based on the ability of viable cells to reduce tetrazolium compound, MTS (3-(4,5-dimethythiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium), into a soluble formazan dye was used. Exponentially growing NS-SV-AC cells were trypsinized, and cell suspensions (1 × 105 cells/ ml) were exposed to radiation (0, 2, 4, 8, or 12 Gy). Cells were seeded in triplicates into 96-well plates at different densities (500 – 3000 cells/well), and treated with 50 M GA 30 minutes later. After 16 h, the drug was washed out and cells were placed in drug-free medium. Cell viability was quantified on day 3 using CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay kit (Promega) as per the manufacturer’s protocol. After 2 h incubation with MTS, absorbance was measured at 490 nm using a multi-well plate spectrometer reader. Absorbance values were normalized to non-irradiated controls, and cell viability was expressed as percentage in relation to controls. The experiment was repeated, and the data were averaged and expressed as mean ± SEM.
Colony Survival Assay
Cells from sub-confluent culture plates were trypsinized and resuspended at 1 × 105 cells/ ml. They were irradiated at 0, 2, 4, 8, or 12 Gy, and seeded in triplicates at different densities in 12-well plates. Cells were similarly treated with GA and thereafter, placed in fresh medium. On day 10 post-radiation, cell colonies were stained with crystal violet and counted. The results are an average of 3 experiments and are expressed as fraction of surviving colonies in irradiated wells compared to non-irradiated (plating efficiency) controls.
Single-Cell Alkaline Gel Electrophoresis (COMET assay)
DNA single and double strand breaks were analyzed by single-cell electrophoresis in alkaline conditions using the Trevigen Comet Assay kit (Trevigen). Sub-confluent cell cultures in 35 mm plates were irradiated (8 Gy) on ice, and cells were collected immediately or allowed to recover at 37 °C. To determine the effect of GA on DNA repair, cells were treated 30 minutes after radiation, and at stated recovery times, cells were trypsinized, washed, and resuspended in Ca+2 and Mg+2 -free ice cold PBS, pH 7.4. An aliquot of resuspended cells was mixed with low melting agarose at 37°C, and 50 l of mix was evenly spread in each well of the comet slide. The agarose was allowed to set at 4°C, 10 minutes, before immersing the slides in prechilled lysis solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris-base, 1% sodium lauryl sarcosinate, 1% Triton X-100, pH 10) overnight at 4 °C. DNA denaturation was performed in alkaline solution (300 mM NaOH, 1 mM EDTA, pH > 13) in the dark for 20 minutes at room temperature before electrophoresis (1 volt/cm, 30 mA) at 4°C for 30 minutes in prechilled alkaline solution. Slides were rinsed in distilled water twice followed by a rinse in 70% ethanol. Slides were dried before staining with 100 l propidium iodide (Sigma; 10 µg/ml in PBS, pH 7.4) for 20 minutes. Images were captured using a TRITC filter mounted on an Olympus epifluorescence microscope, and the DNA tail length and tail moment were measured using CASP software (http://www.casp.of.pl). DNA tail lengths of >50 cells/ treatment group were measured, and the data were statistically evaluated.
FACS analysis
FACSCalibur flow cytometry was carried out at the LSUHSC core facility. Briefly, exponentially growing cells were irradiated (8 Gy) and then treated with drug for indicated times (0, 8, 16 h). Cells were trypsinized, washed with PBS (pH 7.4), and resuspended in 70% ethanol at 4°C. Cells were rinsed and resuspended in PBS before incubation with DNase-free, RNase, (1 unit/1ml; Roche) at 37°C for 30 minutes followed by staining with propidium iodide (0.5 mg/ml; Sigma) before analysis by flow cytometry. The data were analyzed using FACSDiva software version 6.0 (BD Biosciences).
Annexin V/ Propidium Iodide staining
NS-SV-AC were labeled using FITC Annexin V Apoptosis Detection Kit (BD Pharmingen) as per the manufacturer’s protocol. Cells were exposed to 8 Gy prior to treatment with drug for 16 h. Drug was washed out and cells were placed in fresh medium before collection at 48 h. Cells were washed with cold PBS and resuspended at 1×106 cells/ml in binding buffer (0.01 M HEPES, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2). Five µl each of Annexin V-FITC and propidium iodide were added to 100 µl of cell suspension, and cells were incubated in the dark for 15 minutes. The cell mix was diluted with 400 µl binding buffer, and samples were analyzed using a flow cytometer. Cells that were unstained, treated with Annexin V-FITC alone, or treated with PI alone were used as controls for quadrant set-up.
γ-H2AX Immunofluorescence
NS-SV-AC were seeded on coverslips (Warner Instruments) in 12-well plates, and allowed to attach overnight. Cells were exposed, or not, to 8 Gy on ice, and were either immediately washed in cold PBS and fixed in 4% paraformaldehyde/PBS for 20 minutes, or placed at 37 °C and treated with, or without, drug 30 minutes post-radiation. After 16 h drug treatment, cells were washed and fresh medium was added. Twenty-four hours after irradiation, cells were fixed and processed for immunostaining. Non-specific protein interactions were blocked with 5% serum before incubation with anti-mouse γ-H2AX (Millipore) at 4°C, overnight. Coverslips were washed in PBS before addition of Texas Red-conjugated anti-mouse antibody (Vector labs). Cells were washed and mounted with Vector Shield (Vector Labs). Images were acquired using TRITC and DAPI filter sets, and images were overlaid in ImageJ (NIH). The γ-H2AX foci counts were conducted using ImageJ Foci Count, and data analyzed by 2-way ANOVA to determine effects of radiation and drug on phospho-H2AX foci.
Immunoblotting
Total sample protein (30 µg) was electrophoresed on 10% SDS- polyacrylamide gel, and transferred to a PVDF membrane (Bio-Rad). Nonspecific binding was blocked with 5% nonfat dry milk, and the blot was reacted overnight at 4°C with primary antibody to TLK1 (home-made), phospho-S-139-Histone H3 (Millipore), phospho-S-317 Chk1, phospho-T68 Chk2 (Cell Signaling), or β-actin (Sigma) in 1: 1000 in TBS, 0.2% Tween 20. The blot was washed thrice in TBS 0.2% Tween 20 before incubating it with corresponding HRP-conjugated secondary antibody for 1 hour. The protein of interest was visualized after reaction with a chemiluminescent HRP substrate (Thermo Scientific) and exposure to X-ray film.
Measurement of Radiation-Generated Intracellular ROS
The assessment of intracellular ROS was done using fluorescent probe, CM-H2DCFDA (Invitrogen). NS-SV-AC cells were detached and resuspended in medium at 2 × 105 cells/ ml. Five ml of cell suspension was incubated with 5 M CM-H2DCFDA for 30 minutes at 37 °C in CO2 incubator. Cells were centrifuged and unincorporated probe was washed out. Cell suspension was irradiated (8 Gy), or not, before plating 2 × 104 cells/ well in 96 well plates. GA was added 30 minutes later, and fluorescence was analyzed using Synergy 4 Hybrid Microplate Reader (Biotek) at indicated times.
I-Sce I Homologous and Non-homologous DNA Repair Assays
MCF-7 cells with a genome-integrated DR-GFP cassette (a kind gift from Dr. Simon Powell) were used to study homologous recombination (HR). Whereas, HEK293T PC222 cells (a kind gift from Dr. Kum Kum Khanna) with integrated GFP-RFP transgene cassette were used for evaluation of non-homologous end joining (NHEJ). Cells were seeded into 60 mm plates and allowed to adhere overnight. A transfection mix with OPTI-MEM diluted I-SceI plasmid (10 µg) and Lipofectamine 2000 was layered on cells and cultured for 6 h. Cells were detached, and seeded at equal density in three 60 mm plates, and vehicle or GA (50 µM) was added. The drug was washed out after 16 h, and cells cultured in drug-free medium up to 72 h. Cells were lifted-off plates, and single cell suspension was run through a flow cytometer- BD FACS Analyzer. Data of 50,000 viable single cells was acquired using appropriate lasers. The population of fluorescing GFP and RFP cells were identified and reported as percent HR and percent NHEJ. TLK1 inactivation with THD was performed 1 h prior to I-SceI transfection, whereas the TLK1 knock-down experiments were done by transfecting cells with shRNA TLK1 3 days prior to double-strand break induction with I-SceI. The protocol, thereafter, was the same as above.
Animal Irradiation and Collection of Saliva
Six week old male Balb/C mice (Harlan Laboratories) were anesthetized with intramuscular ketamine (150 mg/kg) and xylazine (5mg/kg) mix, and laid supine with extended necks. A 1 cm tissue-equivalent bolus was placed over the head and neck, and the region was irradiated (2 Gy × 5 days) using 6MeV Elekta linear accelerator (LSU Health - Radiation Oncology). After radiation, animals were injected intraperitoneally with 50 mg/kg GA. Animals were kept warm during recovery and once ambulatory, they were housed at the vivarium with access to food and water ad libitum. All procedures were approved by institutional animal care and use committee and they conformed to guidelines for the use of animals in research.
Five weeks after radiation, stimulated salivary flow was assessed. Animals were anesthetized as before, and pilocarpine (6.25 mg/kg) was administered subcutaneously. The head of the animal was placed in a dependent positon, and a pre-weighed cotton swab was placed in the mouth. Whole saliva was collected over 20 minutes, and the difference in weight of the swab pre- and post-collection was determined. Assuming the density of saliva as 1 gm/cm3, the amount of saliva collected was calculated and the flow rate determined. Animals euthanized under anesthesia, and salivary glands extirpated, fixed in 4% paraformaldehyde and paraffin processed for histopathological analysis.
Two-Factor Analysis of Variance (ANOVA) was used to determine significant effects of drug treatment (saline, GA), radiation (no IR, IR) and interaction between drug treatment and radiation on salivary flow. The Bonferroni ad hoc test was used to determine significant two-group comparisons among the four drug-IR interaction groups.
RESULTS
TLK1/1B Activator, Gallic Acid, Suppresses Radiation Cytotoxicity
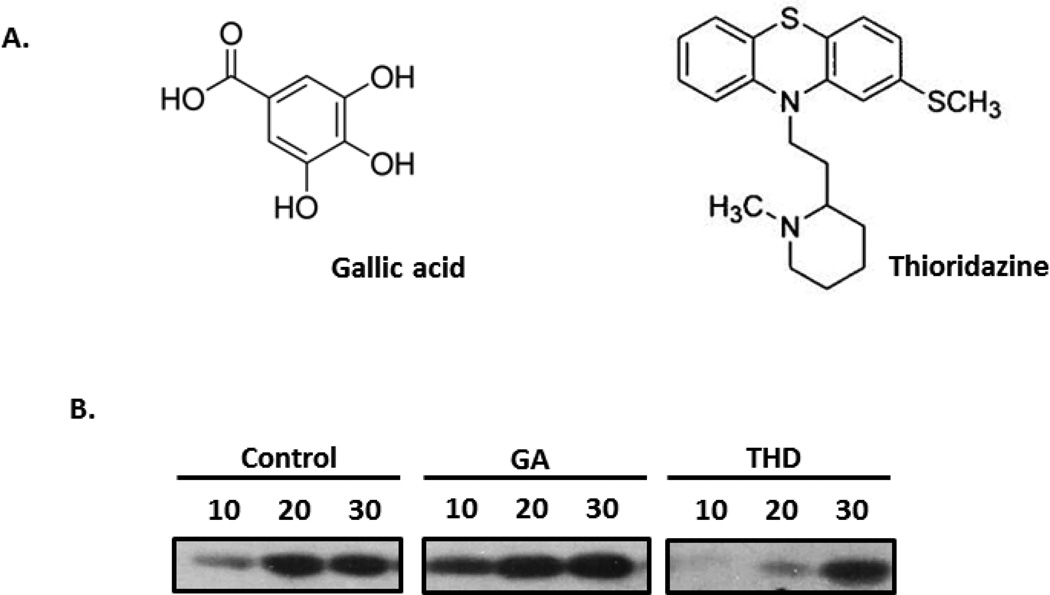
TLK1 is a key player in chromatin assembly, chromosome segregation, and cellular responses to genotoxic stress of ionizing radiation. Compounds that enhance its activity can enhance a cell’s defense mechanism in combating radiation toxicity. In a high-throughput screen of compounds, GA was identified as an activator of TLK1B. Preliminary studies were done to determine cytotoxicity of the compound by incubating NS-SV-AV cells with increasing drug concentrations. The highest drug concentration, 50 µM, that least affected plating efficiency was selected for further studies (data not shown). Assessment of kinase assays in vitro confirmed the stimulatory effect of GA on TLK1-mediated S10 histone H3 phosphorylation, whereas incubation with THD, an inhibitor of TLK1, delayed H3 phosphorylation (Figure 1B).
Figure 1. Modulators of TLK1.
A. Chemical structures of gallic acid (GA) and thiorizadine (THD). B. In vitro kinase reaction with recombinant TLK1B and histone H3 in the presence of GA or THD. Immunoblotted proteins were reacted with antibody to phospho-histone H3 (Ser10).
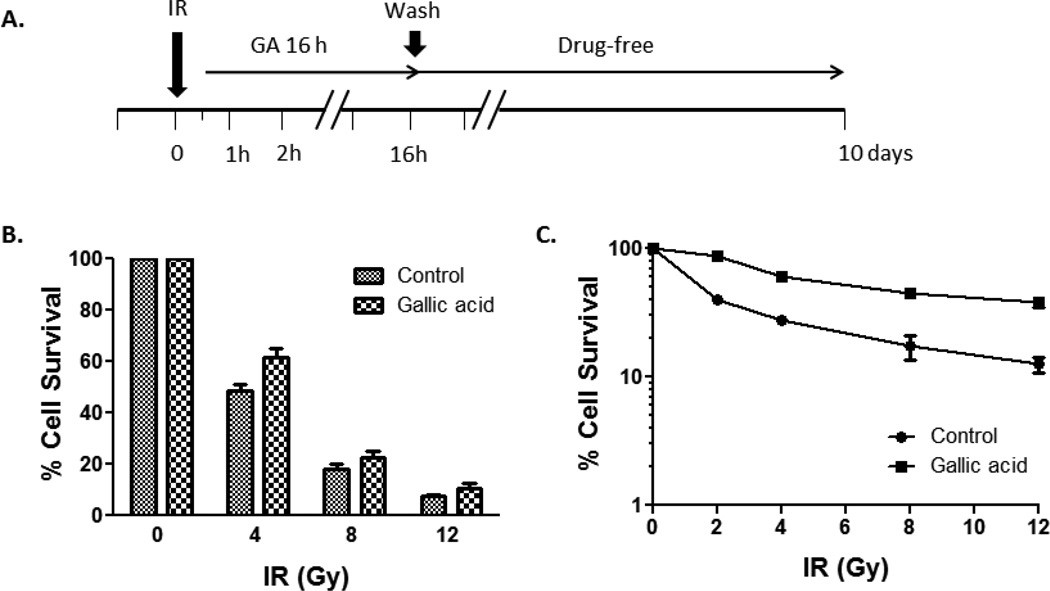
Exogenous expression of TLK1B suppresses radiosensitivity in salivary acinar cells. Because kinase activity is instrumental in promoting cell survival against radiation, we assessed whether activation of cellular TLK1 using small molecules could duplicate the radioresistant phenotype. We chose to study human salivary acinar cells, NS-SV-AC, since preservation of a functioning salivary acinar cell population is critical to forestalling radiation-associated salivary hypofunction. GA is a phenolic compound with known anti-oxidant properties. To minimize its impact on reactive oxygen species-generated DNA damage, drug incubation was initiated 30 minutes following irradiation (Figure 2A). An initial assessment of drug efficacy on cell viability after radiation was done by MTS detection. Improved cell viability was evident when cells were treated with 50 µM GA for 16 h following radiation. Percent cell survival values ± SEM at 4 Gy were 48.39 ± 2.93 for untreated and 61.54 ± 3.50 for GA-treated cells (Figure 2B). A follow-up colony survival assay was performed to validate the effect of drug. The number of colonies formed after exposure of irradiated NS-SV-AC cells to GA was significantly greater than vehicle alone. The mean survival fraction ± SEM at 4 Gy was 59.2 ± 1.77 for GA, and 27.5 ± 0.12 for control. At a higher dose of 12 Gy, the colony number of treated cells was >3-fold that of control (mean ± SEM for control: 12.6 ± 1.71, GA: 38.2 ± 3.61; Figure 2C).
Figure 2. Effect of GA on NS-SV-AC viability after radiation.
A. Experimental design, B. MTS assay, and C. Colony formation assay. NS-SV-AC were irradiated and treated with GA for 16 h. Cells were switched to drug-free medium thereafter, and viability assessed at day 3 and day 10 by MTS assay and colony formation assay, respectively.
To determine if kinase-repressed cells would be susceptible to ionizing radiation induced-cell killing, a known TLK1 inhibitor, thioridazine (THD) was used18. NS-SV-AC cells were extremely sensitive to the drug, and drug incubation was limited to an hour before radiation (Figure S1A, Supplementary Material). THD pretreatment made cells strikingly more radiosensitive than untreated controls. Untreated cells irradiated at 2 Gy demonstrated a mean survival fraction ± SEM of 69.9 ± 3.08 in contrast to 45.1 ± 3.58 in THD-treated (Figure S1B, Supplementary Material).
TLK1 Modulators Influence DNA Damage Response
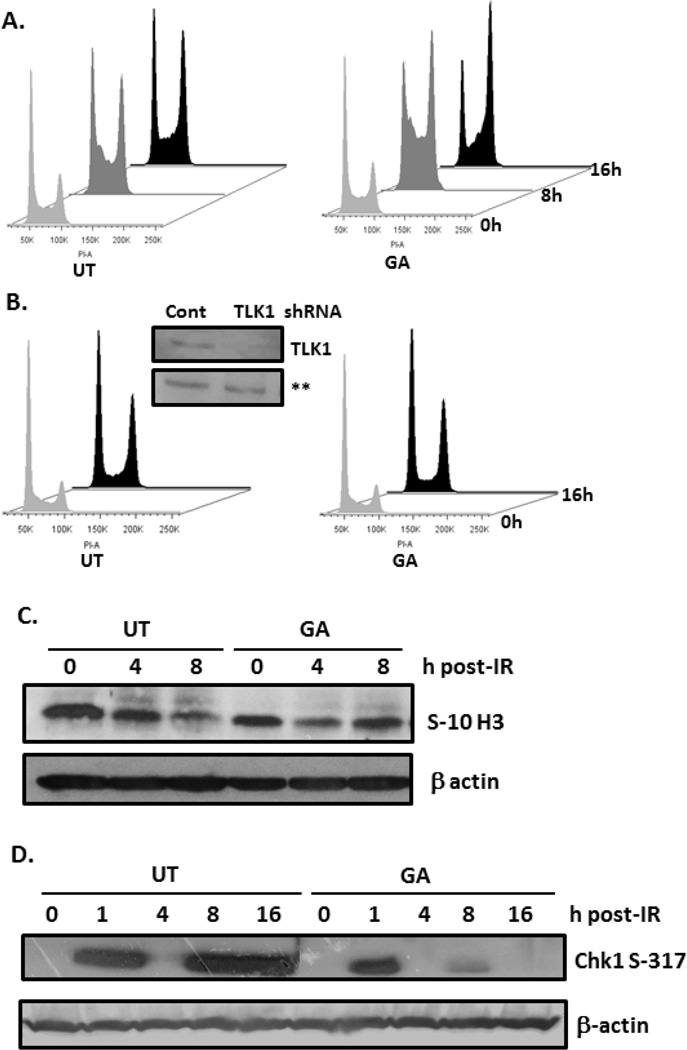
The involvement of TLK1 in DNA damage-induced checkpoint arrest was previously shown. TLK1 is inhibited immediately after radiation with recovery beginning after the first half hour. Therefore, it was anticipated that treatment with GA post-radiation would not affect Chk1-mediated transient suppression of TLK1 activity, but rather hasten its recovery. Compared to irradiated controls, GA treatment increased cell arrest at S and G2/M phases at 8 h and 16 h, respectively. A greater number of GA- treated cells (49%) were at G2/M-phase at 16 h compared to non-drug treated (37%; Figure 3A). To examine whether the cellular response to GA is primarily through TLK1, endogenous TLK1 and its isoforms were knocked-down using shRNA TLK1. Given the role of TLK1 in replication, stably-selected NS-SV-AC shRNA TLK1 cells were slow growing with a nearly 2-fold increase in doubling time (NS-SV-AC: 36.1 h; shRNA TLK1: 68.3 h; data not shown). Cell extracts reacted with TLK1 antiserum confirmed ~80% knockdown of TLK1 protein in shRNA TLK1 cells (Figure 3B). Importantly, treatment of shRNA TLK1 cells with GA failed to elicit robust cell arrest after radiation exposure (Figure 3B). The data suggest that GA facilitates radiation-induced arrest in S and G2/M phases, but the quicker reestablishment of S-10 histone H3 phosphorylation, a marker of chromosome condensation, suggests faster recovery from S-phase arrest in GA-treated cells (Figure 3C). Activated Chk1 is known to transiently suppress TLK1 activity, and correspondingly, kinetics of Chk1 Ser317 phosphorylation was evaluated. As expected Chk1 phosphorylation increased rapidly within an hour after radiation and was undetectable by 4 h, irrespective of treatment. However, a resurgence of phospho-Chk1 seen at 8 h in untreated cells was noticeably suppressed in cells treated with GA (Figure 3D). The activation of Chk2 (T68) and G2 checkpoint proteins, Cdc25C (S216) and CDK1 (Y15), were similar in irradiated cells treated, or not, with GA indicating that radiation-induced G2 arrest occurs regardless of drug treatment (Figure S2A,B, Supplementary Material). Of note, GA increased phosphorylated Cdc25C and CDK1 in non-irradiated cells, but the cells were retained in G1 phase likely through phosphorylation of Cdc25A (data not shown). However, increased levels of phospho-Cdc25C and phospho-CDK1 in non-treated, non-irradiated shRNA-TLK1 cells were unexpected (Figure S2C, Supplementary Material). Taking into consideration that non-irradiated shRNA-TLK1 cells are predominantly in G1 phase (Figure 3D), upregulation of Cdc25C and CDK1 phosphorylation suggests increased endogenous stress in these cells.
Figure 3. Effect of GA on radiation-induced checkpoint delay.
A, B. NS-SV-AC or stably-transfected shRNA TLK1 cells were exposed to 8 Gy, and treated, or not, with GA for indicated times before they were analyzed by propidium iodide flow cytometry. Inset: Immunoblotting of cell lysates with antiserum to TLK1. C, D. Immunoblotting of irradiated NS-SV-AC cells with antibodies to phospho-histone H3 (Ser10), phospho-Chk1 (Ser317), and β-actin. UT: untreated; GA: gallic acid; IR: irradiation.
Gallic Acid Mediated Resolution of Radiation Breaks is TLK1-dependent
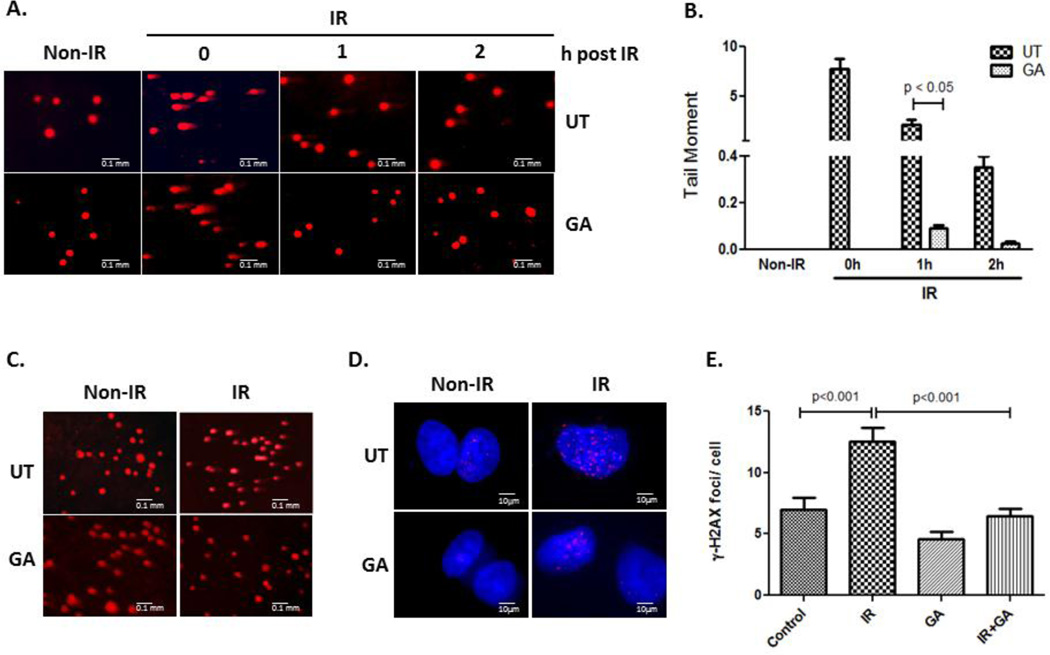
Earlier studies have pointed to a role of TLK1 in chromatin remodeling and DNA double-strand break repair. To investigate whether stimulation of endogenous TLK1 activity with GA evokes a similar response, we used denaturing single-cell gel electrophoresis assay and γ–H2AX foci formation as biomarkers of DNA single- and double- strand breaks, respectively. Radiation damage detected by alkaline comet assay showed a faster resolution of fragmented tail DNA in cells treated with GA compared to untreated cells (Figure 4A). One-way ANOVA test showed significant decrease in tail moment (p <0.0001), and pair wise comparison revealed significant difference between tail moments of control and GA treated cells at 1 h post IR (p < 0.05; Figure 4B). Within 2 h post-irradiation, 61% of cells treated with GA demonstrated complete resolution of tail DNA compared to 0% of untreated cells. Notably, a striking failure to repair DNA breaks in shRNA TLK1 cells and in those treated with inhibitor, THD, demonstrate the significance of TLK1 to DNA repair (Figure S1C,D, Supplementary Material). Correspondingly, cells treated with THD showed increase in apoptosis at 48 h after IR compared to GA suggesting lethality of unrepaired DNA damage in TLK1 compromised cells (THD, 3% late apoptotic cells; GA, 1.5% late apoptotic cells; Figure S3, Supplementary Material).
Figure 4. Resolution of radiation (IR)- induced DNA breaks in NS-SV-AC and shRNA TLK1 cells.
Single cells alkaline gel electrophoresis of A. NS-SV-AC cells and C. shRNA TLK1 cells. Cells were irradiated, and treated, or not, with GA. At indicated times, cells were electrophoresed, and cellular DNA analyzed by propidium iodide staining. B. The quantification of tail moment (mean ± SEM) of irradiated NS-SV-AC cells is graphed. D. Immuno-localization of phospho- S139 H2AX in NS-SV-AC cells 24 h after irradiation (IR). NS-SV-AC cells were irradiated prior to treatment with GA for 16 h. Cells were placed in drug-free medium for 8 h thereafter. Immuno-labeling with antiserum to gamma-H2AX (S139) is shown. E. Quantification of phospho-H2AX foci/ cell is shown. UT: untreated, IR: irradiation.
Unrepaired DNA double strand breaks are one of the most lethal forms of damage. To ascertain the effect of GA on repair of double strand breaks, the kinetics of γ-H2AX foci resolution was used as a biomarker. Immunofluorescence staining of γ-H2AX assessed 24 h after radiation showed markedly reduced foci in GA-treated cells compared to irradiated controls (Figure 4D). Radiation had a significant effect on the number of γ-H2AX foci in the untreated, mean ± SEM 12.52 ± 0.82 vs.7.0 ± 0.87, but had no significant effect in drug-treated cells i.e., mean ± SEM 6.48 ± 0.85 vs. 4.56 ± 0.78 (Figure 4E). A 2-way ANOVA analysis showed a highly significant effect of GA on γ-H2AX foci number (p < 0.01). Bonferroni multiple comparison test revealed significant treatment-radiation interaction (p = 0.02; significance at 5% level) suggesting that the effect of radiation was different among the 2 treatment groups. The data strongly indicate a positive effect of TLK1 modulator, GA, in facilitating repair of double-strand breaks.
Repair of I-SceI targeted breaks in the genome
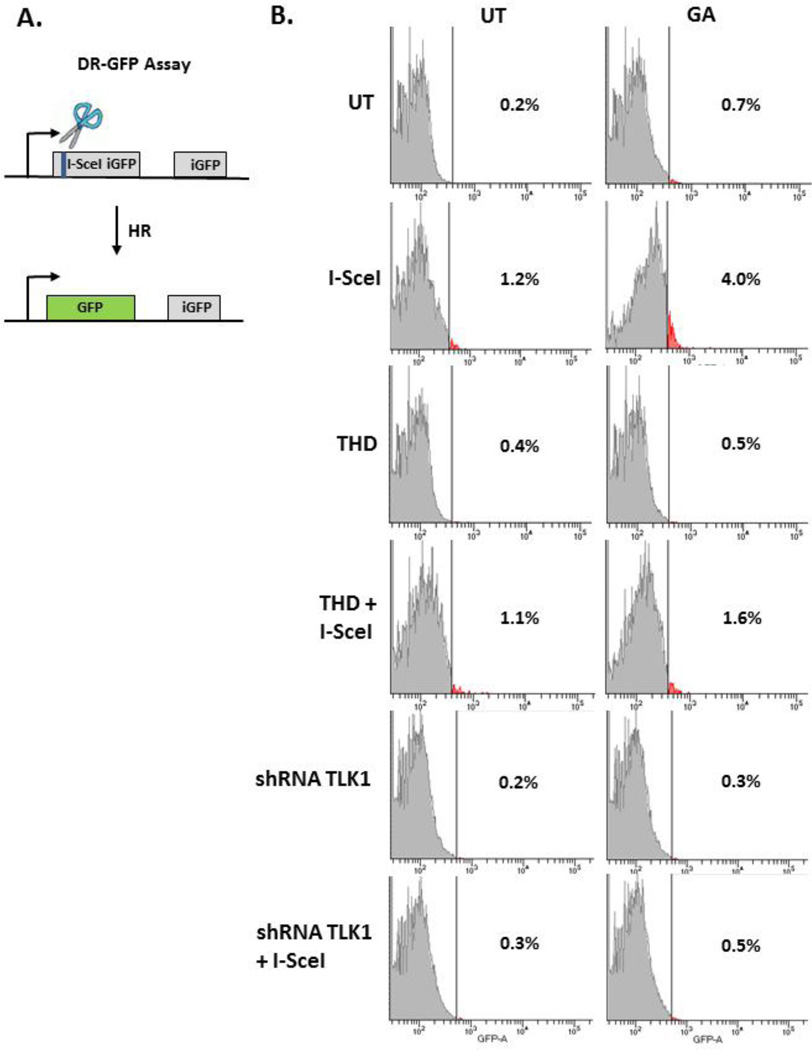
At the concentration used in the study, GA did not significantly alter radiation-generated reactive oxygen species compared to untreated cells (Figure S4, Supplementary Material). To conclusively show that GA effect is not a result of free-radical scavenging property of the compound, but rather its action of DNA repair, restoration of restriction enzyme cleaved DNA ends was studied in cells. Integrated reporter transgenes harboring I-SceI endonuclease sites have been used extensively to elucidate the double-strand break repair pathway of choice. MCF-7 DR-GFP cells contain inactive, dual GFP cassettes, and homologous recombinational repair (HR) at I-SceI break site reconstitutes a functional GFP gene (Figure 5A). Whereas, HEK293T PC222 cells have an integrated inactive RFP cassette, and non-homologous end-joining (NHEJ) at I-SceI introduced breaks restores RFP expression (Figure S5A, Supplementary Material). GA treatment following transient transfection of I-SceI in MCF-7 DR-GFP shows increase in GFP positive cells suggesting an increase in homology-directed double-strand break repair (Untreated: 1.03 ± 0.15% GFP positive cells; GA: 4 ± 0.2 % GFP positive cells). Pre-treatment with THD suppresses GA-facilitated HR (THD: 1.3 ± 0.28 % GFP positive cells, THD+GA: 2.15 ± 0.78 % GFP positive cells). More importantly, a negligible increase in GFP positive cells after I-SceI breaks in shRNA-TLK1 cells suggests a critical role of TLK1 in replication-mediated HR (Untreated: 0.3 % GFP positive cells; GA: 0.5 % GFP positive cells; Figure 5B). Because TLK1 can play a ubiquitous role in chromatin dynamics at break sites, we sought to determine the effect of GA on NHEJ. Study of I-SceI break repair in HEK293T PC222 showed that GA does not significantly change percent RFP-positive cells. Furthermore, THD did not appreciably decrease RFP positive cell population suggesting that activated TLK1 plays a prominent role in HR rather than NHEJ (Figure S5B, Supplementary Material).
Figure 5. Assessment of homologous recombinational repair in MCF-7 DR-GFP cells.
A. Schematic of HR assay, B. Evaluation of I-SceI double strand break repair after treatment with GA and, or, THD in NS-SV-AC and shRNA TLK1 transfected cells. UT: untreated, GA: gallic acid, THD: thioridazine.
Radioprotection of salivary function in vivo
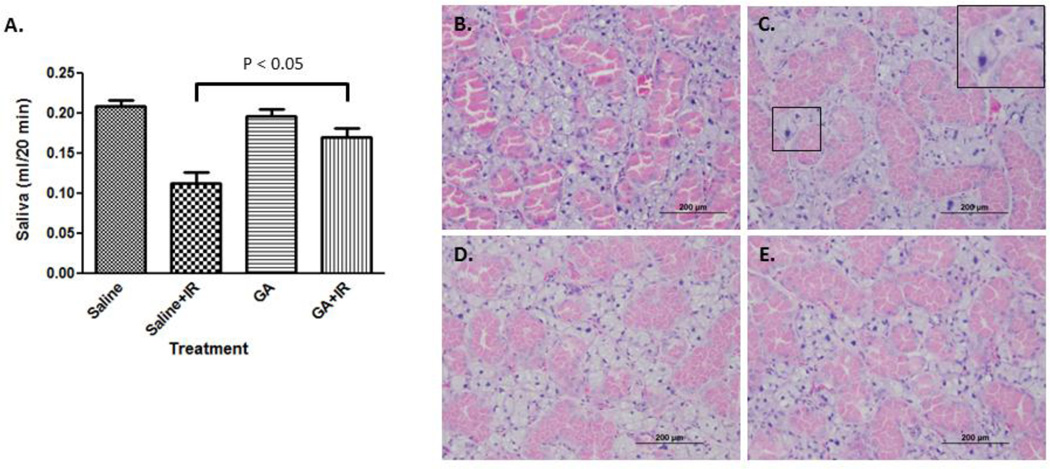
TLK1/1B plays an important role in chromatin dynamics and DNA repair. Having previously established that exogenous TLK1B expression in animal salivary glands protects against radiation-induced hypofunction, we examined the capability of small molecule, GA, in recapitulating the effect. GA was administered after each fraction of radiation, and salivary flow was assessed at 5 weeks. Mean salivary flow in control irradiated animals was significantly lower than non-IR (IR: mean ± SEM, 113.2 ± 39.5 µl/ 20 minutes; non-IR: mean ± SEM, 215 ± 24.1 µl/ 20 minutes; p < 0.01). Animals treated with saline experienced a 48% drop in salivary flow after radiation compared to 14% reduction in GA group (GA: mean ± SEM 196.2 ± 20.9 µl/20 minutes; GA+IR, mean ± SEM 169.6 ± 32.2 µl/ 20 minutes; Figure 6A). Using Bonferroni multiple comparison test, significant two-group comparison among drug-radiation interaction groups were saline and saline + IR groups, and saline + IR and GA + IR groups. Salivary flow was higher among irradiated animals treated with GA than saline, and the difference in mean salivary flow between the groups was significant (p < 0.05). No significant difference in mean salivary flow in GA-treatment groups (p > 0.05) suggests that radiation did not significantly affect salivary output. These results show that GA can allay the negative effects of radiation on salivary glands when administered post-radiation.
Figure 6. Salivary glands post irradiation.
A. The head and neck region of the animal was exposed to 2 Gy × 5 days. Animals were injected with saline or GA (50 mg/kg) each day after radiation. Stimulated saliva was collected at 5 weeks after the onset of radiation. Data shown is mean ± SEM. B–E. Histopathologic images of salivary glands; non-irradiated (B), irradiated (C), gallic acid-treated (D), irradiated and treated with gallic acid (E). Inset: enlargement of select area showing nucleo- and cytomegaly.
Histopathologic analyses of irradiated glands revealed an overall well-preserved salivary gland structure. However, isolated enlarged cells with hyperchromatic, enlarged nuclei (nucleomegaly) and abundant cytoplasm (cytomegaly) were evident after radiation in untreated than GA treated glands (Figure 6B–D). Because of the increase in size of the nucleus as well as the cytoplasm, the nuclear cytoplasmic ratio was not affected.
DISCUSSION
The fluid-producing acinar cells of the salivary glands are sensitive to radiation, and are functionally incapacitated within the first weeks of radiotherapy. There is no effective therapy for salivary hypofunction, and strategies that restore gland function can improve patient care and treatment outcomes. Radioprotection of the acinar cell population can, in principal, be a rational approach to staving off salivary hypofunction. Our past research efforts demonstrated the therapeutic efficacy of TLK1B in sustaining salivary gland function following radiation19–21. Since kinase activity is important for rescue against radiation-induced death, we reasoned that pharmacological stimulation of TLK1/1B could contribute to improved cell viability. We report here the establishment of GA as a facilitator of double strand break repair via a TLK1/1B-dependent mechanism in cell-based studies, and as a mitigator of radiation effect on salivary function in animals. Treatment with the phytochemical, GA, influences repair of radiation-induced DNA breaks and contributes to cell survival.
Previous reports have shown anti-oxidant effects of natural polyphenols in reducing intracellular reactive oxygen species concentration and in parallel, oxidatively damaged DNA in normal cells and tissues14–16. In contrast, studies on cancer cells have demonstrated an increase in levels of intracellular ROS, caspase activation, and poor survival after treatment with the phenolic compound10–13. It is, therefore, likely that GA will have no protective effect on tumor survival, and this will be tested in future studies. Taking into consideration the propensity of pyrogallol compounds to form DNA- topoisomerase complexes, it is proposed that prolonged treatment of cancer cells increases generation of hydrogen peroxide and their susceptibility to cell death9. GA, in particular, was shown to induce cell cycle arrest in a number of cancer cells in culture, and to suppress the growth of xenografts in nude mice22–24. In our studies, GA increased non-irradiated NS-SV-Ac in G1, but heightened S and G2 cell arrests only after radiation. Therefore, cellular context and DNA damage likely dictate the response to GA.
To avert the influence of GA on ROS-generated DNA damage, cells were treated after radiation. But, in essence, treatment post-irradiation served a second purpose. The protein kinase Chk1 is phosphorylated in response to DNA damage in an ATM- and ATR-dependent manner, and activated Chk1 down-regulates TLK1 transiently through phosphorylation at S6958. The temporary inactivation of TLK1 is necessary for Chk1-assisted checkpoint establishment, and knockdown of Chk1 increases radioresistant DNA synthesis25. Accordingly, we anticipated GA treatment after radiation to have little effect on Chk1 activation and checkpoint induction. And, as expected, radiation- induced halt of cell cycle in S and G2 phases was not diminished with GA.
Rapid repair of radiation-induced double-stranded breaks is crucial to averting chromosomal rearrangements and genomic instability. Non-homology dependent NHEJ is associated with repair of a majority of double stranded breaks, and a small percent are resolved by homology driven HR pathway in S and G2 phases. Secondary DNA breaks are known to occur 7–9 h after radiation. These breaks are replication-dependent and are repaired by HR26. The late resurgence of Chk1 activation in NS-SV-AC cells is a likely a result of subsequent DNA breaks resulting from collision of replication forks with unrepaired DNA damage. GA suppressed Chk1 reactivation, which suggests the collapse of fewer replication forks - a likely result of improved repair. Radiation-induced Chk2 activation was not significantly affected indicating that it has a minor role in radiation-induced DNA damage response in these cells.
Cells become sensitive to radiation when either the NHEJ or HR pathway is compromised27. To evaluate the repair pathway choice, restriction enzyme-induced double strand break repair of genome-integrated transgenes is widely used. Unlike radiation, double-strand breaks resulting from I-SceI or HO endonucleases are not influenced by scavengers of reactive oxygen species. The effect of GA on DNA repair was, therefore, validated in this system. Previous studies affirm the antioxidant effects of GA on survival, but in this study we provide evidence of its effect on DNA repair as well. The preferential stimulation of HR by GA suggests a role of TLK1 in this pathway. Rad9, a substrate of TLK1, forms a complex with Rad1 and Hus1 to detect and coordinate DNA repair4. TLK1 phosphorylates Rad9 S328 and T355 within its C-terminal tail, and phosphorylated Rad9 is required to overcome cell arrest in late stages of DNA damage response to radiation28. Rad9 interacts with Rad51, a protein that is instrumental for strand invasion into homologous sequences in sister chromatids during HR29 The preferential stimulation of HR by GA is not surprising given the interaction of TLK1 with Rad9.
The transfection of siRNA TLK1 slows S-phase kinetics7, 28, and the decrease in homology-driven repair in NS-SV-AC shRNA-TLK1 cells alludes to the requirement of replicated sister chromatids in HR. Our findings corroborate a contributory role of TLK1 in HR. Although some polyphenols increase NHEJ repair in vitro30, most studies have focused on their antioxidant property. Studies with GA assert to a direct radical scavenging mechanism of the compound or its role in transcriptional activation of intracellular ROS scavenging enzymes in radiation protection. The demonstration of DNA double strand break repair in this study supports the role of GA beyond that of a radical scavenger. Given the crucial role of DNA damage response in radiation survival, improved DNA repair kinetics can suppress adverse effects of radiation. And, we propose that GA-facilitated reunion of breaks rescues salivary gland function in vivo.
Altogether, our study shows the importance of TLK1 activator, GA, in repair of double-strand breaks. We demonstrate that GA- facilitated HR in S and G2 phases underlies the restoration of DNA integrity and cell survival against radiation. Earlier studies from our laboratory demonstrated the usefulness of exogenous TLK1B in preventing of radiation-inflicted salivary gland dysfunction, and in the current study we show that short-term pharmacological activation of cellular TLK1 is a viable alternative to improving palliative care and therapeutic outcomes in patients undergoing regional radiotherapy.
Highlights.
Gallic acid was identified as a pharmacological activator of Tousled-like kinase 1.
Human salivary acinar cell line, NS-SV-AC, treated with gallic acid after radiation showed improved cell survival.
An analysis of DNA break repair kinetics by single cell alkaline electrophoresis (comet assay) and by resolution of phospho-H2AX foci revealed that gallic acid facilitates repair of radiation-induced DNA breaks.
The study of restriction enzyme, I SceI- induced genomic double-strand break repair in MCF-7 DRGFP cells showed that gallic acid stimulates homologous recombinational repair in cells.
Acknowledgments
We thank Floyd Galiano for conducting the high-throughput screening of small molecule libraries at the Innovative North Louisiana Exploratory Therapeutics (INLET), LSU Health Shreveport and the Feist Weiller Cancer Center, and Priyanka Bajaj for preliminary studies in DNA repair.
FUNDING
The work was supported by the National Institute of Health /National Cancer Institute grant, R03CA169959, and the Feist-Weiller Cancer Center. Funding for open access charge: National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: There is no conflict of interest to disclose.
REFERENCES
- 1.Silljé HH, Takahashi K, Tanaka K, Van Houwe G, Nigg EA. Mammalian homologues of the plant Tousled gene code for cell-cycle-regulated kinases with maximal activities linked to ongoing DNA replication. EMBO J. 1999;18:5691–5702. doi: 10.1093/emboj/18.20.5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, DeFatta R, Anthony C, Sunavala G, De Benedetti A. A translationally regulated Tousled kinase phosphorylates histone H3 and confers radioresistance when overexpressed. Oncogene. 2001;20:726–738. doi: 10.1038/sj.onc.1204147. [DOI] [PubMed] [Google Scholar]
- 3.Silljé HH, Nigg EA. Identification of human Asf1 chromatin assembly factors as substrates of Tousled-like kinases. Curr Biol. 2001;11:1068–1073. doi: 10.1016/s0960-9822(01)00298-6. [DOI] [PubMed] [Google Scholar]
- 4.Sunavala-Dossabhoy G, De Benedetti A. Tousled homolog, TLK1, binds and phosphorylates Rad9; TLK1 acts as a molecular chaperone in DNA repair. DNA Repair (Amst) 2009;8:87–102. doi: 10.1016/j.dnarep.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Sunavala-Dossabhoy G, Balakrishnan SK, Sen S, Nuthalapaty S, De Benedetti A. The radioresistance kinase TLK1B protects the cells by promoting repair of double strand breaks. BMC Mol Biol. 2005;6:19. doi: 10.1186/1471-2199-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canfield C, Rains J, De Benedetti A. TLK1B promotes repair of DSBs via its interaction with Rad9 and Asf1. BMC Mol Biol. 2009;10:110. doi: 10.1186/1471-2199-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sunavala-Dossabhoy G, Li Y, Williams B, De Benedetti A. A dominant negative mutant of TLK1 causes chromosome missegregation and aneuploidy in normal breast epithelial cells. BMC Cell Biol. 2003;4:16. doi: 10.1186/1471-2121-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groth A, Lukas J, Nigg EA, Sillje HHW, Wernstedt C, Bartek J, et al. Human Tousled like kinases are targeted by an ATM- and Chk1-dependent DNA damage checkpoint. EMBO J. 2003;22:1676–1687. doi: 10.1093/emboj/cdg151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López-Lázaro M, Calderón-Montaño JM, Burgos-Morón E, Austin CA. Green tea constituents (−)-epigallocatechin-3-gallate (EGCG) and gallic acid induce topoisomerase I-and topoisomerase II-DNA complexes in cells mediated by pyrogallol-induced hydrogen peroxide. Mutagenesis. 2011;26:489–498. doi: 10.1093/mutage/ger006. [DOI] [PubMed] [Google Scholar]
- 10.Veluri R, Singh RP, Liu Z, Thompson JA, Agarwal R, Agarwal C. Fractionation of grape seed extract and identification of gallic acid as one of the major active constituents causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis. 2006;27:1445–1453. doi: 10.1093/carcin/bgi347. [DOI] [PubMed] [Google Scholar]
- 11.Raina K, Rajamanickam S, Deep G, Singh M, Agarwal R, Agarwal C. Chemopreventive effects of oral gallic acid feeding on tumor growth and progression in TRAMP mice. Mol Cancer Ther. 2008;7:1258–1267. doi: 10.1158/1535-7163.MCT-07-2220. [DOI] [PubMed] [Google Scholar]
- 12.Maurya DK, Nandakumar N, Devasagayam TP. Anticancer property of gallic acid in A549, a human lung adenocarcinoma cell line, and possible mechanisms. J Clin Biochem Nutr. 2011;48:85–90. doi: 10.3164/jcbn.11-004FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell LH, Jr, Mazzio E, Badisa RB, Zhu ZP, Agharahimi M, Oriaku ET, et al. Autoxidation of gallic acid induces ROS-dependent death in human prostate cancer LNCaP cells. Anticancer Res. 2012;32:1595–1602. [PMC free article] [PubMed] [Google Scholar]
- 14.Nair GG, Nair CK. Radioprotective effects of gallic acid in mice. Biomed Res. Int. 2013;2013:953079. doi: 10.1155/2013/953079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandhi NM, Nair CK. Protection of DNA and membrane from gamma radiation induced damage by gallic acid. Mol Cell Biochem. 2005;278:111–117. doi: 10.1007/s11010-005-6940-1. [DOI] [PubMed] [Google Scholar]
- 16.Ferk F, Chakraborty A, Jäger W, Kundi M, Bichler J, Mišík M, et al. Potent protection of gallic acid against DNA oxidation: results of human and animal experiments. Mutat Res. 2011;715:61–71. doi: 10.1016/j.mrfmmm.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Azuma M, Tamatani T, Kasai Y, Sato M. Immortalization of normal human salivary gland cells with duct-, myoepithelial-, acinar-, or squamous phenotype by transfection with SV40 ori- mutant deoxyribonucleic acid. Lab Invest. 1993;69:24–42. [PubMed] [Google Scholar]
- 18.Ronald S, Awate S, Rath A, Carroll J, Galiano F, Dwyer D, et al. Phenothiazine Inhibitors of TLKs Affect Double-Strand Break Repair and DNA Damage Response Recovery and Potentiate Tumor Killing with Radiomimetic Therapy. Genes Cancer. 2013;4:39–53. doi: 10.1177/1947601913479020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palaniyandi S, Odaka Y, Green W, Abreo F, Caldito G, De Benedetti A, Sunavala-Dossabhoy G. Adenoviral delivery of Tousled kinase for the protection of salivary glands against ionizing radiation damage. Gene Ther. 2011;18:275–282. doi: 10.1038/gt.2010.142. [DOI] [PubMed] [Google Scholar]
- 20.Sunavala-Dossabhoy G, Palaniyandi S, Richardson C, De Benedetti A, Schrott L, Caldito G. TAT-mediated delivery of Tousled protein to salivary glands protects against radiation-induced hypofunction. Int J Radiat Oncol Biol Phys. 2012;84:257–265. doi: 10.1016/j.ijrobp.2011.10.064. [DOI] [PubMed] [Google Scholar]
- 21.Timiri Shanmugam PS, Dayton RD, Palaniyandi S, Abreo F, Caldito G, Klein RL, et al. Recombinant AAV9-TLK1B administration ameliorates fractionated radiation-induced xerostomia. Hum Gene Ther. 2013;24:604–612. doi: 10.1089/hum.2012.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal C, Tyagi A, Agarwal R. Gallic acid causes inactivating phosphorylation of cdc25A/cdc25C-cdc2 via ATM-Chk2 activation, leading to cell cycle arrest, and induces apoptosis in human prostate carcinoma DU145 cells. Mol Cancer Ther. 2006;5:3294–3302. doi: 10.1158/1535-7163.MCT-06-0483. [DOI] [PubMed] [Google Scholar]
- 23.Kaur M, Singh RP, Gu M, Agarwal R, Agarwal C. Grape seed extract inhibits in vitro and in vivo growth of human colorectal carcinoma cells. Clin Cancer Res. 2006;12:6194–6202. doi: 10.1158/1078-0432.CCR-06-1465. [DOI] [PubMed] [Google Scholar]
- 24.Ou TT, Wang CJ, Lee YS, Wu CH, Lee HJ. Gallic acid induces G2/M phase cell cycle arrest via regulating 14-3-3β release from Cdc25C and Chk2 activation in human bladder transitional carcinoma cells. Mol Nutr Food Res. 2010;54:1781–1790. doi: 10.1002/mnfr.201000096. [DOI] [PubMed] [Google Scholar]
- 25.Gatei M, Sloper K, Sorensen C, Syljuäsen R, Falck J, Hobson K, et al. Ataxia-telangiectasia-mutated (ATM) and NBS1-dependent phosphorylation of Chk1 on Ser-317 in response to ionizing radiation. J Biol Chem. 2003;278:14806–14811. doi: 10.1074/jbc.M210862200. [DOI] [PubMed] [Google Scholar]
- 26.Groth P, Orta ML, Elvers I, Majumder MM, Lagerqvist A, Helleday T. Homologous recombination repairs secondary replication induced DNA double-strand breaks after ionizing radiation. Nucleic Acids Res. 2012;40:6585–6594. doi: 10.1093/nar/gks315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothkamm K, Kruger I, Thompson LH, Lobrich M. Pathways of DNA double-strand break repair during mammalian cell cycle. Mol Cell Biol. 2003;23:5706–5715. doi: 10.1128/MCB.23.16.5706-5715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly R, Davey SK. Tousled-like kinase-dependent phosphorylation of Rad9 plays a role in cell cycle progression and G2/M checkpoint exit. PLoS One. 2013;8:e85859. doi: 10.1371/journal.pone.0085859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandita RK, Sharma GG, Laszlo A, Hopkins KM, Davey S, Chakhparonian M, et al. Mammalian Rad9 play a role in telomere stability, S- and G2-phase-specific cell survival and homologous recombinational repair. Mol Cell Biol. 2006;26:1850–1864. doi: 10.1128/MCB.26.5.1850-1864.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charles C, Nachtergael A, Ouedraogo M, Belayew A, Duez P. Effects of chemopreventive natural products on non-homologous end joining DNA double-strand break repair. Mutat Res. 2014;768:33–41. doi: 10.1016/j.mrgentox.2014.04.014. [DOI] [PubMed] [Google Scholar]